Constraining Polymers into β-Turns: Miscibility and Phase Segregation Effects in Lipid Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
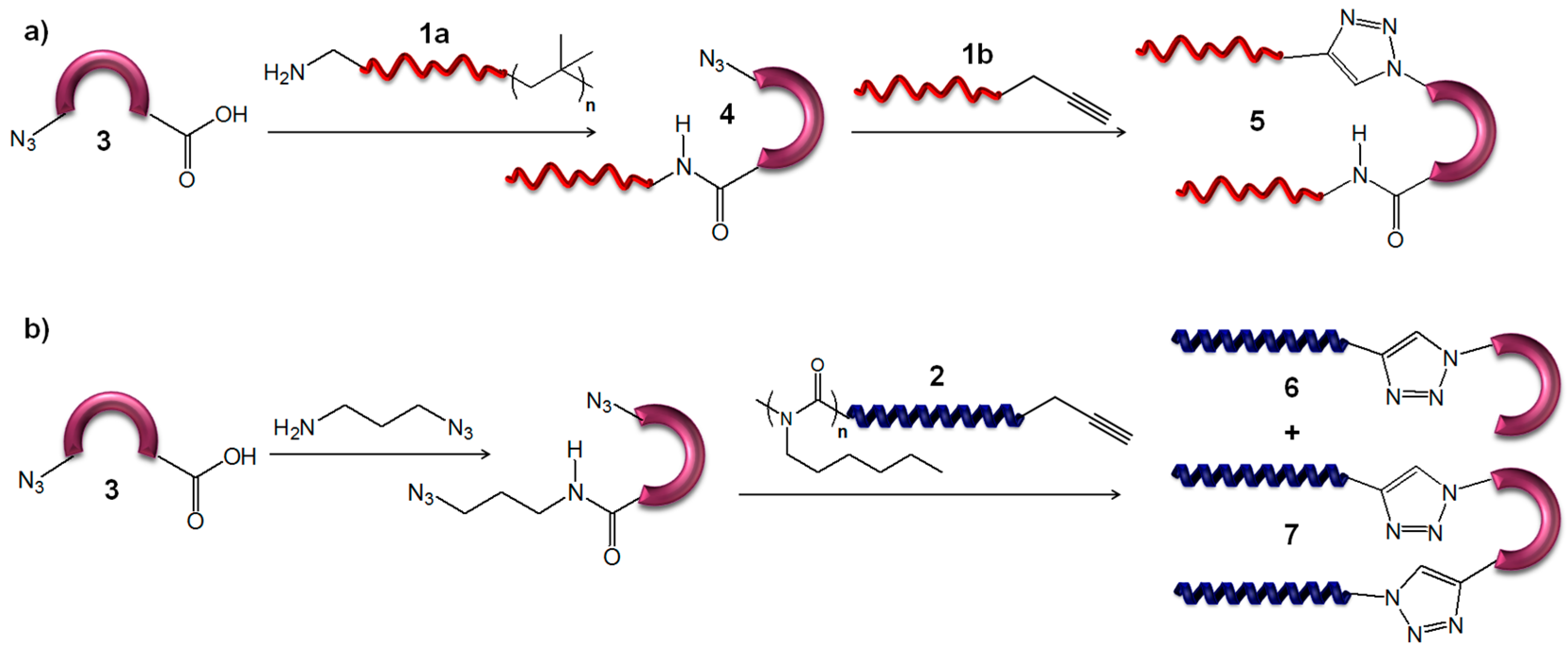
2.3. General Procedure for the Synthesis of β-Turn Mimetic Conjugates
3. Results and Discussion
3.1. Polyisobutylene Conjugates
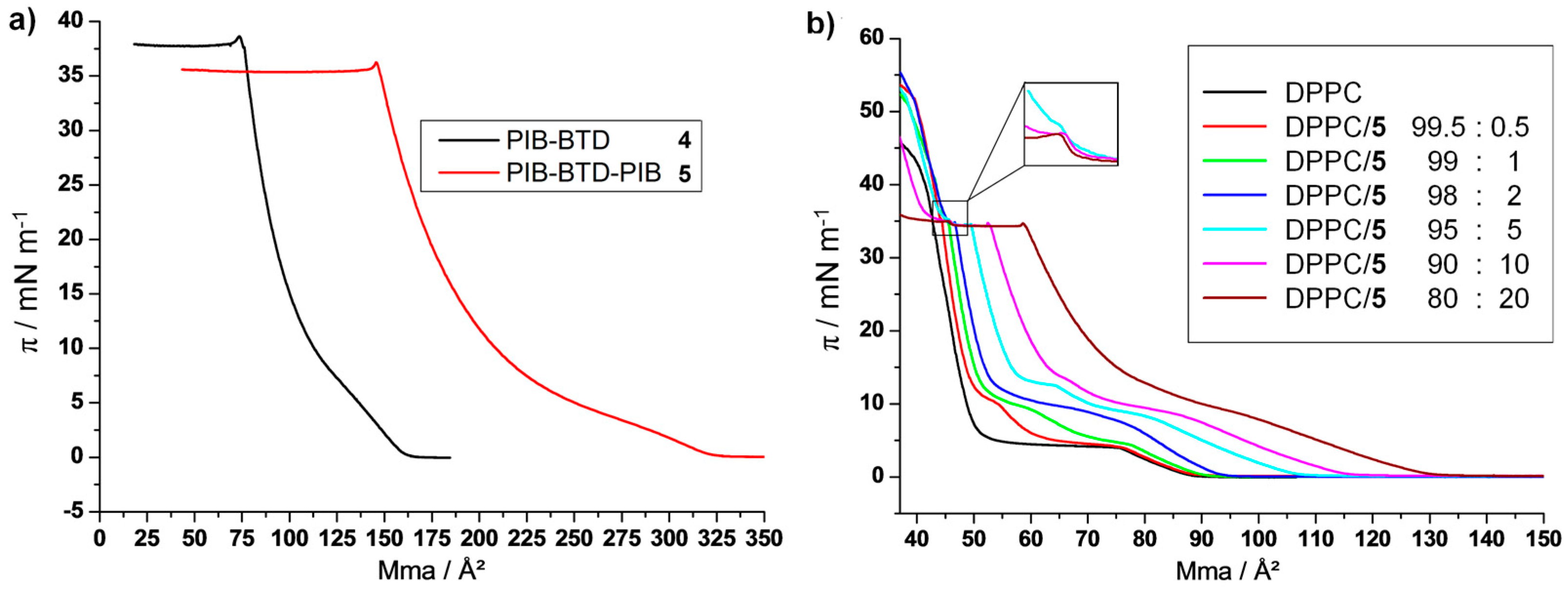
3.1.1. π-A Isotherms
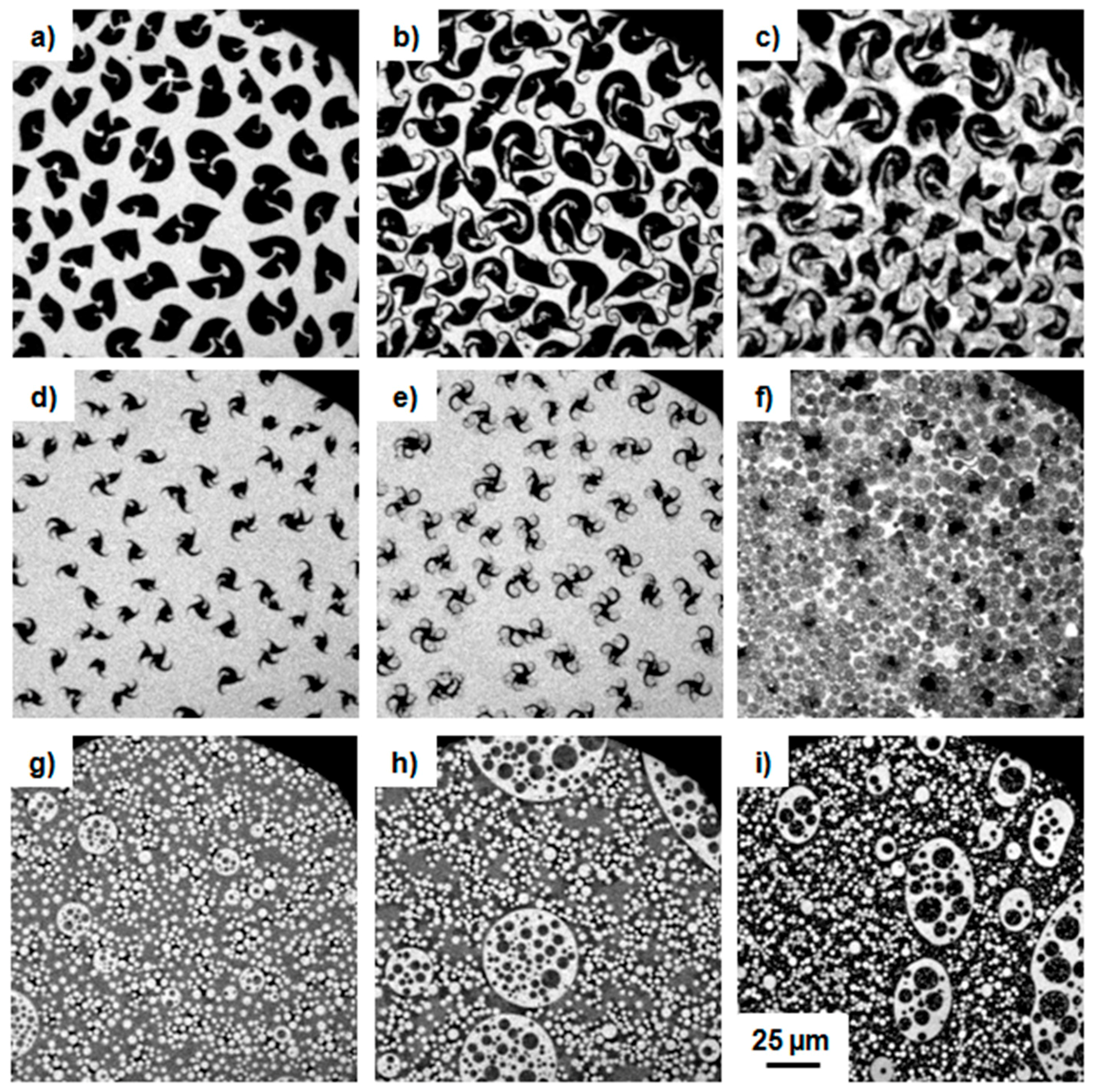
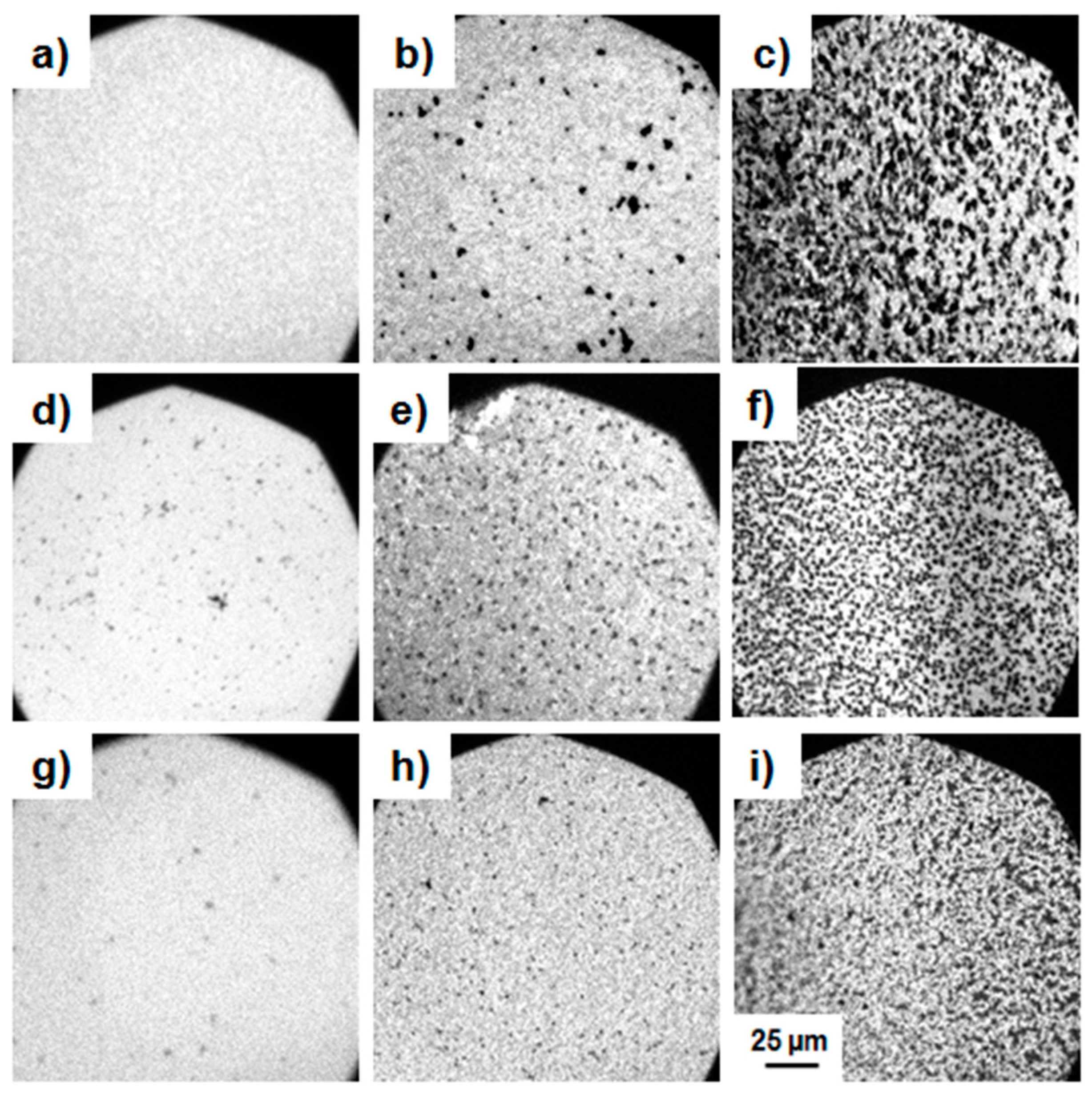
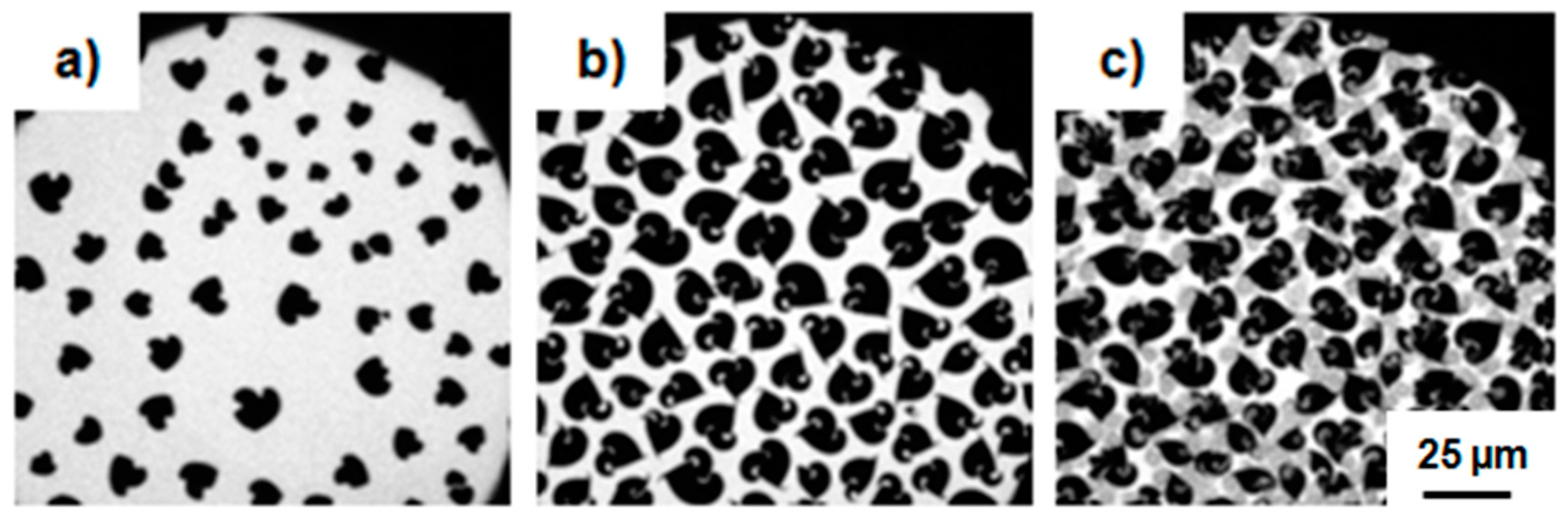
3.1.2. Epifluorescence Microscopy
3.1.3. AFM
3.2. Poly(n-Hexyl Isocyanate) Conjugates
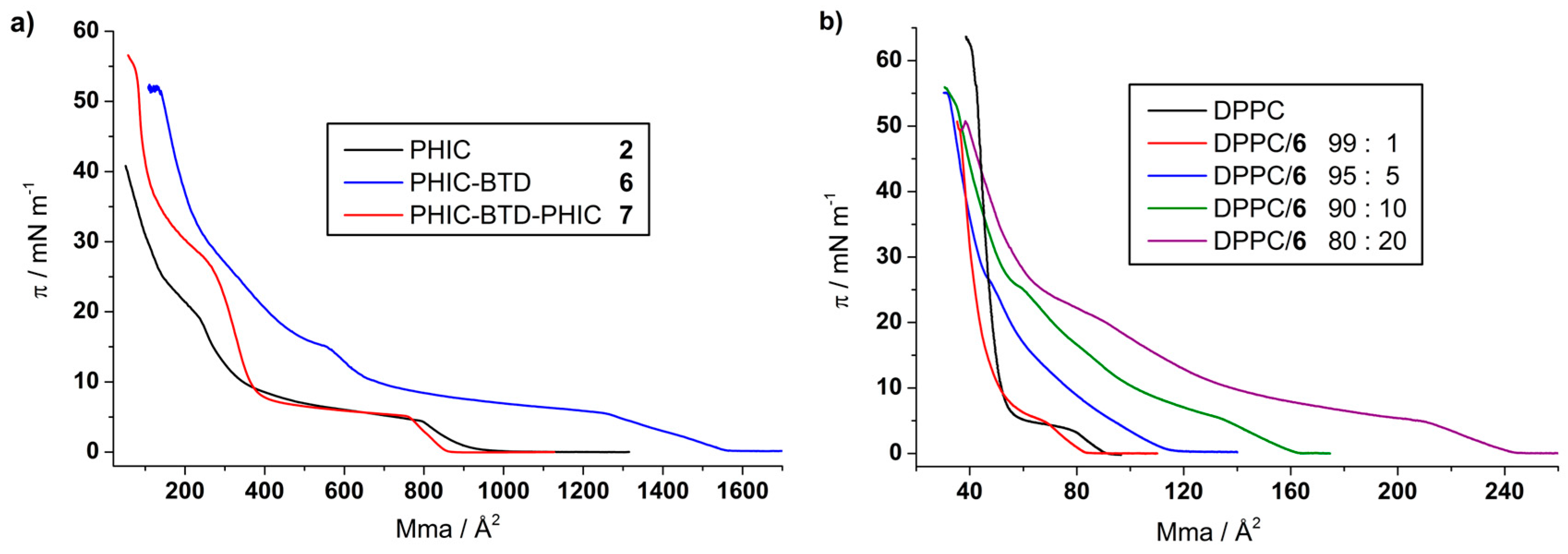
3.2.1. π-A Isotherms
3.2.2. Epifluorescence Microscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, A.; Glaser, N.; Le Meins, J.; Lecommandoux, S. Block copolymer vesicle permeability measured by osmotic swelling and shrinking. Langmuir 2011, 27, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Schulz, M.; Binder, W.H. Mixed hybrid lipid/polymer vesicles as a novel membrane platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the lipid-bilayer: Interaction of polymers and nanoparticles with membranes. Soft Matter. 2012, 8, 4849–4864. [Google Scholar] [CrossRef]
- Binder, W.H.; Barragan, V.; Menger, F.M. Domains and rafts in lipid membranes. Angew. Chem. Int. Ed. 2003, 42, 5802–5827. [Google Scholar] [CrossRef] [PubMed]
- Olubummo, A.; Schulz, M.; Lechner, B.-D.; Scholtysek, P.; Bacia, K.; Blume, A.; Kressler, J.; Binder, W.H. Controlling the localization of polymer-functionalized nanoparticles in mixed lipid/polymer membranes. ACS Nano. 2012, 6, 8713–8727. [Google Scholar] [CrossRef] [PubMed]
- Marsat, J.-N.; Heydenreich, M.; Kleinpeter, E.; Berlepsch, H.V.; Böttcher, C.; Laschewsky, A. Self-assembly into multicompartment micelles and selective solubilization by hydrophilic-lipophilic-fluorophilic block copolymers. Macromolecules 2011, 44, 2092–2105. [Google Scholar] [CrossRef]
- Amado, E.; Kressler, J. Triphilic block copolymers with perfluorocarbon moieties in aqueous systems and their biochemical perspectives. Soft Matter. 2011, 7, 7144–7149. [Google Scholar] [CrossRef]
- Marsat, J.-N.; Stahlhut, F.; Laschewsky, A.; Berlepsch, H.; Böttcher, C. Multicompartment micelles from silicone-based triphilic block copolymers. Colloid Polym. Sci. 2013, 291, 2561–2567. [Google Scholar] [CrossRef]
- Schwieger, C.; Achilles, A.; Scholz, S.; Ruger, J.; Bacia, K.; Saalwaechter, K.; Kressler, J.; Blume, A. Binding of amphiphilic and triphilic block copolymers to lipid model membranes: The role of perfluorinated moieties. Soft Matter. 2014, 10, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ichihara, H.; Hino, M.; Umebayashi, M.; Ueoka, R. Therapeutic effects of hybrid liposomes without drugs for rheumatoid arthritis. Drug Deliv. 2015, 22, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Hattori, S.; Kariya, R.; Komizu, Y.; Kudo, E.; Goto, H.; Taura, M.; Ueoka, R.; Kimura, S.; Okada, S. Inhibition of HIV-1 entry by the tricyclic coumarin gut-70 through the modification of membrane fluidity. Biochem. Biophys. Res. Commun. 2015, 457, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, H.; Yamasaki, S.; Hino, M.; Ueoka, R.; Matsumoto, Y. Therapeutic effects of hybrid liposomes with downregulation of inflammatory cytokine for model mice of rheumatoid arthritis in vivo. Bioorg. Med. Chem. Lett. 2015, 25, 2686–2689. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, H.; Komizu, Y.; Ichihara, H.; Goto, K.; Ueoka, R. Hybrid liposomes inhibit tumor growth and lung metastasis of murine osteosarcoma cells. Cancer/ Med. 2013, 2, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hino, M.; Ichihara, H.; Matsumoto, Y.; Ueoka, R. Anti-tumor effects of cationic hybrid liposomes against colon carcinoma along with apoptosis in vitro. Biol. Pharm. Bull. 2012, 35, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Tanaka, K.; Komizu, Y.; Tamiya-Koizumi, K.; Murate, T.; Ueoka, R.; Kyogashima, M.; Usukura, J.; Takahashi, T.; Suzuki, M. Hybrid liposomes affect cellular lipid constituents and caveolae structures. Bioorg. Med. Chem. Lett. 2012, 22, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, R.; Matsumoto, Y.; Goto, K.; Ichihara, H.; Komizu, Y. Membrane targeted chemotherapy with hybrid liposomes for tumor cells leading to apoptosis. Curr. Pharm. Des. 2011, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Komizu, Y.; Nakata, S.; Goto, K.; Matsumoto, Y.; Ueoka, R. Membrane-targeted nanotherapy with hybrid liposomes for tumor cells leading to apoptosis. ACS Med. Chem. Lett. 2011, 2, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, H.; Zako, K.; Komizu, Y.; Goto, K.; Ueoka, R. Therapeutic effects of hybrid liposomes composed of phosphatidylcholine and docosahexaenoic acid on the hepatic metastasis of colon carcinoma along with apoptosis in vivo. Biol. Pharm. Bull. 2011, 34, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Komizu, Y.; Nakata, S.; Goto, K.; Matsumoto, Y.; Ueoka, R. Clustering of lipid rafts in plasma membranes by hybrid liposomes for leukemia cells along with apoptosis. Chem. Lett. 2010, 39, 1291–1293. [Google Scholar] [CrossRef]
- Schulz, M.; Werner, S.; Bacia, K.; Binder, W.H. Controlling molecular recognition with lipid/polymer domains in vesicle membranes. Angew. Chem. Int. Ed. 2013, 52, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Wu, D.; Mikhalevich, V.; Palivan, C.G.; Meier, W. Hybrid polymer-lipid films as platforms for directed membrane protein insertion. Langmuir 2015, 31, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Hussain, H.; Schwieger, C.; Schulz, M.; Binder, W.H.; Kressler, J. Molecular arrangement of symmetric and non-symmetric triblock copolymers of poly(ethylene oxide) and poly(isobutylene) at the air–water interface. J. Colloid Interface Sci. 2015, 437, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Olubummo, A.; Bacia, K.; Binder, W.H. Lateral surface engineering of hybrid lipid-bcp vesicles and selective nanoparticle embedding. Soft Matter. 2014, 10, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Olubummo, A.; Schulz, M.; Schöps, R.; Kressler, J.; Binder, W.H. Phase changes in mixed lipid/polymer membranes by multivalent nanoparticle recognition. Langmuir 2014, 30, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Glatte, D.; Meister, A.; Scholtysek, P.; Kerth, A.; Blume, A.; Bacia, K.; Binder, W.H. Hybrid lipid/polymer giant unilamellar vesicles: Effects of incorporated biocompatible pib-peo block copolymers on vesicle properties. Soft Matter. 2011, 7, 8100–8110. [Google Scholar] [CrossRef]
- Sachsenhofer, R.; Binder, W.H.; Farnik, D.; Zirbs, R. Polymersome-embedded nanoparticles. Macromol. Symp. 2007, 254, 375–377. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R.; Farnik, D.; Blaas, D. Guiding the location of nanoparticles into vesicular structures: A morphological study. Phys. Chem. Chem. Phys. 2007, 9, 6435–6441. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Kerschner, H.; Georgopoulos, A.; Barragan-Montero, V.; Einzmann, M. Chemistry with liposomes: Controlling membrane-structure by macromolecules. In Proceedings of the Joint Meeting on Medicinal Chemistry, Vienna, Austria, 20–23 June 2005; pp. 113–116. [Google Scholar]
- Binder, W.H. Supramolecular assembly of nanoparticles at liquid-liquid interfaces. Angew. Chem. Int. Ed. 2005, 44, 5172–5175. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Einzmann, M.; Knapp, M.; Köhler, G. Domain formation in lipid bilayer membranes: Control of membrane nanostructure by molecular architecture. Monatsheft Chem. 2004, 135, 13–21. [Google Scholar] [CrossRef]
- Malke, M.; Barqawi, H.; Binder, W.H. Synthesis of an amphiphilic β-turn mimetic polymer conjugate. ACS Macro. Lett. 2014, 3, 393–397. [Google Scholar] [CrossRef]
- Freidinger, R.M.; Perlow, D.S.; Veber, D.F. Protected lactam-bridged dipeptides for use as conformational constraints in peptides. J. Org. Chem. 1982, 47, 104–109. [Google Scholar] [CrossRef]
- Graf von Roedern, E.; Lohof, E.; Hessler, G.; Hoffmann, M.; Kessler, H. Synthesis and conformational analysis of linear and cyclic peptides containing sugar amino acids. J. Am. Chem. Soc. 1996, 118, 10156–10167. [Google Scholar] [CrossRef]
- Schneider, J.P.; Kelly, J.W. Synthesis and efficacy of square planar copper complexes designed to nucleate b-sheet structure. J. Am. Chem. Soc. 1995, 117, 2533–2546. [Google Scholar] [CrossRef]
- Aemissegger, A.; Kräutler, V.; van Gunsteren, W.F.; Hilvert, D. A photoinducible β-hairpin. J. Am. Chem. Soc. 2005, 127, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, P.; Geyer, A. Coupled hydrogen-bonding networks in polyhydroxylated peptides. Angew. Chem. Int. Ed. 2004, 43, 5789–5791. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Geyer, A. Nmr spectroscopic characterization of the membrane affinity of polyols. Magn. Reson. Chem. 2005, 43, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Akbar, Z.; Phoenix, D.A.; Snape, T.J. Interactions between suitably functionalised conformationally distinct benzanilides and phospholipid monolayers. Soft Matter. 2012, 8, 3258–3264. [Google Scholar] [CrossRef]
- Dennison, S.R.; Snape, T.J.; Phoenix, D.A. Thermodynamic interactions of a cis and trans benzanilide with escherichia coli bacterial membranes. Eur. Biophys. J. 2012, 41, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Baier, G.; Siebert, J.M.; Landfester, K.; Musyanovych, A. Surface click reactions on polymeric nanocapsules for versatile functionalization. Macromolecules 2012, 45, 3419–3427. [Google Scholar] [CrossRef]
- Deike, S.; Binder, W.H. Induction of chirality in β-turn mimetic polymer conjugates via postpolymerization “click” coupling. Macromolecules 2017, 50, 2637–2644. [Google Scholar] [CrossRef]
- Adekunle, O.; Herbst, F.; Hackethal, K.; Binder, W.H. Synthesis of nonsymmetric chain end functionalized polyisobutylenes. J. Polym. Sci. A 2011, 49, 2931–2940. [Google Scholar] [CrossRef]
- Morgan, D.L.; Martinez-Castro, N.; Storey, R.F. End-quenching of TiCl4-catalyzed quasiliving polyisobutylene with alkoxybenzenes for direct chain end functionalization. Macromolecules 2010, 43, 8724–8740. [Google Scholar] [CrossRef]
- Li, H.; Sachsenhofer, R.; Binder, W.H.; Henze, T.; Thurn-Albrecht, T.; Busse, K.; Kressler, J. Hierarchical organization of poly(ethylene oxide)-block-poly(isobutylene) and hydrophobically modified Fe2O3 nanoparticles at the air–water interface and on solid supports. Langmuir 2009, 25, 8320–8329. [Google Scholar] [CrossRef] [PubMed]
- Schröter, J.A.; Plehnert, R.; Tschierske, C.; Katholy, S.; Janietz, D.; Penacorada, F.; Brehmer, L. Monolayer properties of a new family of amphiphiles with an unusual head-group topology. Langmuir 1997, 13, 796–800. [Google Scholar] [CrossRef]
- Reynolds, P.A.; McGillivray, D.J.; Gilbert, E.P.; Holt, S.A.; Henderson, M.J.; White, J.W. Neutron and x-ray reflectivity from polyisobutylene-based amphiphiles at the air−water interface. Langmuir 2003, 19, 752–761. [Google Scholar] [CrossRef]
- Weis, R.M. Fluorescence microscopy of phospholipid monolayer phase transitions. Chem. Phys. Lipids 1991, 57, 227–239. [Google Scholar] [CrossRef]
- Scholtysek, P.; Li, Z.; Kressler, J.; Blume, A. Interactions of dppc with semitelechelic poly(glycerol methacrylate)s with perfluoroalkyl end groups. Langmuir 2012, 28, 15651–15662. [Google Scholar] [CrossRef] [PubMed]
- El Kirat, K.; Besson, F.; Prigent, A.-F.; Chauvet, J.-P.; Roux, B. Role of calcium and membrane organization on phospholipase d localization and activity: Competition between a soluble and an insoluble substrate. J. Biol. Chem. 2002, 277, 21231–21236. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Yamamoto, M.; Kurauchi, N.; Kato, T. Surface pressure and fluorescence microscopy studies of poly(n-hexyl isocyanate) films spread at the air−water interface. Langmuir 1999, 15, 1388–1391. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Ishikawa, R.; Yamamoto, M.; Kuki, T.; Kato, T. Molecular weight dependence of interfacial properties of poly(n-hexyl isocyanate) films at the air−water and oil−water interfaces. Langmuir 2001, 17, 384–387. [Google Scholar] [CrossRef]
- Morioka, T.; Shibata, O.; Kawaguchi, M. Molecular weight dependence of lb morphology of poly(n-hexyl isocyanate) (phic). Langmuir 2010, 26, 18189–18193. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Shibata, O.; Kawaguchi, M. Molecular weight dependence of surface dilatational moduli of poly(n-hexyl isocyanate) films spread at the air−water interface. Langmuir 2010, 26, 14058–14063. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, L.; Becerra, N.; Sandoval, C.; Pitsikalis, M.; Hadjichristidis, N.; Leiva, A.; Radic, D. Amphiphilic diblock copolymers containing poly(n-hexylisocyanate): Monolayer behavior at the air–water interface. J. Appl. Polym. Sci. 2011, 122, 1395–1404. [Google Scholar] [CrossRef]
- Kim, J.-H.; Rahman, M.S.; Lee, J.-S.; Park, J.-W. Liquid crystalline ordering in the self-assembled monolayers of tethered rodlike polymers. J. Am. Chem. Soc. 2007, 129, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves da Silva, A.M.; Filipe, E.J.M.; d’Oliveira, J.M.R.; Martinho, J.M.G. Interfacial behavior of poly(styrene)−poly(ethylene oxide) diblock copolymer monolayers at the air−water interface. Hydrophilic block chain length and temperature influence. Langmuir 1996, 12, 6547–6553. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deike, S.; Malke, M.; Lechner, B.-D.; Binder, W.H. Constraining Polymers into β-Turns: Miscibility and Phase Segregation Effects in Lipid Monolayers. Polymers 2017, 9, 369. https://doi.org/10.3390/polym9080369
Deike S, Malke M, Lechner B-D, Binder WH. Constraining Polymers into β-Turns: Miscibility and Phase Segregation Effects in Lipid Monolayers. Polymers. 2017; 9(8):369. https://doi.org/10.3390/polym9080369
Chicago/Turabian StyleDeike, Stefanie, Marlen Malke, Bob-Dan Lechner, and Wolfgang H. Binder. 2017. "Constraining Polymers into β-Turns: Miscibility and Phase Segregation Effects in Lipid Monolayers" Polymers 9, no. 8: 369. https://doi.org/10.3390/polym9080369




