TEMPO-Oxidized Cellulose with High Degree of Oxidation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
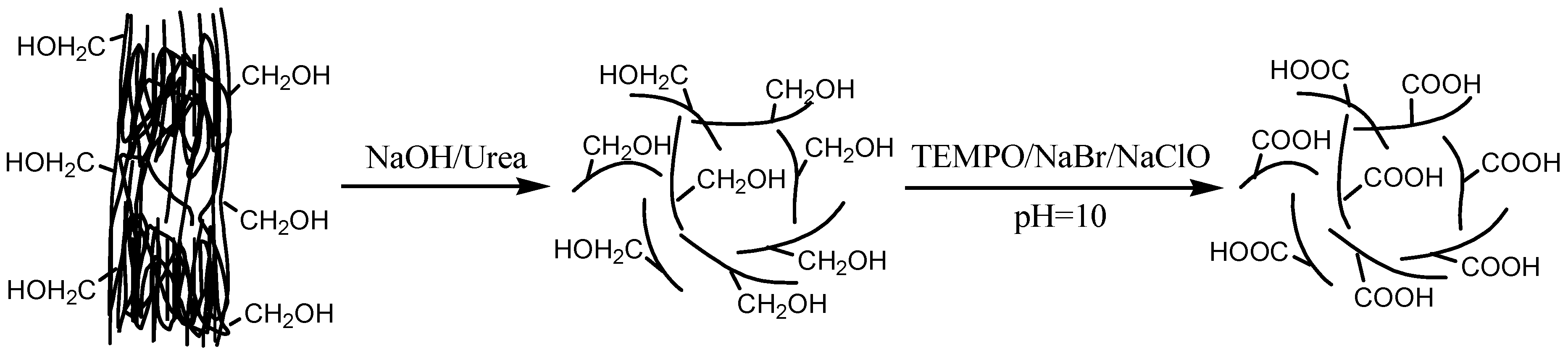
2.2. Preparation of TEMPO-Oxidized Cellulose
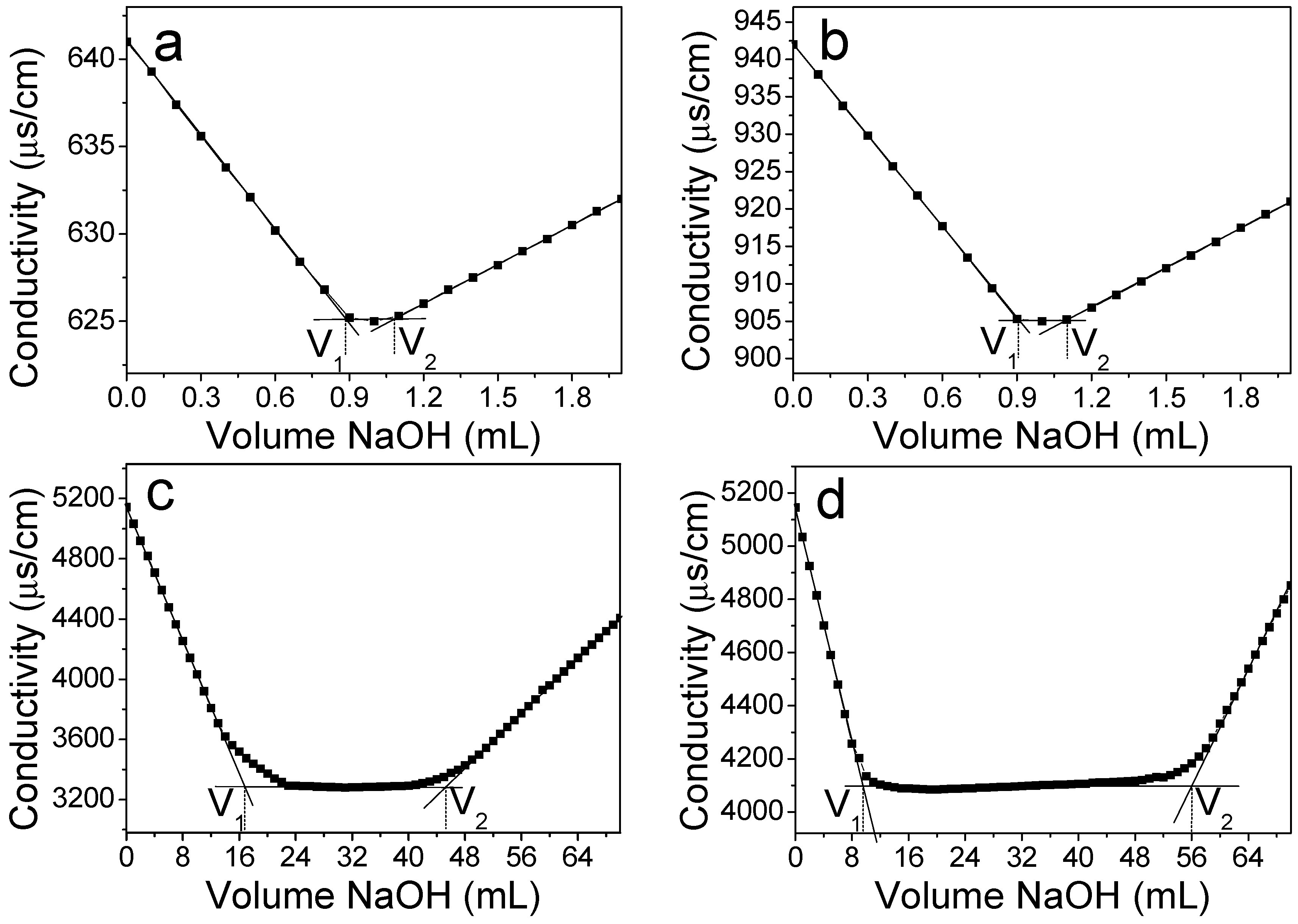
2.3. Degree of Oxidation (DO)
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5. Fiber Analyzer
2.6. Wide Angle X-ray Diffraction (WXRD)
2.7. Transmission Electron Microscopy (TEM)
3. Results and Discussion
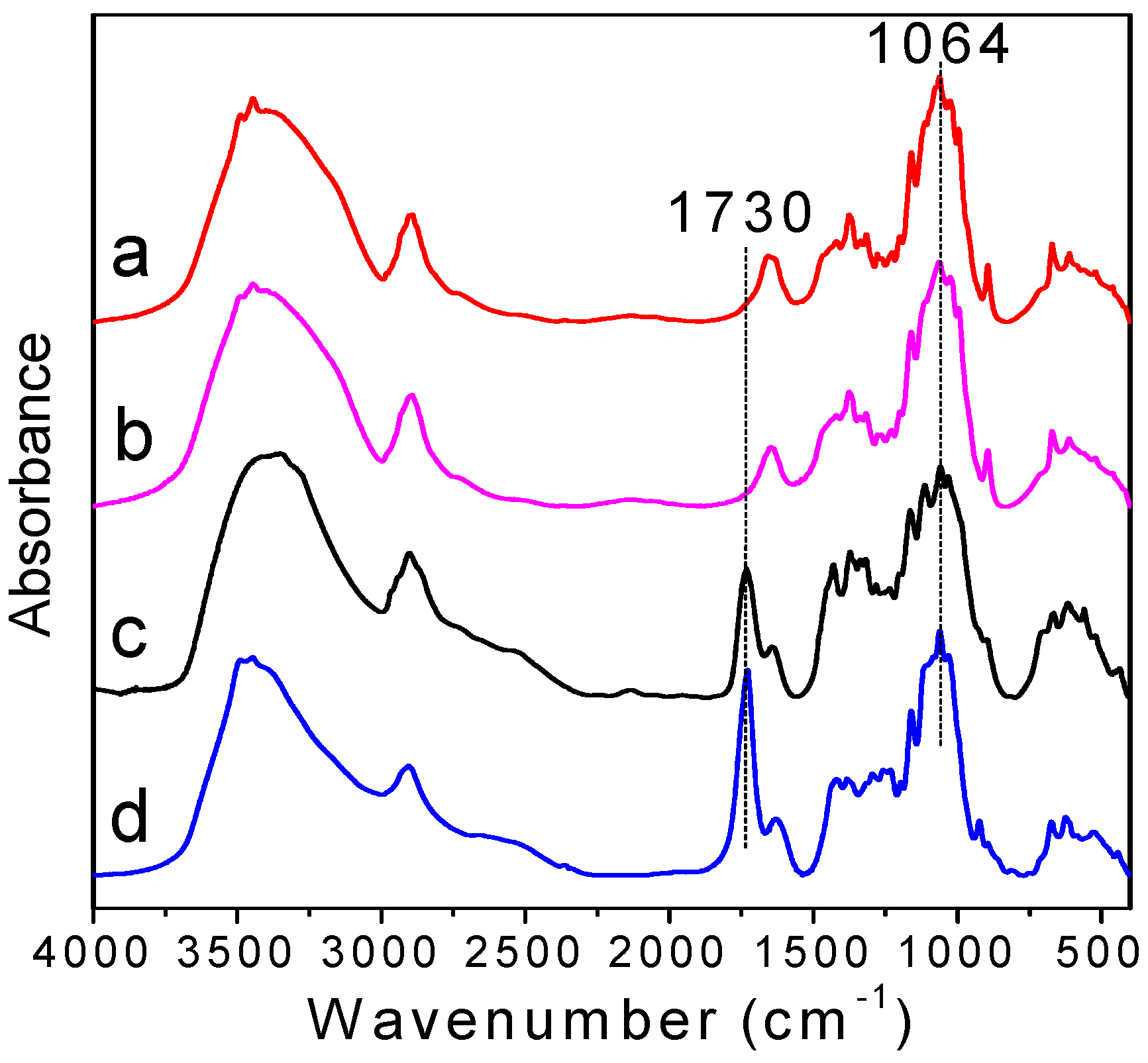
3.1. Infrared Analysis of Oxidized Cellulose
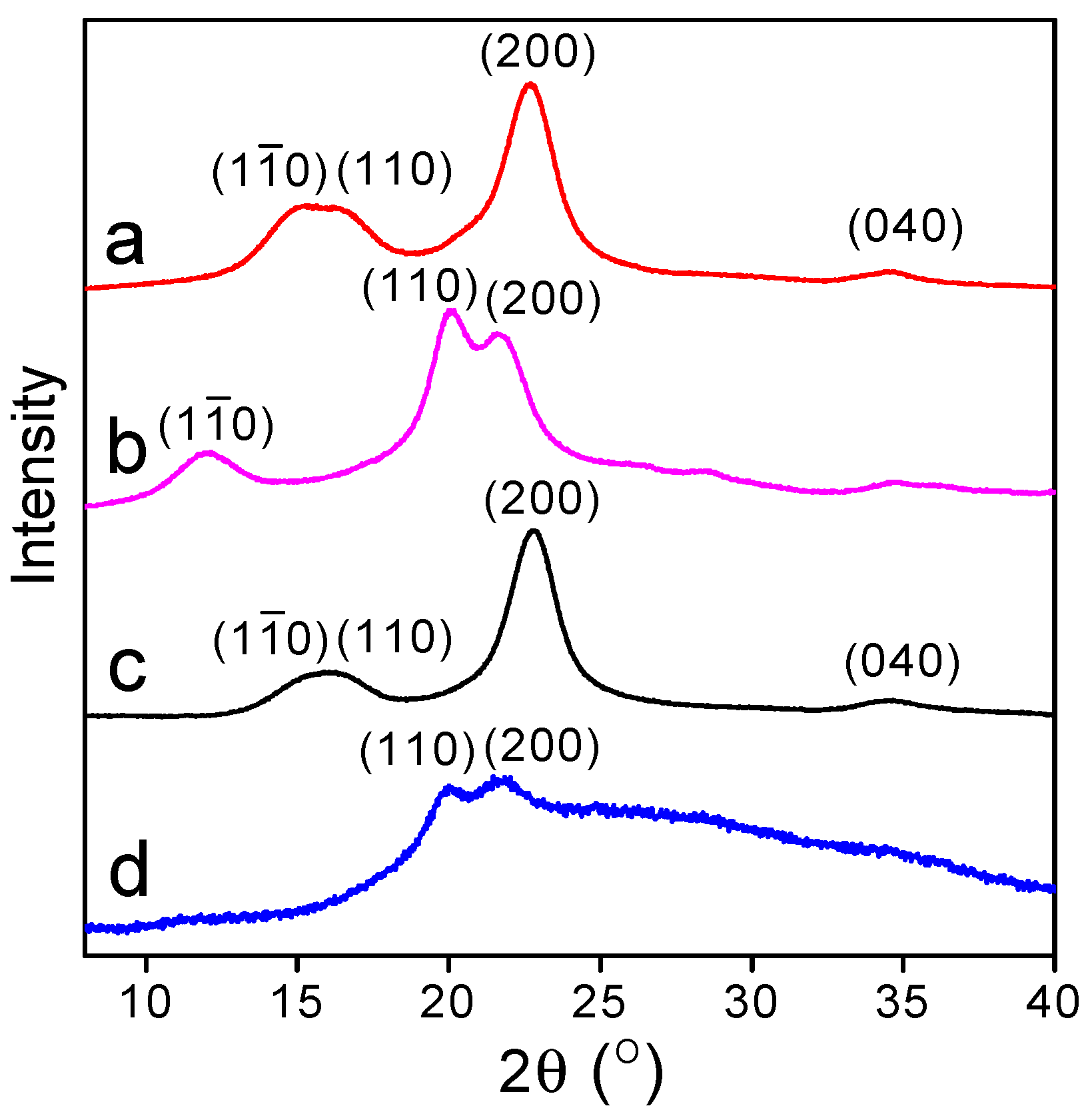
3.2. XRD Analysis of Oxidized Cellulose
3.3. DO of Oxidized Cellulose
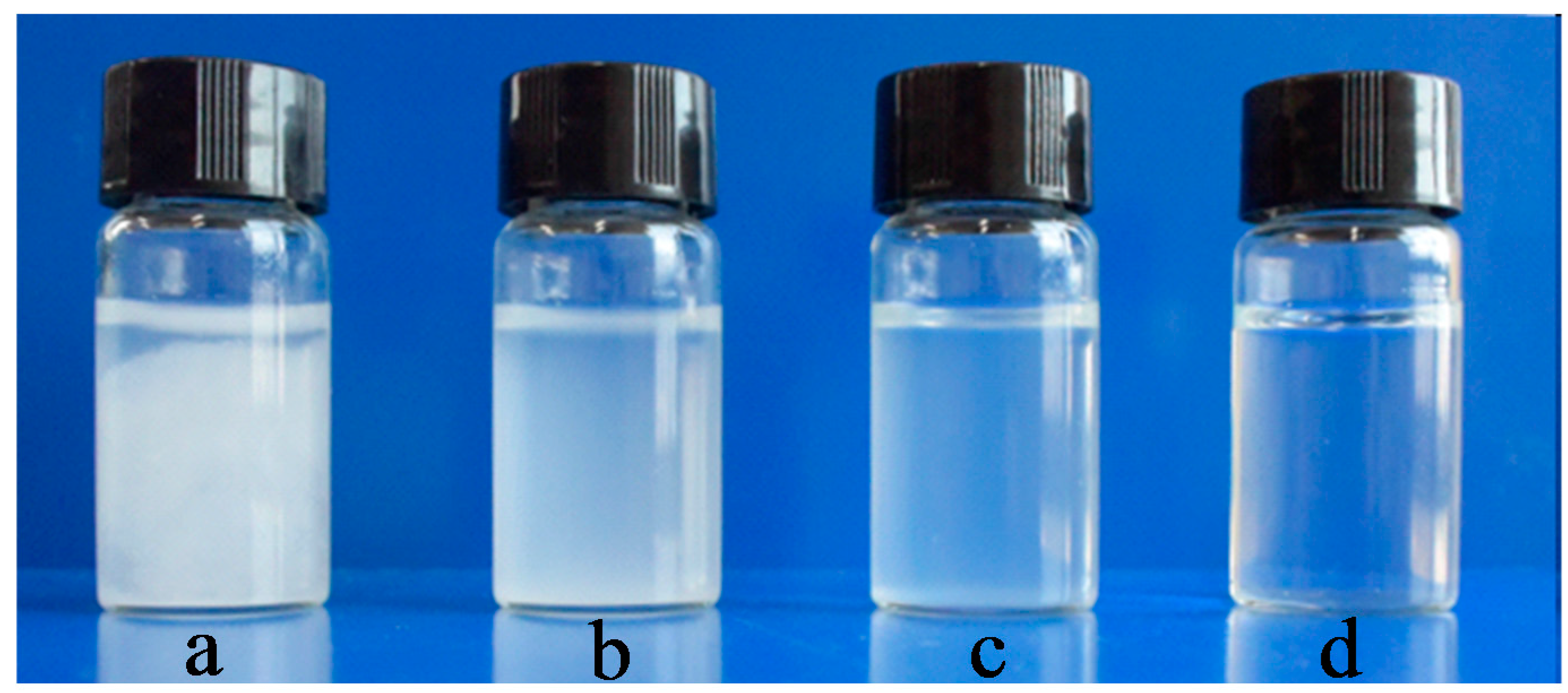
3.4. Dispersion States of Oxidized Cellulose
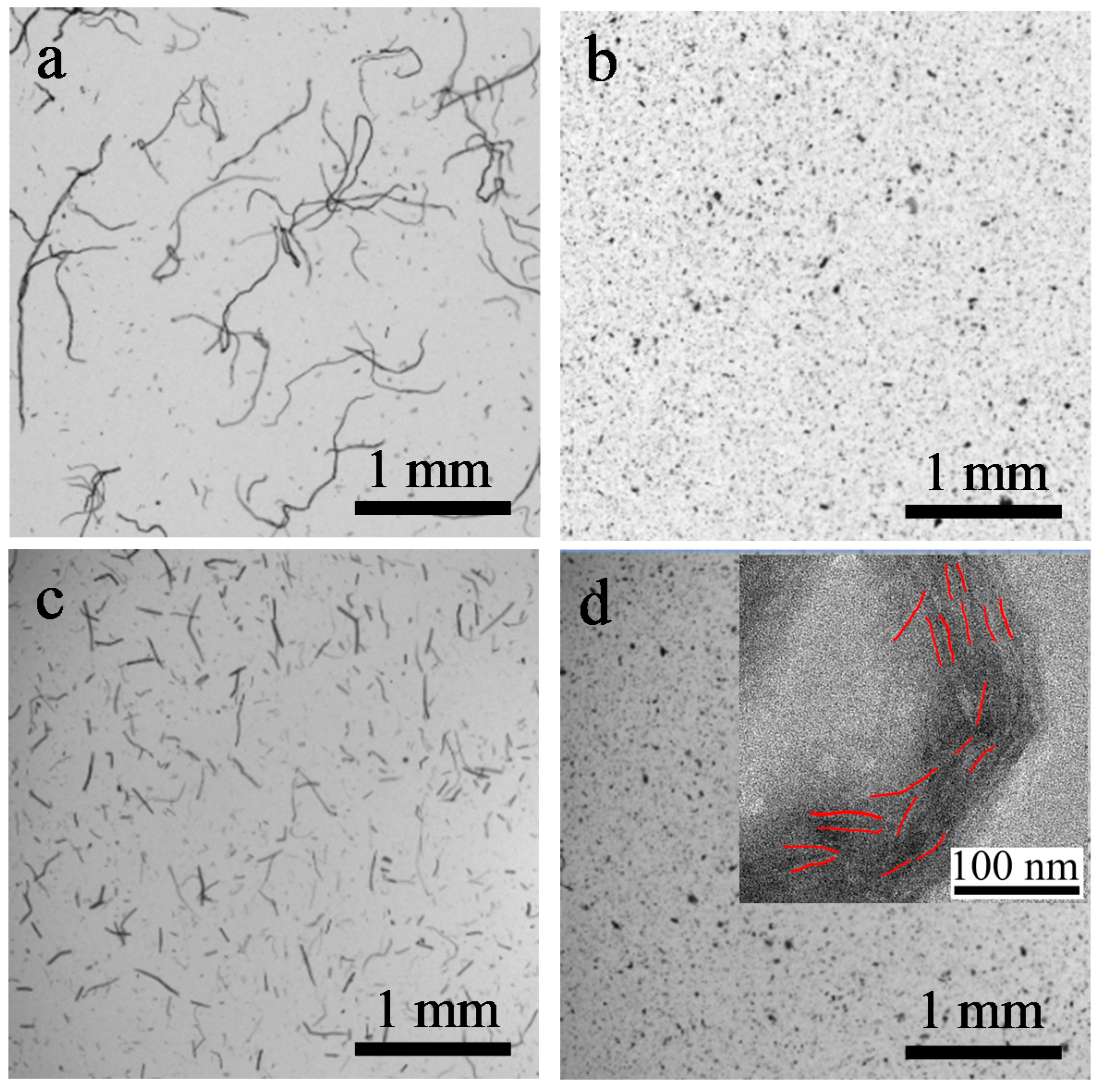
3.5. Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Amin, M.; Abbas, N.S.; Hussain, M.A.; Edgar, K.J.; Tahir, M.N.; Tremel, W.; Sher, M. Cellulose ether derivatives: A new platform for prodrug formation of fluoroquinolone antibiotics. Cellulose 2015, 22, 2011–2022. [Google Scholar] [CrossRef]
- Tian, H.; He, J. Cellulose as a Scaffold for Self-Assembly: From Basic Research to Real Applications. Langmuir 2016, 32, 12269–12282. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Li, M.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose Nanoparticles: Structure–morphology–rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Lin, X.; Ma, W.; Wu, H.; Cao, S.; Huang, L.; Chen, L.; Takahara, A. Superhydrophobic magnetic poly(DOPAm-co-PFOEA)/Fe3O4/cellulose microspheres for stable liquid marbles. Chem. Commun. 2016, 52, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, X.; White, K.L.; Lin, S.; Wu, H.; Cao, S.; Huang, L.; Chen, L. Effect of the degree of substitution on the hydrophobicity of acetylated cellulose for production of liquid marbles. Cellulose 2016, 23, 811–821. [Google Scholar] [CrossRef]
- An, X.Y.; Wen, Y.B.; Cheng, D.; Zhu, X.H.; Ni, Y.H. Preparation of cellulose nano-crystals through a sequential process of cellulase pretreatment and acid hydrolysis. Cellulose 2016, 23, 2409–2420. [Google Scholar] [CrossRef]
- Weng, R.; Chen, L.; Lin, S.; Zhang, H.; Wu, H.; Liu, K.; Cao, S.; Huang, L. Preparation and Characterization of Antibacterial Cellulose/Chitosan Nanofiltration Membranes. Polymers 2017, 9, 116. [Google Scholar] [CrossRef]
- Lin, X.; Ma, W.; Wu, H.; Huang, L.; Chen, L.; Takahara, A. Fabrication of cellulose based superhydrophobic microspheres for the production of magnetically actuatable smart liquid marbles. J. Bioresour. Bioprod. 2017, 2, 110–115. [Google Scholar]
- De Nooy, A.E.J.; Besemer, A.C.; van Bekkum, H. Highly selective tempo mediated oxidation of primary alcohol groups in polysaccharides. Recueil des Travaux Chimiques des Pays-Bas 1994, 113, 165–166. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.S.; Robyt, J.F. Oxidation of primary alcohol groups of naturally occurring polysaccharides with 2,2,6,6-tetramethyl-1-piperidine oxoammonium ion. J. Carbohydr. Chem. 1996, 15, 819–830. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Perez, D.D.; Montanari, S.; Vignon, M.R. TEMPO-mediated oxidation of cellulose III. Biomacromolecules 2003, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Burger, C.; Ma, H.; Chu, B.; Hsiao, B.S. Exploring the nature of cellulose microfibrils. Biomacromolecules 2015, 16, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Dai, L.; Wang, B.; Long, Z.; Chen, L.; Zhang, D.; Guo, S. Properties of hydroxypropyl guar/TEMPO-oxidized cellulose nanofibrils composite films. Cellulose 2015, 22, 3117–3126. [Google Scholar] [CrossRef]
- Sone, A.; Saito, T.; Isogai, A. Preparation of aqueous dispersions of TEMPO-oxidized cellulose nanofibrils with various metal counterions and their super deodorant performances. ACS Macro Lett. 2016, 5, 1402–1405. [Google Scholar] [CrossRef]
- De Carvalho, R.A.; Veronese, G.; Carvalho, A.; Barbu, E.; Amaral, A.; Trovatti, E. The potential of TEMPO-oxidized nanofibrillar cellulose beads for cell delivery applications. Cellulose 2016, 23, 3399–3405. [Google Scholar] [CrossRef]
- Cheng, D.; Wen, Y.B.; An, X.Y.; Zhu, X.H.; Ni, Y.H. TEMPO-oxidized cellulose nanofibers (TOCNs) as a green reinforcement for waterborne polyurethane coating (WPU) on wood. Carbohydr. Polym. 2016, 151, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Lu, Y.; Yin, Y.; Yang, D.; Sun, J.; She, X.; Xia, Y. High frequency ultrasound preparation of TEMPO-oxided ultrafine cellulose nanofibrils of angstrom-scale. Acta Polym. Sin. 2017, 683–691. [Google Scholar] [CrossRef]
- Hao, J.; Xu, S.Y.; Xu, N.Y.; Li, D.X.; Linhardt, R.J.; Zhang, Z.Q. Impact of degree of oxidation on the physicochemical properties of microcrystalline cellulose. Carbohydr. Polym. 2017, 155, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.L.; Ma, X.J.; Lin, L.; Huang, F.; Huang, L.L.; Chen, L.H. Morphological and chemical characterization of green bamboo (dendrocalamopsis oldhami (Munro) Keng f.) for dissolving pulp production. Bioresources 2014, 9, 4528–4539. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.N.; Zhou, J.P.; Li, H.; Chen, H.; Jin, H.M. Novel fibers prepared from cellulose in NaOH/urea aqueous solution. Macromol. Rapid Commun. 2004, 25, 1558–1562. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L.N. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Zini, E.; Scandola, M.; Gatenholm, P. Heterogeneous acylation of flax fibers. Reaction kinetics and surface properties. Biomacromolecules 2003, 4, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.; Baiardo, M.; Scandola, M.; Lednická, D.; Cnockaert, M.C.; Mergaert, J.; Swings, J. Natural cellulose fibers: Heterogeneous acetylation kinetics and biodegradation behavior. Biomacromolecules 2001, 2, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Matsunaga, H. Crystal structure of native cellulose. Macromolecules 1991, 24, 3968–3969. [Google Scholar] [CrossRef]
- Kolpak, F.J.; Blackwell, J. Determination of the structure of cellulose II. Macromolecules 1976, 9, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Stahl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Sun, B.; Gu, C.J.; Ma, J.H.; Liang, B.R. Kinetic study on TEMPO-mediated selective oxidation of regenerated cellulose. Cellulose 2005, 12, 59–66. [Google Scholar] [CrossRef]
- O’Sullivan, A. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
| Samples | Crystallinity Index (%) | Degree of Oxidation (%) |
|---|---|---|
| Pristine cellulose | 71.0 | 0.3 |
| Direct TEMPO-oxidized cellulose | 69.5 | 53.0 |
| NaOH/urea-treated cellulose | 63.2 | 0.3 |
| NaOH/urea-treated TEMPO-oxidized cellulose | 26.6 | 91.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Li, W.; Lin, X.; Xiao, H.; Miao, Q.; Huang, L.; Chen, L.; Wu, H. TEMPO-Oxidized Cellulose with High Degree of Oxidation. Polymers 2017, 9, 421. https://doi.org/10.3390/polym9090421
Tang Z, Li W, Lin X, Xiao H, Miao Q, Huang L, Chen L, Wu H. TEMPO-Oxidized Cellulose with High Degree of Oxidation. Polymers. 2017; 9(9):421. https://doi.org/10.3390/polym9090421
Chicago/Turabian StyleTang, Zuwu, Wenyan Li, Xinxing Lin, He Xiao, Qingxian Miao, Liulian Huang, Lihui Chen, and Hui Wu. 2017. "TEMPO-Oxidized Cellulose with High Degree of Oxidation" Polymers 9, no. 9: 421. https://doi.org/10.3390/polym9090421
APA StyleTang, Z., Li, W., Lin, X., Xiao, H., Miao, Q., Huang, L., Chen, L., & Wu, H. (2017). TEMPO-Oxidized Cellulose with High Degree of Oxidation. Polymers, 9(9), 421. https://doi.org/10.3390/polym9090421





