A New Technique for Improved Use of Thermal Energy from Waste Effluents
Abstract
:1. Introduction
2. Material and Methods
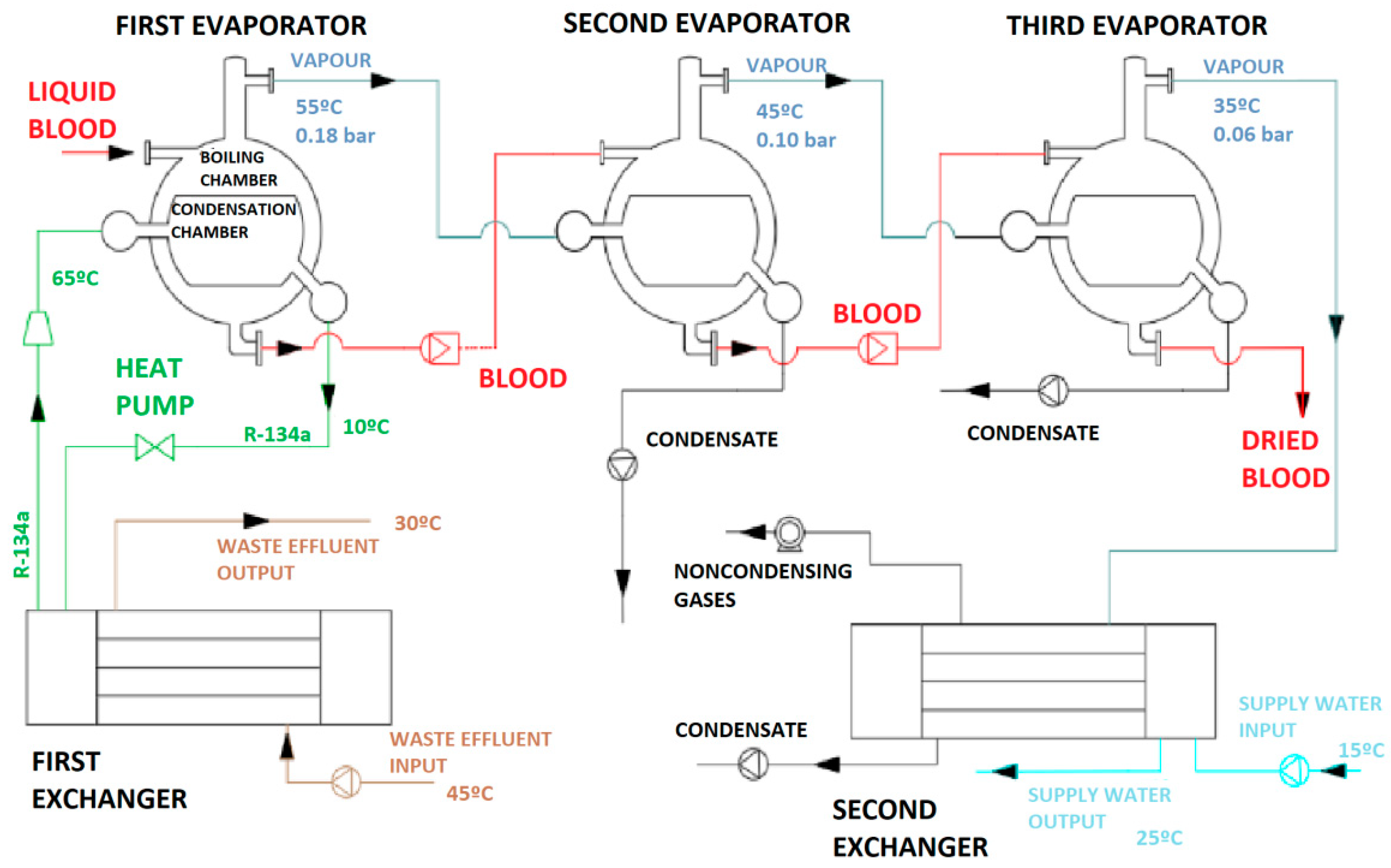
2.1. Layout of the Proposed Technique: Heating of a Processed Fluid by Means of a Waste Effluent and an Interposed Third Fluid
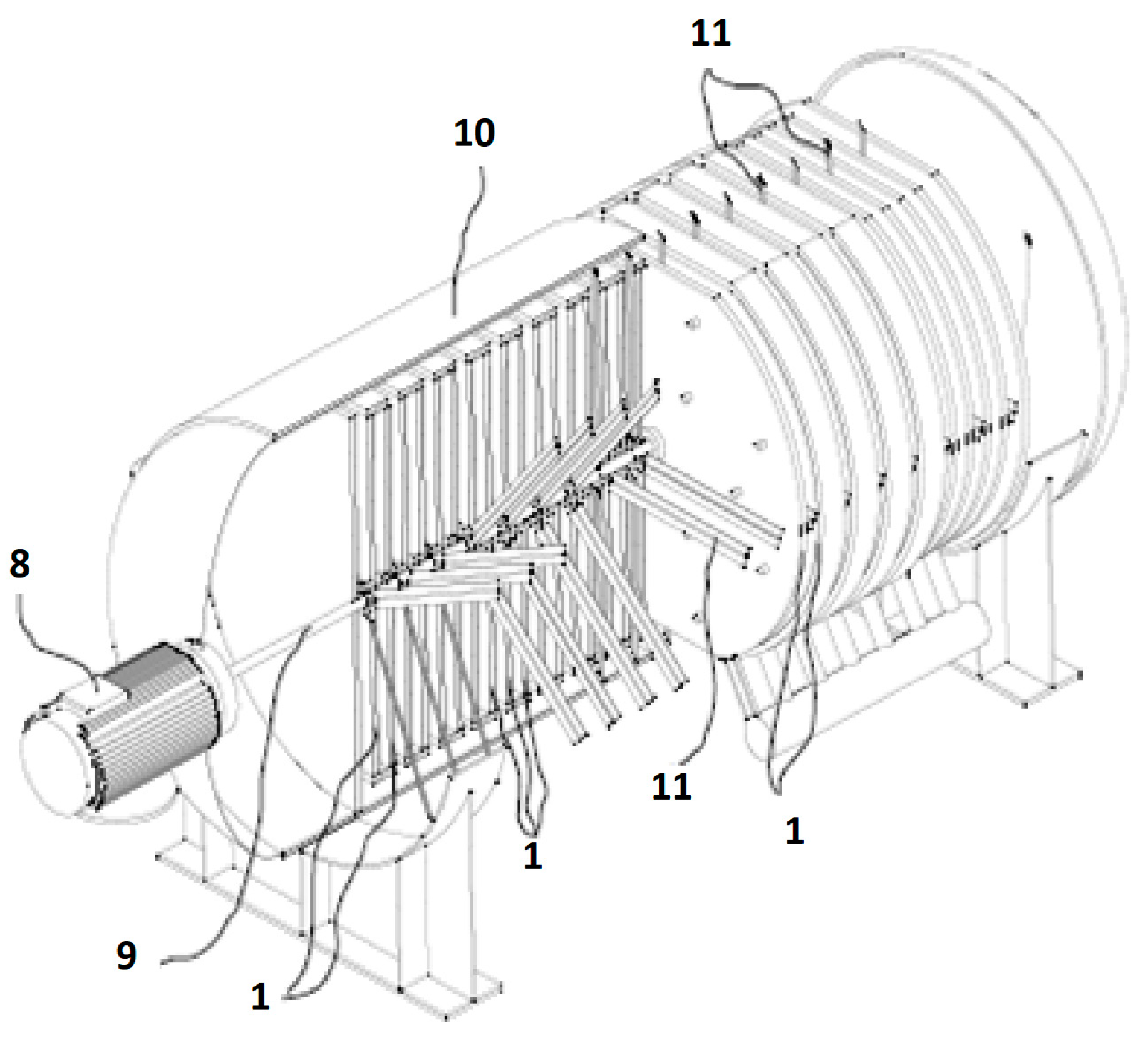
2.2. Description of a Prototype Apparatus
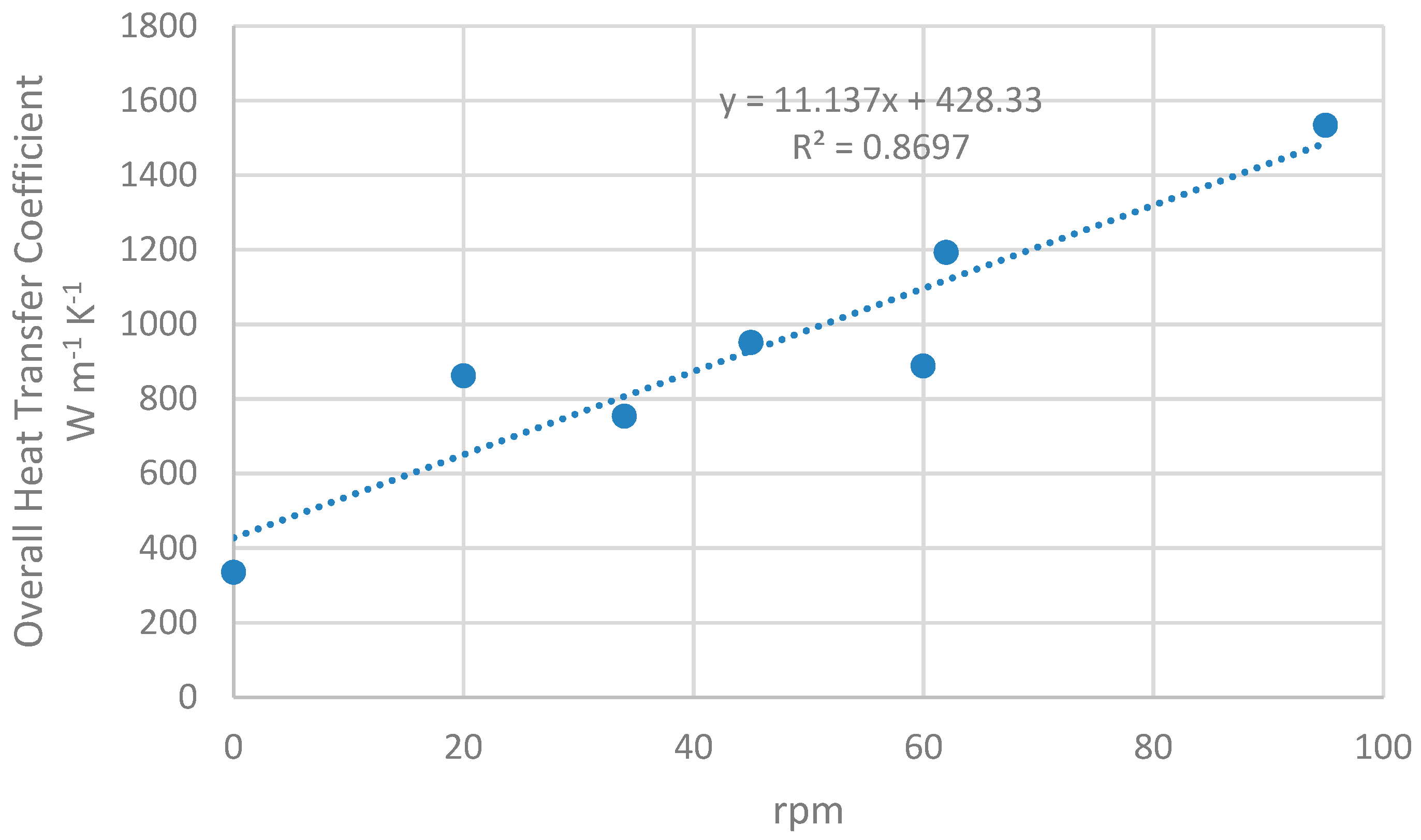
2.3. Heat Exchanger Performance
2.4. Drying Blood into Blood Meal
2.5. Economic Feasibility
3. Results
3.1. Heat Exchanger Performance
3.2. Drying Blood into Blood Meal
3.3. Economic Feasibility
4. Discussion
4.1. Heat Exchanger Performance
4.2. Drying Blood into Blood Meal
4.3. Economic Feasibility
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazari, L.; Sarathy, S.; Santoro, D.; Ho, D.; Ray, M.B.; Xu, C. Recent advances in energy recovery from wastewater sludge. In Direct Thermochemical Liquefaction for Energy Applications, 1st ed.; Rosendahl, L., Ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 67–100. [Google Scholar]
- Moran, M.; Shapiro, H. Fundamentals of Engineering Thermodynamics, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 357–512. [Google Scholar]
- Schmid, F. Sewage water: Interesting heat source for heat pumps and chillers. In Proceedings of the 9th International IEA Heat Pump Conference, Zürich, Switzerland, 20–22 May 2008. [Google Scholar]
- Blanco-Orús, R.; García-Ramos, F.J. Calefacción de instalaciones de porcino con suelo radiante de agua caliente. Mundo Ganad. 2013, 252, 48–52. [Google Scholar]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Cipolla, S.S.; Maglionico, M. Heat recovery from urban wastewater: Analysis of the variability of flow rate and temperature in the sewer of Bologna, Italy. Energy Procedia 2014, 45, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Haddad, C.; Périlhon, C.; Danlos, A.; François, M.X.; Descombes, G. Some efficient solutions to recover low and medium waste heat: Competitiveness of the thermoacoustic technology. Energy Procedia 2014, 50, 1056–1069. [Google Scholar] [CrossRef]
- Hao, X.; Li, J.; van Loosdrecht, M.C.M.; Jiang, H.; Liu, R. Energy recovery from wastewater: Heat over organics. Water Res. 2019, 161, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, A.S.; Hoppock, D.C.; Webber, M.E. Energy Recovery from Wastewater Treatment Plants in the United States: A Case Study of the Energy-Water Nexus. Sustainability 2010, 2, 945–962. [Google Scholar] [CrossRef] [Green Version]
- McCabe, W.; Smith, J.; Harriot, P. Unit Operations of Chemical Engineering, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2004; pp. 273–750. [Google Scholar]
- Yunta, F.; Di Foggia, M.; Bellido-Díaz, V.; Morales-Calderón, M.; Tessarin, P.; López-Rayo, S.; Tinti, A.; Kovács, K.; Klencsár, Z.; Fodor, F.; et al. Blood Meal-Based Compound. Good Choice as Iron Fertilizer for Organic Farming. J. Agric. Food Chem. 2013, 61, 3995–4003. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineer’s Handbook, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2009; pp. 150–1570. [Google Scholar]
- Coulson, J.M.; Richardson, J.F.; Sinnott, R.K. Coulson and Richardson’s Chemical Engineering: Chemical Engineering Design, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 358–512. [Google Scholar]
- Khanam, S.; Mohanty, B. Energy Reduction Schemes for Multiple Effect Evaporator Systems. Appl. Energy 2010, 87, 1102–1111. [Google Scholar] [CrossRef]
- Mujumdar, A.S. Handbook of Industrial Drying, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 915–1200. [Google Scholar]
- Kudra, T.; Mujumdar, A.S. Advanced Drying Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 57–250. [Google Scholar]
- Afonso, I.M.; Cruz, P.; Maia, J.M.; Melo, L.F. Simplified numerical simulation to obtain heat transfer correlations for stirred yoghurt in a plater heat exchanger. Food Bioprod. Process. 2008, 87, 296–303. [Google Scholar] [CrossRef]
- Afonso, I.M.; Hes, L.; Maia, J.M.; Melo, L.F. Heat transfer and rheology of stirred yoghurt during cooling in plate heat exchangers. J. Food Eng. 2003, 57, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.S.; Dias, R.; Nóbrega, J.M.; Afonso, I.M.; Melo, L.F.; Maia, J.M. Simulation of stirred yoghurt processing in plate heat exchangers. J. Food Eng. 2005, 69, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Kanaris, A.G.; Mouza, A.A.; Paras, S.V. Optimal design of a plate heat exchanger with undulated surfaces. Int. J. Therm. Sci. 2009, 48, 1184–1195. [Google Scholar] [CrossRef]
- Pinson, F.; Gregoire, O.; Quintard, M.; Prat, M.; Simonin, O. Modeling of turbulent heat transfer and thermal dispersion for flows in flat plate heat exchangers. Int. J. Heat Mass Transf. 2007, 50, 1500–1515. [Google Scholar] [CrossRef]
- Gut, J.A.W.; Fernandes, R.; Pinto, J.M.; Tadini, C.C. Thermal model validation of plate heat exchangers with generalized configurations. Chem. Eng. Sci. 2004, 59, 4591–4600. [Google Scholar] [CrossRef]
- Abd, A.A.; Kareem, M.Q.; Naji, S.Z. Performance analysis of shell and tube heat exchanger: Parametric study. Case Stud. Therm. Eng. 2018, 12, 563–568. [Google Scholar] [CrossRef]
- Deng, W.; Su, Y.; Yu, W. Theoretical calculation of heat transfer coefficient when sludge drying in a nara-type paddle dryer using different heat carriers. Procedia Environ. Sci. 2013, 18, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Sahu, H.S. CFD Analysis of Heat Transfer Enhancement in Shell and Tube Type Heat Exchanger creating Triangular Fin on the Tubes. Int. J. Trend Sci. Res. Dev. 2018, 2, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Gowthamam, P.S.; Satish, S. Analysis of Segmental and Helical Baffle in Shell and tube Heat Exchanger. Int. J. Curr. Eng. Technol. 2014, 2, 625–628. [Google Scholar] [CrossRef]
- Gupta, V.; Dewangan, R. Analytical Comparison of Different Geometrical Shapes of Tubes of Shell & Tube Heat Exchanger. Int. J. Sci. Res. Dev. 2017, 5, 508–511. [Google Scholar]
- Shendre, M.; Biradar, S. Experimental Study on Heat Transfer and Fluid Flow Characteristics of Shell and Tube Heat Exchanger using hiTRAN Wire Inserts. Int. J. Trend Sci. Res. Dev. 2018, 2, 572–579. [Google Scholar]
- Ockerman, H.W.; Hansen, C.L. Animal By-Product Processing & Utilization, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 210–253. [Google Scholar]
- Warris, P.D. Meat Science: An Introductory Text, 2nd ed.; CABI: Wallingford, UK, 2009; pp. 45–157. [Google Scholar]
- Babale, D.M.; Charity, A.M.; Shehu, J.F. Economic Evaluation of replacing Fishmeal meal with Blood Meal in broiler production in Mubi, Adamawa State, Nigeria. Int. J. Manag. Soc. Sci. Res. 2012, 1, 36–40. [Google Scholar]


| Technique | Temperature (°C) | Recovered Energy (MJ t−1) | Blood Meal (kg t−1) | Valorization (€ t−1) |
|---|---|---|---|---|
| Conditioning of Dwellings | 40–80 | 167.47 | - | 3.20 |
| Preheating of Process Fluids | 40–80 | 167.47 | - | 3.20 |
| Drying with a Heat Pump | 20–80 | 251.21 | 16.69 | 6.70 |
| Drying with a Heat Pump and Three Evaporators | 20–80 | 251.21 | 16.69 | 9.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magide-Ameijide, J.M.; Varela-Rodríguez, H.; López-Fabal, A. A New Technique for Improved Use of Thermal Energy from Waste Effluents. Agronomy 2020, 10, 97. https://doi.org/10.3390/agronomy10010097
Magide-Ameijide JM, Varela-Rodríguez H, López-Fabal A. A New Technique for Improved Use of Thermal Energy from Waste Effluents. Agronomy. 2020; 10(1):97. https://doi.org/10.3390/agronomy10010097
Chicago/Turabian StyleMagide-Ameijide, José Manuel, Hiram Varela-Rodríguez, and Adolfo López-Fabal. 2020. "A New Technique for Improved Use of Thermal Energy from Waste Effluents" Agronomy 10, no. 1: 97. https://doi.org/10.3390/agronomy10010097





