Effects of Mixed Hardwood and Sugarcane Biochar as Bark-Based Substrate Substitutes on Container Plants Production and Nutrient Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurements
2.2.1. Potting Mix Physical and Chemical Properties
2.2.2. Plant Growth
2.3. Statistical Analysis
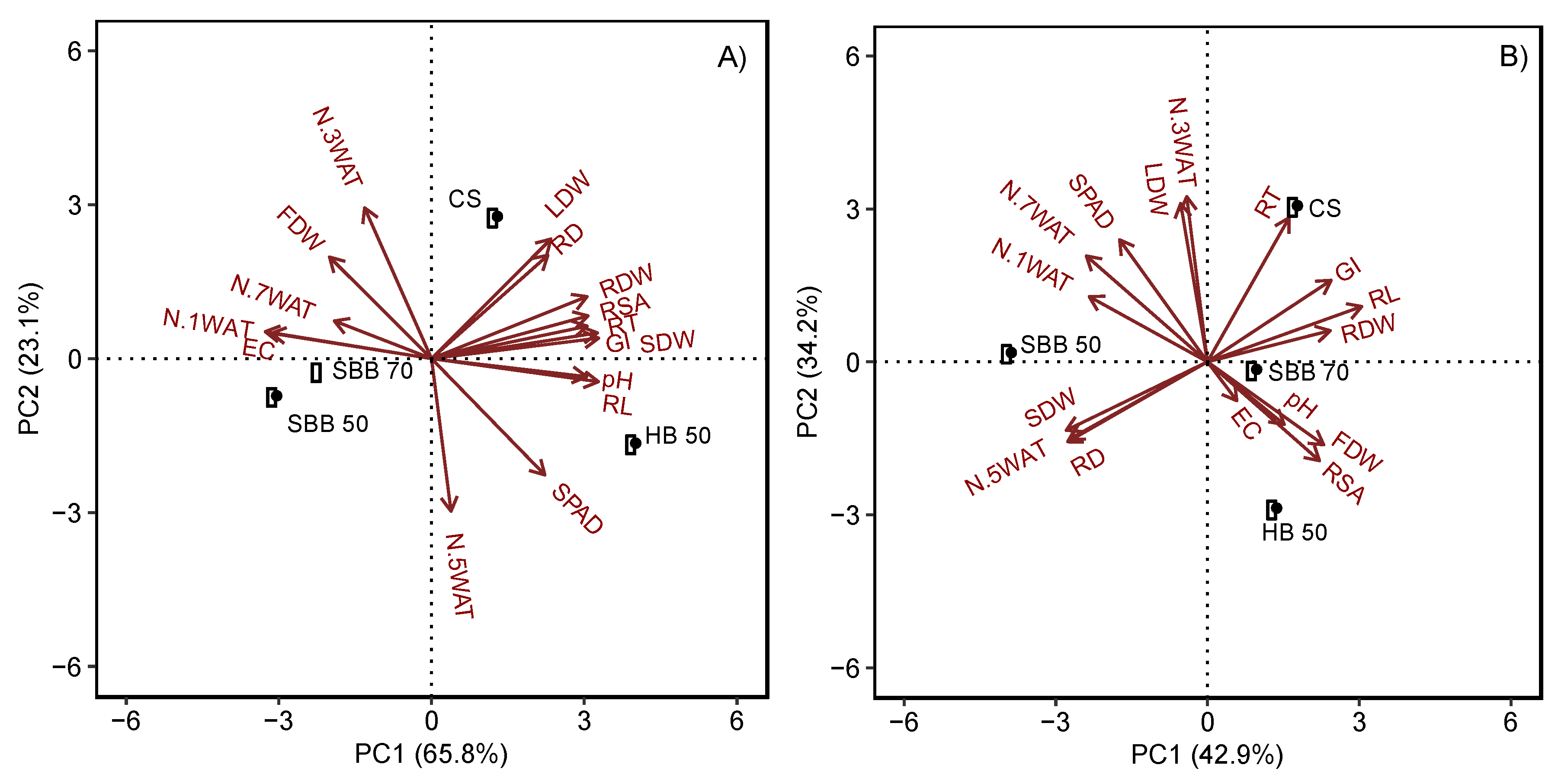
3. Results
3.1. Potting Mix Physical and Chemical Properties
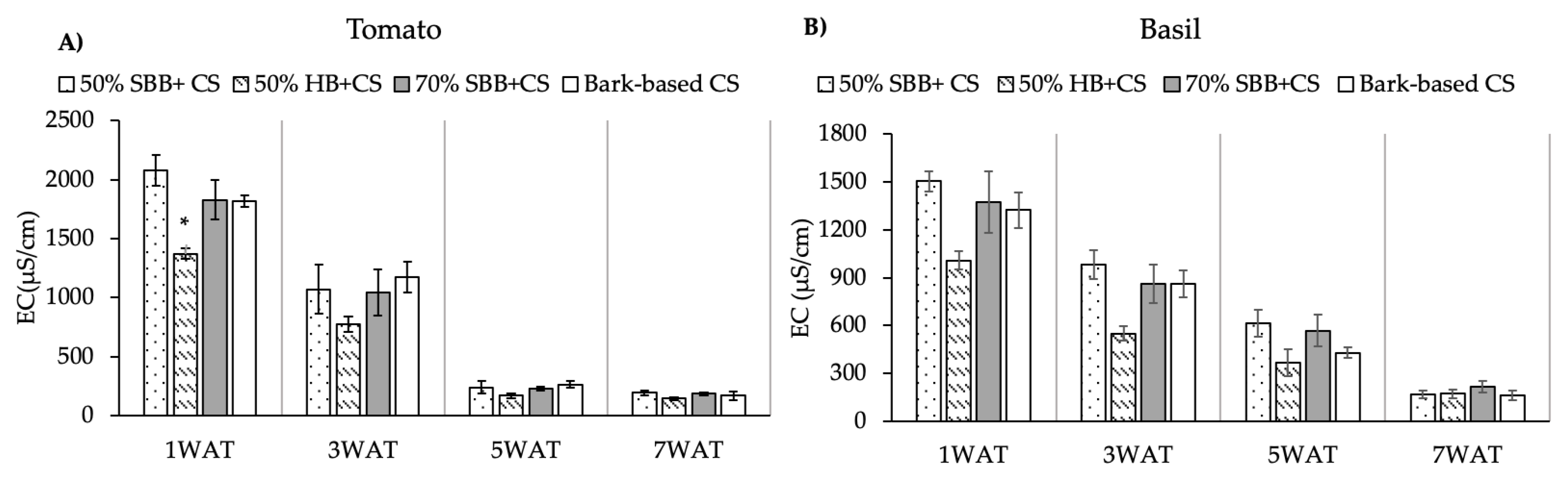
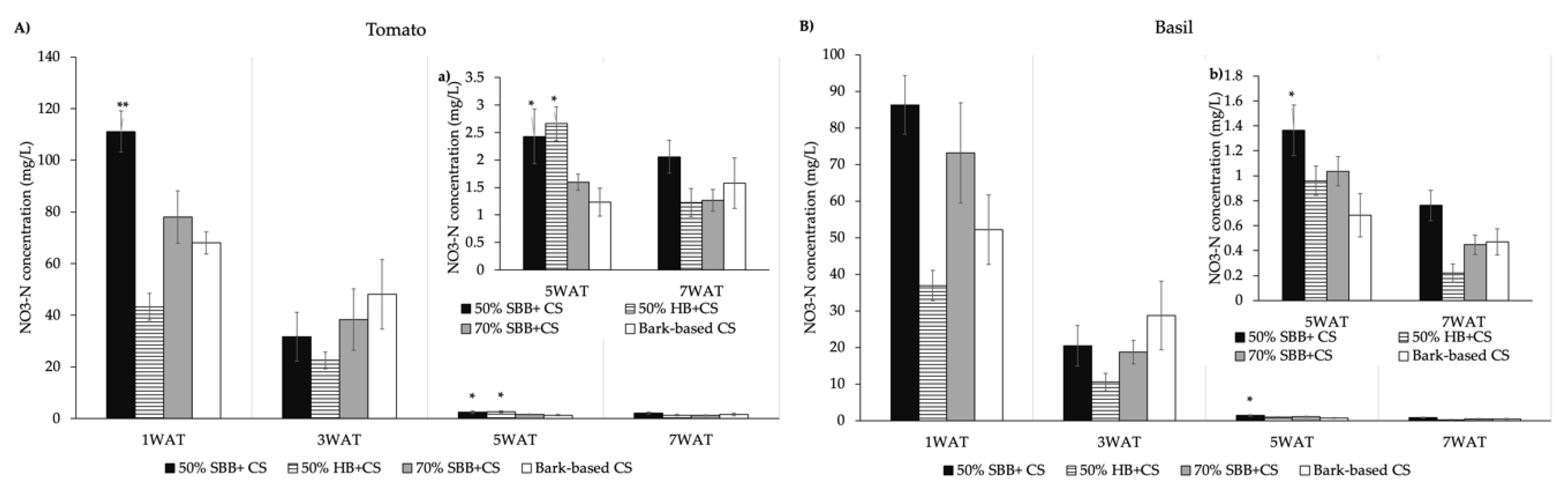
3.2. Leachate NO3–N
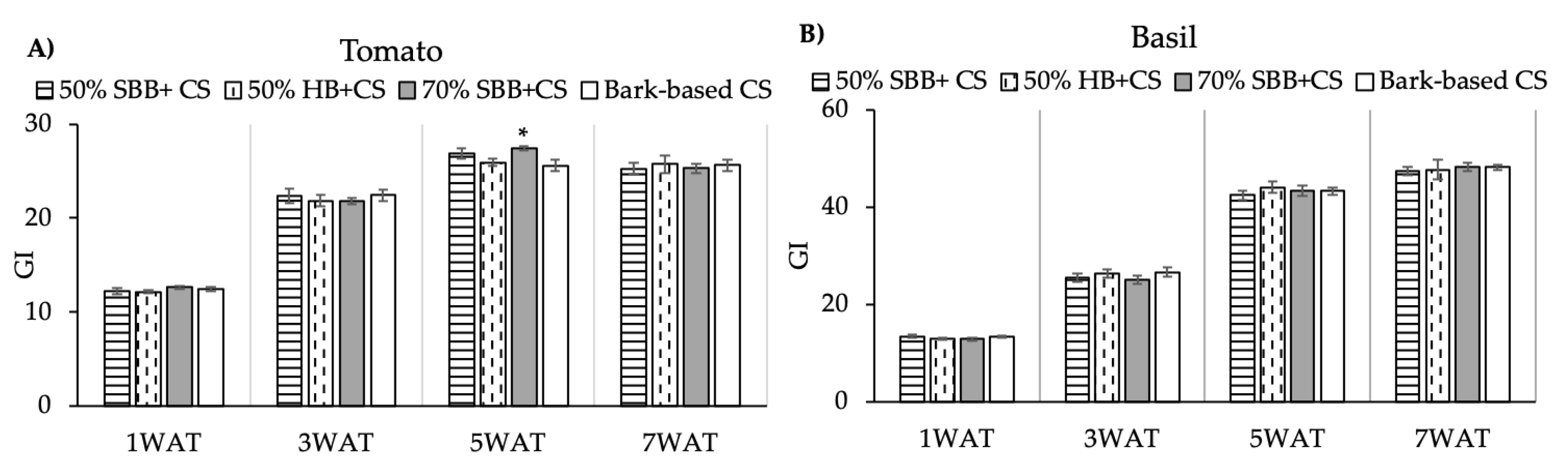
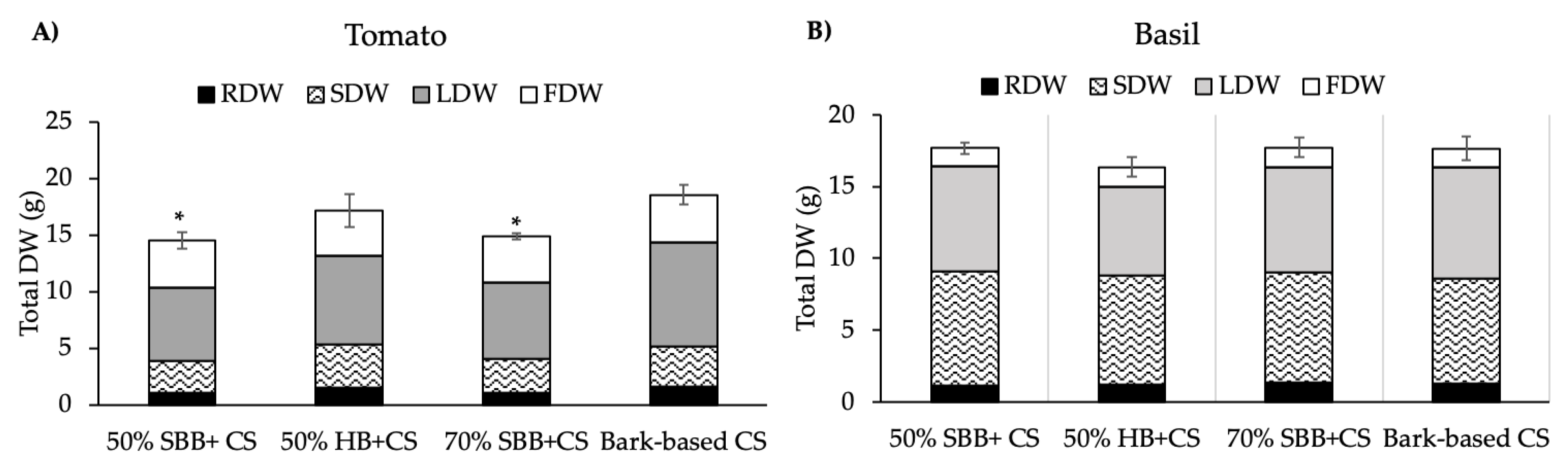
3.3. Plant Growth
4. Discussion
4.1. Potting Mix Physical and Chemical Properties
4.2. Biochar Effects on Leachate NO3–N
4.3. Biochar Effects on Plants Growth
4.4. Treatment Factors Determined Plants and Mix Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and organic growing media have distinct community structure, stability and functionality in soilless culture systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.; Martinez-Nicolas, J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and physiological Responses of tomato plants Grown in Different Soilless Culture systems with saline WATer under Greenhouse Conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef] [PubMed]
- Kılıc, P.; Erdal, I.; Aktas, H. Effect of different substrates on yield and fruit quality of tomato grown in soilless culture. Infrastruktura i Ekologia Terenów Wiejskich 2018. [Google Scholar] [CrossRef]
- Sedaghat, M.; Kazemzadeh-Beneh, H.; Azizi, M.; Momeni, M. Optimizing Growing Media for Enhancement to Vegetative Growth, Yield and Fruit Quality of Greenhouse Tomato Productionin Soilless Culture System. World J. Agric. Sci 2017, 13, 82–89. [Google Scholar]
- Mairapetyan, S.; Alexanyan, J.; Tovmasyan, A.; Daryadar, M.; Stepanian, B.; Mamikonyan, V. Productivity, biochemical indices and antioxidant activity of peppermint (Mentha piperita L.) and basil (Ocimum basilicum L.) in conditions of hydroponics. J. Aquac. Res. Dev 2016, 7, 1–3. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Currey, C.J.; Flax, N.J.; Litvin, A.G.; Metz, V.C. Substrate Volumetric WATer Content Controls Growth and Development of Containerized Culinary Herbs. Agronomy 2019, 9, 667. [Google Scholar] [CrossRef] [Green Version]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2019, 261, 109001. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wei, X. Controlled-Release Fertilizers as a Means to Reduce Nitrogen Leaching and Runoff in Container-Grown Plant Production. Nitrogen Agric. Updates 2018, 33. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- Bilderback, T.; Boyer, C.; Chappell, M.; Fain, G.; Fare, D.; Gilliam, C.; Jackson, B.; Lea-Cox, J.; LeBude, A.; Niemiera, A. Best Management Practices: Guide for Producing Nursery Crops; Southern Nursery Association: Acworth, Goregia, 2013. [Google Scholar]
- Ngaatendwe, M.; Ernest, M.; Moses, M.; Tuarira, M.; Ngenzile, M.; Tanyaradzwa, Z. Use of vermicompost as supplement to pine bark for seedling production in nurseries. World J. Agric. Res. 2015, 3, 123–128. [Google Scholar]
- El Sharkawi, H.M.; Ahmed, M.A.; Hassanein, M.K. Development of treated Rice Husk as an alternative substrate medium in cucumber soilless culture. J. Agric. Environ. Sci. 2014, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-S.; Zhao, Y.; Dou, H.; Cai, X.; Gu, M.; Yu, F. Effects of biochar mixtures with pine-bark based substrates on growth and development of horticultural crops. Hortic. Environ. Biotechnol. 2018, 59, 345–354. [Google Scholar] [CrossRef]
- Gu, M.; Li, Q.; Steele, P.H.; Niu, G.; Yu, F. Growth of ‘Fireworks’ gomphrena grown in substrates amended with biochar. J. Food Agric. Environ. 2013, 11, 819–821. [Google Scholar]
- Buamscha, M.G.; Altland, J.E.; Sullivan, D.M.; Horneck, D.A. Micronutrient availability in fresh and aged Douglas fir bark. HortScience 2007, 42, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Torres-Quezada, E.A.; Santos, B.M.; Zotarelli, L.; Treadwell, D.A. Soilless Media and Containers for Bell Pepper Production. Int. J. Veg. Sci. 2015, 21, 177–187. [Google Scholar] [CrossRef]
- Wright, R.D.; Jackson, B.E.; Barnes, M.C.; Browder, J.F. The landscape performance of annual bedding plants grown in pine tree substrate. HortTechnology 2009, 19, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Cole, D.M.; Sibley, J.L.; Blythe, E.K.; Eakes, D.J.; Tilt, K.M. Evaluation of cotton gin compost as a horticultural substrate. In A Research Paper Presented at the Southern Nursery Association Researcher’s Conference; Department of Horticulture, Auburn University: Auburn, AL, USA, 2002; Volume 47, pp. 264–276. [Google Scholar]
- Haynes, R.W. An Analysis of the Timber Situation in the United States: 1952 to 2050; Gen. Tech. Rep. PNW-GTR-560; US Department of Agriculture Forest Service Pacific Northwest Research Station: Corvallis, OR, USA, 2003; Volume 560, p. 254.
- Lu, W.; Sibley, J.L.; Gilliam, C.H.; Bannon, J.S.; Zhang, Y. Estimation of US bark generation and implications for horticultural industries. J. Environ. Hortic. 2006, 24, 29–34. [Google Scholar]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sour. 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Mate. Sci. Eng. 2014, 2014, 715398. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Niu, G.; Starman, T.; Volder, A.; Gu, M. Poinsettia Growth and Development Response to Container Root Substrate with Biochar. Horticulturae 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Niu, G.; Starman, T.; Gu, M. Growth and development of Easter lily in response to container substrate with biochar. J. Hortic. Sci. Biotechnol. 2018, 94, 80–86. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of Biochar on Container Substrate Properties and Growth of Plants—A Review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Niu, G.; Feagley, S.E.; Gu, M. Evaluation of a hardwood biochar and two composts mixes as replacements for a peat-based commercial substrate. Ind. Crop Prod. 2019, 129, 549–560. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.-Y.; Tian, Y.; Gong, X.-Q. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 2014, 176, 70–78. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Nieto, A.; Gascó, G.; Paz-Ferreiro, J.; Fernández, J.; Plaza, C.; Méndez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Headlee, W.L.; Brewer, C.E.; Hall, R.B. Biochar as a substitute for vermiculite in potting mix for hybrid poplar. Bioenergy Res. 2014, 7, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available WATer capacity and plant growth in two contrasting soil types. Soil Tillage Res. 2016, 161, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.; Koskinen, W.; Baker, J.; Reicosky, D. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 2010, 333, 443–452. [Google Scholar] [CrossRef]
- Hina, K.; Bishop, P.; Arbestain, M.C.; Calvelo-Pereira, R.; Maciá-Agulló, J.A.; Hindmarsh, J.; Hanly, J.; Macìas, F.; Hedley, M. Producing biochars with enhanced surface activity through alkaline pretreatment of feedstocks. Soil Res. 2010, 48, 606–617. [Google Scholar] [CrossRef]
- Locke, J.C.; Altland, J.E.; Ford, C.W. Gasified rice hull biochar affects nutrition and growth of horticultural crops in container substrates. J. Environ. Hortic. 2013, 31, 195–202. [Google Scholar]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Webber, C.L., III; White, P.M., Jr.; Gu, M.; Spaunhorst, D.J.; Lima, I.M.; Petrie, E.C. Sugarcane and Pine Biochar as Amendments for Greenhouse Growing Media for the Production of Bean (Phaseolus vulgaris L.) Seedlings. J. Agric. Sci. 2018, 10, 58. [Google Scholar]
- Yeager, T.; Fare, D.; Lea-Cox, J.; Ruter, J.; Bilderback, T.; Gilliam, C.; Niemiera, A.; Warren, S.; Whitewell, T.; White, R. Best Management Practices: Guide for Producing Container-Grown Plants; Southern Nursery Association: Marietta, Georgia, 2007. [Google Scholar]
- Fonteno, W.; Hardin, C.; Brewster, J. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; North Carolina State University: Raleigh, NC, USA, 1995. [Google Scholar]
- LeBude, A.; Bilderback, T. Pour-through extraction procedure: A nutrient management tool for nursery crops. N.C. Coop. Ext. 2009, 1–8. [Google Scholar]
- Gell, K.; van Groenigen, J.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard. Mate. 2011, 186, 2017–2025. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Spaunhorst, D.J.; Lima, I.M.; Petrie, E.C. Sugarcane Biochar as an Amendment for Greenhouse Growing Media for the Production of Cucurbit Seedlings. J. Agric. Sci. 2018, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Niu, H.; Xu, J.; Wang, X. Nitrate-nitrogen leaching and modeling in intensive agriculture farmland in China. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Luce, M.S.; Whalen, J.K.; Ziadi, N.; Zebarth, B.J. Nitrogen dynamics and indices to predict soil nitrogen supply in humid temperate soils. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 112, pp. 55–102. [Google Scholar]
- Wang, X.; Xing, Y. Evaluation of the effects of irrigation and fertilization on tomato fruit yield and quality: A principal component analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.V. Greenhouse Operation and Management; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Badr, M.; Hussein, S.A.; El-Tohamy, W.; Gruda, N. Nutrient uptake and yield of tomato under various methods of fertilizer application and levels of fertigation in arid lands. Gesunde Pflanzen 2010, 62, 11–19. [Google Scholar] [CrossRef]
- Vinten, A.; Vivian, B.; Wright, F.; Howard, R. A comparative study of nitrate leaching from soils of differing textures under similar climatic and cropping conditions. J. Hydrol. 1994, 159, 197–213. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Eller, F.J.; Evangelista, R.L.; Moser, B.R.; Lee, E.; Wagner, R.E.; Peterson, S.C. Evaluation of biochar-anaerobic potato digestate mixtures as renewable components of horticultural potting media. Ind. Crops Prod. 2015, 65, 467–471. [Google Scholar] [CrossRef]
- Dunlop, S.J.; Arbestain, M.C.; Bishop, P.A.; Wargent, J.J. Closing the loop: Use of biochar produced from tomato crop green waste as a substrate for soilless, hydroponic tomato production. HortScience 2015, 50, 1572–1581. [Google Scholar] [CrossRef]
- Liu, R.; Gu, M.; Huang, L.; Yu, F.; Jung, S.-K.; Choi, H.-S. Effect of pine wood biochar mixed with two types of compost on growth of bell pepper (Capsicum annuum L.). Hortic. Environ. Biotechnol. 2019, 60, 313–319. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.; Duvall, M.; Sohi, S. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Lobet, G.; Dinneny, J.R. Environmental control of root system biology. Ann. Rev. Plant Biol. 2016, 67, 619–642. [Google Scholar] [CrossRef] [Green Version]
- USDA-NASS. Agricultural Statistics; USDA, Ed.; United States Government Printing Office Washington: Washington, DC, USA, 2018; pp. 202–210.


| Composition | pH | EC µS/cm | TP% | CC % | AS % | BD g/cm3 |
|---|---|---|---|---|---|---|
| SBB | 5.9 | 753 | 74 | 71 | 3 | 0.11 |
| HB | 10.1 | 1058 | 87 | 66 | 20 | 0.13 |
| 50%SBB + 50%CS | 6.3 | 2073 | 81 | 75 | 7 | 0.13 |
| 50%HB + 50%CS | 7.5 | 1370 | 78 | 62 | 17 | 0.13 |
| 70%SBB + 30%CS | 6.4 | 1830 | 89 | 76 | 13 | 0.14 |
| CS | 6.5 | 1819 | 97 | 85 | 12 | 0.15 |
| Suitable range Z | - | - | 50–80 | 45–65 | 10–30 | 0.19–0.7 |
| Mixes | Root Length (cm) | Root Surface Area (cm2) | Average Root Diameter (mm) | Number of Root Tips |
|---|---|---|---|---|
| Tomato | ||||
| 50%SBB + 50%CS | 1214 ± 60 | 442 ±37 * | 1.2 ± 0.1 | 2650 ± 94 * |
| 50%HB + 50%CS | 1454 ± 67 | 557 ± 24 | 1.2 ± 0.1 | 3349 ± 171 |
| 70%SBB + 30%CS | 1234 ± 74 | 421 ± 25 * | 1.1 ± 0.1 | 2970 ± 196 |
| CS | 1324 ± 40 | 543 ± 19 | 1.3 ± 0.1 | 3227 ± 157 |
| Basil | ||||
| 50%SBB + 50%CS | 1415 ± 48 *** | 819 ± 18 | 1.9 ± 0.1 *** | 3092 ± 166 ** |
| 50%HB + 50%CS | 1887 ± 117 * | 866 ± 23 | 1.5 ± 0.1 * | 3006 ± 149 ** |
| 70%SBB + 30%CS | 1850 ± 115 * | 870 ± 19 | 1.5 ± 0.1 * | 3528 ± 222 |
| CS | 2240 ± 74 | 832 ± 26 | 1.2 ± 0.0 | 4003 ± 80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Huang, L.; Li, Q.; Lima, I.M.; White, P.M.; Gu, M. Effects of Mixed Hardwood and Sugarcane Biochar as Bark-Based Substrate Substitutes on Container Plants Production and Nutrient Leaching. Agronomy 2020, 10, 156. https://doi.org/10.3390/agronomy10020156
Yu P, Huang L, Li Q, Lima IM, White PM, Gu M. Effects of Mixed Hardwood and Sugarcane Biochar as Bark-Based Substrate Substitutes on Container Plants Production and Nutrient Leaching. Agronomy. 2020; 10(2):156. https://doi.org/10.3390/agronomy10020156
Chicago/Turabian StyleYu, Ping, Lan Huang, Qiansheng Li, Isabel M. Lima, Paul M. White, and Mengmeng Gu. 2020. "Effects of Mixed Hardwood and Sugarcane Biochar as Bark-Based Substrate Substitutes on Container Plants Production and Nutrient Leaching" Agronomy 10, no. 2: 156. https://doi.org/10.3390/agronomy10020156






