Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review
Abstract
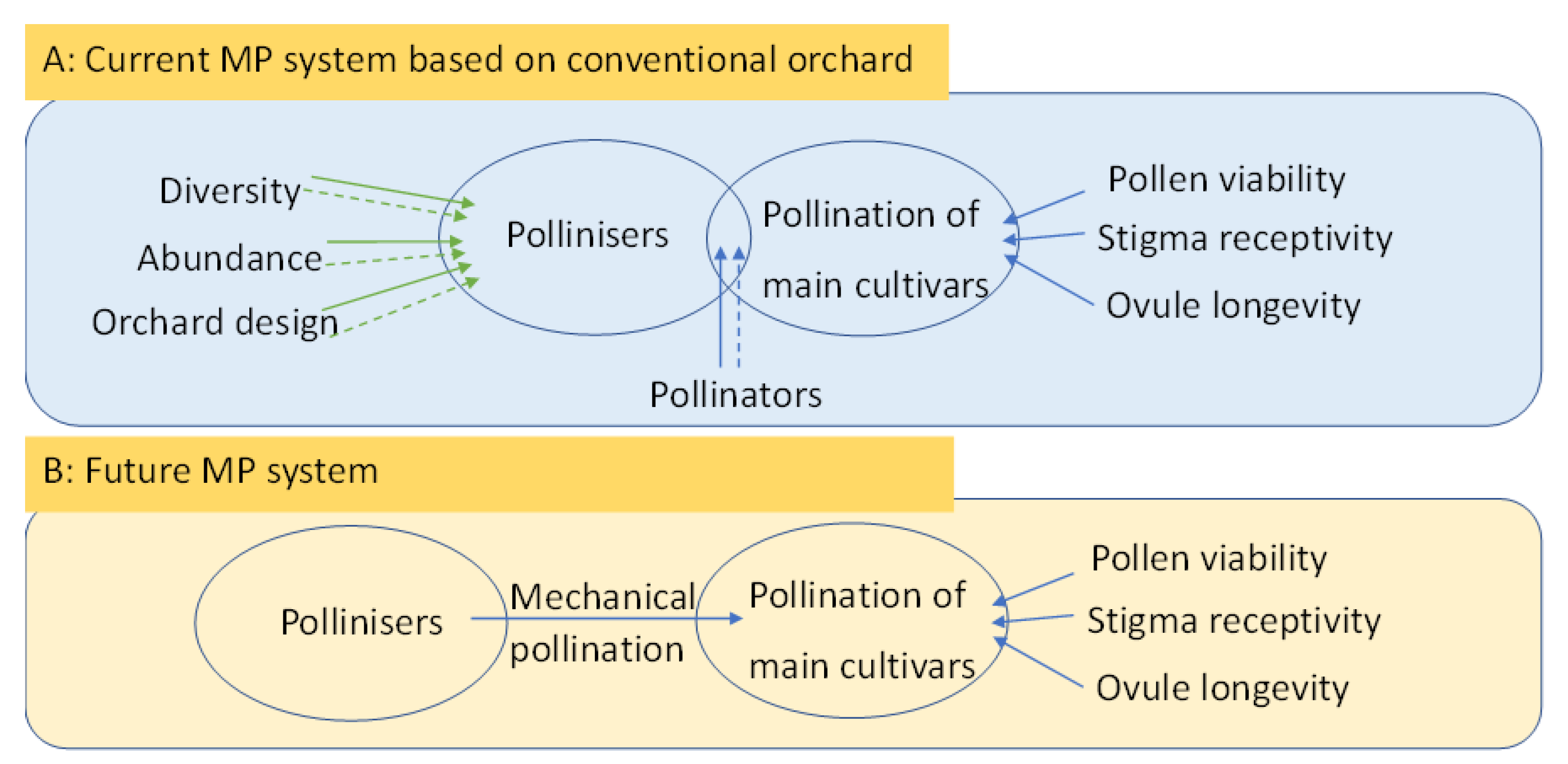
:1. Introduction
2. Benefits of Mechanical Pollination Systems
3. Processes of Mechanical Pollination
3.1. Pollen Collection
3.2. Pollen Handling
3.3. Pollen Storage
3.4. Pollen Delivery
4. Potential Risks of Mechanical Pollination
4.1. Pollen Transmission of Bacteria and Viruses
4.2. Reducing Yield and/or Quality
5. What Factors to Consider When Predicting the Likelihood of Success of MP for Fruit and Nut Crops
5.1. Pollen Type
5.2. Flower Morphology
5.3. Flowering Pattern and Stigma Receptivity
5.4. Training System
5.5. Tree Height
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreitzman, M.; Toensmeier, E.; Chan, K.M.A.; Smukler, S.; Ramankutty, N. Perennial staple crops: Yields, distribution, and nutrition in the global food system. Front. Sustain. Food Syst. 2020, 4, 1–21. [Google Scholar] [CrossRef]
- Statista 2020. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 11 March 2021).
- Serna 2020. Available online: https://www.nutfruit.org/industry/technical-resources?category=statistical-yearbooks (accessed on 11 March 2021).
- Rezaei, M.; Hokmabadi, H.; Khadivi, A.; Safari-Khuzani, A.; Heidari, P. The selection of superior pistachio (Pistacia vera L.) genotypes among seedling trees originated from open-pollination. Sci. Hortic. 2019, 251, 88–100. [Google Scholar] [CrossRef]
- Simon, S.; Lesueur-Jannoyer, M.; Plénet, D.; Lauri, P.É.; Le Bellec, F. Methodology to design agroecological orchards: Learnings from on-station and on-farm experiences. Eur. J. Agron. 2017, 82, 320–330. [Google Scholar] [CrossRef]
- Bosch, J.; Osorio-Canadas, S.; Sgolastra, F.; Vicens, N. Use of a managed solitary bee to pollinate almonds: Population sustainability and increased fruit set. Insects 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Mustiga, G.; Romero, A.; Northfield, T.D.; Lambert, S.; Motamayor, J.C. Supplemental and synchronized pollination may increase yield in Cacao. HortScience 2019, 54, 1718–1727. [Google Scholar] [CrossRef] [Green Version]
- Howlett, B.G.; Nelson, W.R.; Pattemore, D.E.; Gee, M. Pollination of macadamia: Review and opportunities for improving yields. Sci. Hortic. 2015, 197, 411–419. [Google Scholar] [CrossRef]
- Toledo-Hernández, M.; Wanger, T.C.; Tscharntke, T. Neglected pollinators: Can enhanced pollination services improve cocoa yields? A review. Agric. Eco. Environ. 2017, 247, 137–148. [Google Scholar] [CrossRef]
- Pardo, A.; Borges, P.A.V. Worldwide importance of insect pollination in apple orchards: A review. Agric. Ecosyt. Environ. 2020, 293, 106839. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, R.; Valls, A.; Cebrián, N.; Zornoza, C.; Breijo, F.G.; Armiñana, J.R.; Garmendia, A.; Merle, H. Effect of temperature on pollen germination for several Rosaceae species: Influence of freezing conservation time on germination patterns. PeerJ 2019, 7, e8195. [Google Scholar] [CrossRef]
- Carpenedo, S.; Bassols, M.D.C.; Franzon, R.C.; Byrne, D.H.; Silva, J.B.D. Stigmatic receptivity of peach flowers submitted to heat stress, Acta Scientiarum. Agronomy 2020, 42, e42450. [Google Scholar]
- Nunes-Silva, P.; Witter, S.; da Rosa, J.M.; Halinski, R.; Schlemmer, L.M.; Arioli, C.J.; Ramos, J.D.; Botton, M.; Blochtein, B. Diversity of floral visitors in apple Orchards: Influence on fruit characteristics depends on apple cultivar. Neotrop. Entomol. 2020, 49, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Samnegård, U.; Hambäck, P.A.; Smith, H.G. Pollination treatment affects fruit set and modifies marketable and storable fruit quality of commercial apples. R. Soc. Open Sci. 2019, 6, 190326. [Google Scholar] [CrossRef] [Green Version]
- Brittain, C.; Kremen, C.; Garber, A.; Klein, A.M. Pollination and plant resources change the nutritional quality of almonds for human health. PLoS ONE 2014, 9, e90082. [Google Scholar] [CrossRef]
- Whiting, M. Advancing Precision Pollination Systems to Improve Yield Security; Final project report; Washington State University: Pullman, WA, USA, 2019; p. 11. Available online: https://treefruitresearch.org/report/advancing-precision-pollination-systems-to-improve-yield-security/ (accessed on 11 March 2021).
- Cerovíc, R.; Akšíc, M.F.; Meland, M. Success rate of individual pollinizers for the pear cultivars ‘Ingeborg’ and ‘Celina’ in a nordic climate. Agronomy 2020, 10, 970. [Google Scholar] [CrossRef]
- Quinlan, G.; Milbrath, M.; Otto, C.; Smart, A.; Iwanowicz, D.; Cornman, R.S.; Isaacs, R. Honey bee foraged pollen reveals temporal changes in pollen protein content and changes in forager choice for abundant versus high protein flowers. Agric. Ecosys. Environ. 2021, 322, 107645. [Google Scholar] [CrossRef]
- Quinet, M.; Mabeluanga, T.; Moquet, L.; Jacquemart, A.L. Introduction of new tools to improve pollination in European pear orchards. Sci. Hortic. 2016, 213, 5–12. [Google Scholar] [CrossRef]
- Sáez, A.; Negri, P.; Viel, M.; Aizen, M.A. Pollination efficiency of artificial and bee pollination practices in kiwifruit. Sci. Hortic. 2019, 246, 1017–1021. [Google Scholar] [CrossRef]
- Ascari, L.; Cristofori, V.; Macri, F.; Botta, R.; Silvestri, C.; De Gregorio, T.; Herta, E.S.; Di Berardino, M.; Kaufmann, S.; Sinisalco, C. Hazelnut pollen phenotyping using label-free impedance flow cytometry. Front. Plant Sci. 2020, 11, 1935. [Google Scholar] [CrossRef]
- Padilla, F.; Soria, N.; Oleas, A.; Rueda, D.; Manjunatha, B.; Kundapur, R.R.; Maddela, N.R.; Rajeswari, B. The effects of pesticides on morphology, viability, and germination of Blackberry (Rubus glaucus Benth.) and Tree tomato (Solanum betaceum Cav.) pollen grains. 3 Biotech 2017, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Estrada, A.; Cuevas, J. Pollination designs in ‘Manzanillo’ olive orchards. Sci. Hortic. 2020, 261, 108918. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, Y.; Liu, Y.; Zhang, T.; Peng, S.; Deng, J. Novel classification forms for xenia. HortScience 2020, 55, 980–987. [Google Scholar] [CrossRef]
- FAO. The Pollination of Cultivated Plants: A Compendium for Practitioners; Roubik, D.W., Ed.; FAO: Rome, Italy, 2018; Volume 2, Available online: https://www.fao.org/3/i9184en/I9184EN.pdf (accessed on 11 March 2021).
- Isaacs, R.; Williams, N.; Ellis, J.; Pitts-Singer, T.L.; Bommarco, R.; Vaughan, M. Integrated crop pollination: Combining strategies to ensure stable and sustainable yields of pollination-dependent crops. Basic Appl. Ecol. 2017, 22, 44–60. [Google Scholar] [CrossRef]
- Goodrich, B.K.; Williams, J.C.; Goodhue, R.E. The great bee migration: Supply analysis of honey bee colony shipments into California for almond pollination services. Am. J. Agric. Econ. 2019, 101, 1353–1372. [Google Scholar] [CrossRef]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A complex parasite, crippling honey bees worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef]
- Guichard, M.; Dietemann, V.; Neuditschko, M.; Dainat, B. Advances and perspectives in selecting resistance traits against the parasitic mite Varroa destructor in honey bees. Genet. Sel. Evol. 2020, 52, 71. [Google Scholar] [CrossRef]
- Rader, R.; Cunningham, S.A.; Howlet, B.G.; Inouye, D.W. Non-bee insects as visitors and pollinators of crops: Biology, ecology, and management. Ann. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef] [Green Version]
- Knapp, J.L.; Bartlett, L.J.; Osborne, J.L. Re-evaluating strategies for pollinator-dependent crops: How useful is parthenocarpy? J. Appl. Ecol. 2017, 54, 1171–1179. [Google Scholar] [CrossRef]
- Hu, Y.; Resende, M.F.R.; Bombarely, A.; Brym, M.; Bassil, E.; Chambers, A.H. Genomics-based diversity analysis of Vanilla species using a Vanilla planifolia draft genome and Genotyping-By-Sequencing. Sci. Rep. 2019, 9, 3416. [Google Scholar] [CrossRef]
- Pinillos, V.; Cuevas, J. Artificial pollination in tree crop production. In Horticultural Reviews; Janick, J., Ed.; Wiley and Sons, Inc.: Hoboken, NJ, USA, 2008; Chapter 34; pp. 239–276. [Google Scholar]
- Pattemore, D.E.; Evans, L.E.; McBrydie, H.M.; Dag, A.; Howlett, B.G.; Cutting, B.; Goodwin, R.M. Understanding pollination processes in avocado (Persea americana) orchards. Acta Hortic. 2020, 1299, 317–328. [Google Scholar] [CrossRef]
- Howlett, B.G.; Read, S.F.J.; Alavi, M.; Cutting, B.T.; Nelson, W.R.; Goodwin, R.M.; Cross, S.; Thorp, T.G.; Pattemore, D.E. Cross-pollination enhances macadamia yields, even with branch-level resource limitation. HortScience 2019, 54, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.d.S.; Milfont, M.d.O.; Silva, M.M.; Carneiro, L.T.; Castro, C.C. Butterflies provide pollination services to macadamia in northeastern Brazil. Sci. Hortic. 2020, 259, 108818. [Google Scholar] [CrossRef]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Billiard, S.; Gallina, S.; Essalouh, L.; Mhaïs, A.; Moukhli, A.; El Bakkali, A.; Barcaccia, G.; et al. Elucidation of the genetic architecture of self-incompatibility in olive: Evolutionary consequences and perspectives for orchard management. Evol. Appl. 2017, 10, 867–880. [Google Scholar] [PubMed]
- Montemurro, C.; Dambruoso, G.; Bottalico, G.; Sabetta, W. Self-incompatibility assessment of some italian olive genotypes (Olea europaea L.) and cross-derived seedling selection by SSR markers on seed endosperms. Front. Plant Sci. 2019, 10, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, R. High-Tech Bee Boosters. Cherry Growers Turn to Tech Companies to Supplement Honey Bee Pollination. 2021. Available online: https://www.goodfruit.com/high-tech-bee-boosters/?fbclid=IwAR3QfeQggrBT0_9_aomEvhY9SbZDg_3Ldkn7jHtxwcaX8yAPA818wJbxfLg (accessed on 11 March 2021).
- Mu, L.; Liu, H.; Cui, Y.; Fu, L.; Gejima, Y. Mechanized technologies for scaffolding cultivation in the kiwifruit industry: A review. Inf. Processing Agric. 2018, 5, 401–410. [Google Scholar] [CrossRef]
- Edete. Available online: https://www.edetepta.com/ (accessed on 11 March 2021).
- AgroPalm Machinery. Available online: https://www.agropalmsmachinery.com/ (accessed on 11 March 2021).
- Kiwifruit Vine Health. Available online: https://www.kvh.org.nz/ (accessed on 11 March 2021).
- Fraser Gear. Available online: https://frasergear.co.nz/ (accessed on 11 March 2021).
- Vera-Chang, J.; Cabrera-Verdezoto, R.; Morán-Morán, J.; Neira-Rengifo, K.; Haz-Burgos, R.; Vera-Barahona, J.; Molina-Triviño, H.; Moncayo-Carreño, O.; Díaz-Ocampo, E.; Cabrera-Verdesoto, C. Evaluación de tres métodos de polinización artificial en clones de cacao (Theobroma cacao L.) CCN-51. Idesia 2016, 34, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.A. Pollination of date palm (Phoenix dactylifera L.) cv. Khenazy by pollen grain-water suspension spray. J. Food Agric. Environ. 2010, 8, 313–317. [Google Scholar]
- Al-Wusaibai, N.A.; Ben Abdallah, A.; Al-Husainai, M.S.; Al-Salman, H.; Elballaj, M. A comparative study between mechanical and manual pollination in two premier Saudi Arabian date palm cultivars. Indian J. Sci. Tech. 2012, 5, 2487–2490. [Google Scholar] [CrossRef]
- Munir, M.; Al-Hajhoj, M.R.; Ghazzawy, H.S.; Sallam, A.K.M.; Al-Bahigan, A.M.; Al-Muiweed, M.A. A comparative study of pollination methods effect on the changes in fruit yield and quality of date palm cultivar Khalas. Asian J. Agric. Bio. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Akhavan, F.; Kamgar, S.; Nematollahi, M.A.; Golneshan, A.A.; Nassiri, S.M.; Mousavi Khaneghah, A. Design, development, and performance evaluation of a ducted fan date palm (Phoenix dactylifera L.) pollinator. Sci. Hortic. 2021, 277, 109808. [Google Scholar] [CrossRef]
- Asteggiano, L.; Giordani, L.; Bevilacqua, A.; Vittone, G.; Costa, G. Ten years of research on complementary pollination of kiwifruit. Acta Hortic. 2011, 913, 615–620. [Google Scholar] [CrossRef]
- Lim, K.H.; Lee, S.H. Effect of sodium chloride, PGDO and Arabic gum in pollen liquid diluent on suspensibility of kiwi pollen. J. Appl. Bot. Food Qual. 2013, 86, 133–137. [Google Scholar]
- Goodwin, R.M.; McBrydie, H.M. Use of Pollen Blowers and Pollen Dispensers to Pollinate Kiwifruit Artificially; Available online: http://www.kvh.org.nz/vdb/document/92559 (accessed on 11 March 2021).
- Tacconi, G.; Michelotti, V.; Cacioppo, O.; Vittone, G. Kiwifruit pollination: The interaction between pollen quality, pollination systems and flowering stage. J. Berry Res. 2016, 6, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Pozo, M.I.; Vendeville, J.; Kay, C.; Wackers, F. Entomovectoring technology in kiwifruit pollination. Acta Hortic. 2018, 1218, 381–390. [Google Scholar] [CrossRef]
- Shi, F.; Jiang, Z.; Ma, C.; Zhu, Y.; Liu, Z. Controlled parameters of targeted pollination deposition by air-liquid nozzle. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mach. 2019, 50, 115–124. [Google Scholar]
- Sakamoto, D.; Hayama, H.; Ito, A.; Kashimura, Y.; Moriguchi, T.; Nakamura, Y. Spray pollination as a labor-saving pollination system in Japanese pear (Pyrus pyrifolia (Burm.f.) Nakai): Development of the suspension medium. Sci. Hortic. 2009, 119, 280–285. [Google Scholar] [CrossRef]
- Cacioppo, O.; Michelotti, V.; Vittone, G.; Tacconi, G. Pollination of kiwifruit: 30 years of applied research leads to a model system for studying the interaction between pollination and flowering stage. Acta Hortic. 2018, 1229, 355–363. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. Profitability of artificial pollination in ‘Manzanillo’ olive orchards. Agronomy 2020, 10, 652. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Evans, M.J.; Neave, M.; Armstrong, J.; Barton, P.S. Pollination and resource limitation as interacting constraints on almond fruit set. Plant Biol. 2020, 22, 113–119. [Google Scholar] [CrossRef]
- Fichtner and Wilson. Can Mechanically Applied Pollen Either Supplement Bees, Or Ensure an Almond Crop in the Event of Bee Inefficacy or Unavailability? 2016. Available online: https://thealmonddoctor.com/2016/01/10/supplemental_pollen_trials_2015/ (accessed on 11 March 2021).
- Ellena, M.; Sandoval, P.; Gonzalez, A.; Galdames, R.; Jequier, J.; Contreras, M.; Azocar, G. Preliminary results of supplementary pollination on hazelnut in south Chile. Acta Hortic. 2014, 1052, 121–128. [Google Scholar] [CrossRef]
- Ascari, L.; Guastella, D.; Sigwebela, M.; Engelbrecht, G.; Stubbs, O.; Hills, D.; De Gregorio, T.; Siniscalco, C. Artificial pollination on hazelnut in South Africa: Preliminary data and perspectives. Acta Hortic. 2018, 1226, 141–147. [Google Scholar] [CrossRef]
- Guastella, D.; Sigwebela, M.; Suarez, E.; Stubbs, O.; Acevedo, J.; Engelbrecht, G. Effect of photo-selective shade nets on pollination process and nut development of Corylus avellana L. Front. Plant Sci. 2020, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, H.; Karimi, H.R.; Shamshiri, M.H.; Tajabadipur, A. Preliminary evaluation of artificial pollination in pistachio using pollen suspension spray. Plant Knowl. J. 2013, 2, 94–98. [Google Scholar]
- Karimi, H.R.; Mohammadi, N.; Estaji, A.; Esmaeilizadeh, M. Effect of supplementary pollination using enriched pollen suspension with Zn on fruit set and fruit quality of pistachio. Sci. Hortic. 2017, 216, 272–277. [Google Scholar] [CrossRef]
- Karimi, H.R.; Zeraatkar, H. Effects of artificial pollination using pollen suspension spray on nut and kernel quality of pistachio cultivar Owhadi. Int. J. Fruit Sci. 2016, 16, 171–181. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, Y.; Yu, Y.; Gao, H.; Lai, C.; Luo, X.; Huang, X. Effects of cross-pollination by ‘Murcott’ tangor on the physicochemical properties, bioactive compounds and antioxidant capacities of ‘Qicheng 52’ navel orange. Food Chem. 2019, 270, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.P.; Sharkey, T.D. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant Cell Environ. 2019, 42, 2759–2775. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Law, S.E.; Mccoy, D.; Wetzstein, H.Y. Stigma development and receptivity in almond (Prunus dulcis). Ann. Bot. 2006, 97, 57–63. [Google Scholar] [CrossRef]
- Kargar, M.H.; Imani, A. Effects of fungicides on pollen germination peach and nectarine in vitro. Afr. J. Plant Sci. 2011, 5, 643–647. [Google Scholar]
- Pollen Plus. Available online: https://www.pollenplus.co.nz/pure-pollen (accessed on 11 March 2021).
- Parker, A.J.; Tran, J.L.; Ison, J.L.; Bai, J.D.K.; Weis, A.E.; Thomson, J.D. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod-Plant Interact. 2015, 9, 197–203. [Google Scholar] [CrossRef]
- Oster, R.; DeBell, J. Development of a backpack-mounted pollen vacuum. Tree Plant. Notes 2016, 59, 42–47. [Google Scholar]
- Wizenberg, S.B.; Weis, A.E.; Campbell, L.G. Comparing methods for controlled capture and quantification of pollen in Cannabis sativa. Appl. Plant Sci. 2020, 8, e11389. [Google Scholar] [CrossRef] [PubMed]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. Effects of desiccation and dilution on germinability of almond pollen. J. Hortic. Sci. Biotechnol. 1999, 74, 321–327. [Google Scholar] [CrossRef]
- Coutney, R.; Mullinax, T.J. No Bees, but a lot of Buzz about Artificial Pollination (Video). 2016. Available online: https://www.goodfruit.com/no-bees-but-a-lot-of-buzz-about-artificial-pollination-video/) (accessed on 11 March 2021).
- No.1 Pollen, 2019 Pollination Handbook. Available online: https://www.trevelyan.co.nz/wp-content/uploads/2019/10/PollinationHandbook2019_booklet_ver1.0.pdf (accessed on 11 March 2021).
- Firman Pollen. Available online: https://firmanpollen.com/ (accessed on 11 March 2021).
- PollenPro. Available online: https://www.pollenpro.net/ (accessed on 11 March 2021).
- Cruzatty, L.G.; Rivero, M.; Droppelmann, F. Effect of temperature and drying on the longevity of stored Nothofagus alpina pollen. N. Zeal. J. Bot. 2015, 53, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.H.; Brown, C.D. Pollen has higher water content when dispersed in a tricellular state than in a bicellular state. Acta Bot. Bras. 2018, 32, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Buitink, J.; Claessens, M.M.A.E.; Hemminga, M.A.; Hoekstra, F.A. Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiol. 1998, 118, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivanna, K.R.; Johri, B.M. The Angiosperm Pollen: Structure and Function; Wiley Eastern: Louisville, KY, USA, 1985. [Google Scholar]
- Williams, J.H.; Taylor, M.L.; O’Meara, B.C. Repeated evolution of tricellular (and bicellular) pollen. Am. J. Bot. 2014, 101, 559–571. [Google Scholar] [CrossRef]
- Brewbaker, J.L. The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in the angiosperms. Am. J. Bot. 1967, 54, 1069–1083. [Google Scholar] [CrossRef]
- Towill, L. Cryopreservation of Plant Cells and Organs; Kartha, K.K., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 171–198. [Google Scholar]
- Lin, L.; He, Y.; Xiao, Z.; Zhao, K.; Dong, T.; Nie, P. Rapid-detection sensor for rice grain moisture based on NIR spectroscopy. Appl. Sci. 2019, 9, 1654. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, W. Moisture content detection of maize seed based on visible/near-infrared and near-infrared hyperspectral imaging technology. Int. J. Food Sci. Technol. 2020, 55, 631–640. [Google Scholar] [CrossRef]
- Flying Doctors. Available online: https://www.biobestgroup.com/en/news/precision-pollination-in-kiwi-with-the-flying-doctors%C2%AE (accessed on 11 March 2021).
- Lahdenperä, M.L. A case study: Use of prestop® mix biofungicide in entomovectoring on apple against storage rot diseases. In Entomovectoring for Precision Biocontrol and Enhanced Pollination of Crops; Springer: Cham, Switzerland, 2020; pp. 137–145. [Google Scholar]
- Duke, M.; Barnett, J.; Bell, J.; Jones, M.H.; Martinsen, P.; McDonald, B.; Nejati, M.; Scarfe, A.; Schaare, P.; Seabright, M.; et al. Pollination of Kiwifruit Flowers. In Proceedings of the 7th Asian-Australasian Conference on Precision Agriculture (7ACPA), Hamilton, New Zealand, 1–5 October 2017; Available online: https://zenodo.org/record/895619#.YIpCmrUzZaQ (accessed on 11 March 2021).
- On Target Spray System. Available online: https://ontargetspray.com/ (accessed on 11 March 2021).
- Pollensmart. Available online: https://www.pollensmart.co.nz/ (accessed on 11 March 2021).
- Progressive Ag Inc. Available online: https://proaginc.com/ (accessed on 11 March 2021).
- Dropcopter. Available online: https://www.dropcopter.com/ (accessed on 11 March 2021).
- Zhang, C.; Valente, J.; Kooistra, L.; Guo, L.; Wang, W. Opportunities of UVAs in orchard management. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci-ISPRS Arch. 2019, 42, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Chechetka, S.A.; Yu, Y.; Tange, M.; Miyako, E. Materially engineered artificial pollinators. Chem 2017, 2, 224–239. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Miyako, E. Soap bubble pollination. iScience 2020, 23, 101188. [Google Scholar] [CrossRef]
- Zhang, L.; Ferguson, L.; Whiting, M.D. Temperature effects on pistil viability and fruit set in sweet cherry. Sci. Hortic. 2018, 241, 8–17. [Google Scholar] [CrossRef]
- Badger, M.; Ortega-Jimenez, V.M.; von Rabenau, L.; Smiley, A.; Dudley, R. Electrostatic charge on flying hummingbirds and its potential role in pollination. PLoS ONE 2015, 10, e0138003. [Google Scholar] [CrossRef]
- Broussard, M.A.; Goodwin, M.; McBrydie, H.M.; Evans, L.J.; Pattemore, D.E. Pollination requirements of kiwifruit (Actinidia chinensis Planch.) differ between cultivars ‘Hayward’ and ‘Zesy002’. N. Zeal. J. Crop Hortic. Sci. 2021, 49, 30–40. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Buriani, G.; Mauri, S.; Kay, C.; Tacconi, G.; Spinelli, F. Pathways of flower infection and pollen-mediated dispersion of Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. Hortic. Res. 2018, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Tontou, R.; Giovanardi, D.; Stefani, E. Pollen as a possible pathway for the dissemination of Pseudomonas syringae pv. actinidiae and bacterial canker of kiwifruit. Phytopathol. Mediterr. 2014, 53, 333–339. [Google Scholar]
- Vanneste, J. The scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of Kiwifruit (Pseudomonas syringae pv. actinidiae). Ann. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Isogai, M.; Miyoshi, K.; Watanabe, M.; Yoshikawa, N. Characterization of horizontal transmission of blueberry latent spherical virus by pollen. Arch. Virol. 2020, 165, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Card, S.D.; Pearon, M.N.; Clover, G.R.G. Plant pathogens transmitted by pollen. Australas. Plant Pathol. 2007, 36, 455–461. [Google Scholar] [CrossRef]
- Beaver-Kanuya, E.; Harper, S.J. Detection and quantification of four viruses in Prunus pollen: Implications for biosecurity. J. Virol. Methods 2019, 271, 113673. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Hendrix, S.D.; Clough, Y.; Scofield, A.; Kremen, C. Interacting effects of pollination, water and nutrients on fruit tree performance. Plant Bio. 2015, 17, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Herbert, S.W.; Walton, D.A.; Wallace, H.M. The influence of pollen-parent and carbohydrate availability on macadamia yield and nut size. Sci. Hortic. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Webber, S.M.; Garratt, M.P.D.; Lukac, M.; Bailey, A.P.; Huxley, T.; Potts, S.G. Quantifying crop pollinator-dependence and pollination deficits: The effects of experimental scale on yield and quality assessments. Agric. Ecosyst. Environ. 2020, 304, 107106. [Google Scholar] [CrossRef]
- Sáez, A.; Aizen, M.A.; Medici, S.; Viel, M.; Villalobos, E.; Negri, P. Bees increase crop yield in an alleged pollinator-independent almond variety. Sci. Rep. 2020, 10, 3177. [Google Scholar] [CrossRef] [Green Version]
- Roubik, D.W. Feral African bees augment neotropical coffee yield. In Pollinating Bees—The Conservation Link between Agriculture and Nature; Kevan, P.G., Impereatriz-Fonseca, V., Eds.; Ministry of the Environment: Brasilia, Brazil, 2002; pp. 255–266. [Google Scholar]
- Ramírez, F.; Davenport, T.L. Mango (Mangifera indica L.) pollination: A review. Sci. Hortic. 2016, 203, 158–168. [Google Scholar] [CrossRef]
- Arrington, M.; DeVetter, L.W. Floral morphology differs among new Northern Highbush blueberry cultivars. J. Hortic. 2018, 5, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Usman, K.; Munir, M.; Khan, M.S. Quantitative and qualitative characteristics of date palm cv. Gulistan in response to pollination times. Sarhad J. Agric. 2018, 34, 40–46. [Google Scholar] [CrossRef]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Flowering and fruit set in olive: A review. Iran. J. Plant Physiol. 2015, 5, 1263–1272. [Google Scholar]
- Alves, G.; Gelain, J.; Vidal, G.S.; Nesi, C.N.; de Mio, L.L.M.; Biasi, L.A. Flowering period and fruit quality of peach trees selections and cultivars in the metropolitan region of Curitiba. Rev. Bras Frutic. 2018, 40, e-991. [Google Scholar] [CrossRef]
- Sanzol, J.; Rallo, P.; Herrero, M. Asynchronous development of stigmatic receptivity in the pear (Pyrus communis; Rosaceae) flower. Am. J. Bot. 2003, 90, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Đorđević, M.; Cerovic, R.; Radičević, S.; Nikolić, D.; Marić, S.; Glišić, M. Influence of pollination mode on fruit set in plum (Prunus domestica). Acta Hortic. 2016, 1139, 347–352. [Google Scholar] [CrossRef]
- Azizi-Gannouni, T.; Ammari, Y. Flowering of sweet cherries “Prunus avium” in Tunisia. In Prunus; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Cavalcante, M.C.; Oliveira, F.F.; Maués, M.M.; Freitas, B.M. Pollination requirements and the foraging behavior of potential pollinators of cultivated brazil nut (Bertholletia excelsa Bonpl.) trees in central amazon rainforest. Psyche 2012, 2012, 978019. [Google Scholar]
- Eradasappa, E.; Mohana, G.S. Investigations on self-compatibility and extent of self and cross- pollination in cashew. J. Plant. Crops 2019, 47, 72–81. [Google Scholar]
- Larue, C.; Austruy, E.; Basset, G.; Petit, R. Revisiting pollination mode in chestnut (Castanea spp.): An integrated approach. Bot. Lett. 2021, 168, 348–372. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.; Liang, Y.; Hao, M.; Li, Y. Flowering and pollination characteristics of chinese-grown pecan (Carya illinoinensis). Acta Hortic. 2015, 1070, 43–51. [Google Scholar] [CrossRef]
- Kallsen, C.E.; Parfitt, D.E.; Maranto, J. UC Pistachio Cultivars Show Improved Nut Quality and Are Ready for Harvest Earlier than ‘Kerman’. April–June 89–93. 2020. Available online: http://calag.ucanr.edu (accessed on 20 March 2022).
- Prado, S.G.; Collazo, J.A.; Irwin, R.E. Resurgence of specialized shade coffee cultivation: Effects on pollination services and quality of coffee production. Agric. Ecosyst. Environ. 2018, 265, 567–575. [Google Scholar] [CrossRef]
- Pacini, E.; Dolferus, R. Pollen developmental arrest: Maintaining pollen fertility in a world with a changing climate. Front. Plant Sci. 2019, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- von Bennewitz, E.; Ramírez, C.; Muñoz, D.; Cazanga-Solar, R.; Lošak, T.; Alba-Mejía, J.E.; Maureira-Butler, I. Phenology, pollen synchronization and fruit characteristics of European hazelnut (Corylus avellana L.) cv. “tonda de giffoni” in three sites of Central Chile. Rev. Fac. De Cienc. Agrar. 2019, 51, 55–67. [Google Scholar]
- Howlett, B.G. Optimising Pollination of Macadamia and Avocado in Australia. Horticulture Innovation Australia; Final report; Project Number: MT13060. 2016. Available online: https://www.horticulture.com.au/growers/help-your-business-grow/research-reports-publications-fact-sheets-and-more/mt13060/ (accessed on 11 March 2021).
- Dallabetta, N.; Guerra, A.; Pasqualini, J.; Fazio, G. Performance of semi-dwarf apple rootstocks in two-dimensional training systems. HortScience 2021, 56, 234–241. [Google Scholar] [CrossRef]
- Legun, K.; Burch, K. Robot-ready: How apple producers are assembling in anticipation of new AI robotics. J. Rural. Stud. 2021, 82, 380–390. [Google Scholar] [CrossRef]
- Aarnio, C. Researchers to Create Robotic System for Fruit Flower Pollination, 2020. Available online: https://dailyevergreen.com/81901/news/researchers-to-create-robotic-system-for-fruit-flower-pollination/ (accessed on 11 March 2021).
- Louarn, G.; Song, Y. Two decades of functional-structural plant modelling: Now addressing fundamental questions in systems biology and predictive ecology. Ann. Bot. 2020, 126, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Boudon, F.; Persello, S.; Jestin, A.; Briand, A.S.; Grechi, I.; Fernique, P.; Guédon, Y.; Léchaudel, M.; Lauri, P.É.; Normand, F. V-Mango: A functional-structural model of mango tree growth, development and fruit production. Ann. Bot. 2020, 126, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi, I.; Hanan, J.S. Investigating tree and fruit growth through functional-structural modelling: Implications of carbon autonomy at different scales. Ann. Bot. 2020, 126, 775–788. [Google Scholar] [CrossRef]
- Sales, L.P.; Rodrigues, L.; Masiero, R. Climate change drives spatial mismatch and threatens the biotic interactions of the Brazil nut. Glob. Ecol. Biogeogr. 2021, 30, 117–127. [Google Scholar] [CrossRef]
- Darbyshire, R.; López, J.N.; Song, X.; Wenden, B.; Close, D. Modelling cherry full bloom using ‘space-for-time’ across climatically diverse growing environments. Agric. For. Meteorol. 2020, 284, 107901. [Google Scholar] [CrossRef]
- Imbach, P.; Fung, E.; Hannah, L.; Navarro-Racines, C.E.; Roubik, D.W.; Ricketts, T.H.; Harvey, C.A.; Donatti, C.I.; Läderach, P.; Locatelli, B.; et al. Coupling of pollination services and coffee suitability under climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 10438–10442. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, K.S.; Leclercq, N.; Vereecken, N.J. Honey bees (Hymenoptera: Apidae) outnumber native bees in Tasmanian apple orchards: Perspectives for balancing crop production and native bee conservation. Austral Entomol. 2021, 60, 422–435. [Google Scholar] [CrossRef]
- Marqués, A.; Juan, A.; Ruíz, M.; Traveset, A.; Leza, M. Improvement of almond production using Bombus terrestris (Hymenoptera: Apidae) in Mediterranean conditions. J. Appl. Entomol. 2019, 143, 1132–1142. [Google Scholar] [CrossRef]
- Karun, K.; Sjini, K.K.; Niral, V.; Amarnth, C.H.; Remya, P.; Rajesh, M.K.; Samsudeen, K.; Jerard, B.A.; Engelmann, F. Coconut (Cocos Nucifera L.) pollen cryopreservation. CryoLetters 2014, 35, 407–417. [Google Scholar] [PubMed]
- Quinet, M.; Jacquemart, A.L. Troubles in pear pollination: Effects of collection and storage method on pollen viability and fruit production. Acta Oecologica 2020, 105, 103558. [Google Scholar] [CrossRef]
- Kadri, N.E.; Mimoun, M.B. In vitro germination of different date palm (Phoenix Dactylifera L.) pollen sources from southern Tunisia under the effect of three storage temperatures. Int. J. Fruit Sci. 2020, 20, S1519–S1529. [Google Scholar] [CrossRef]
- Mesnoua, M.; Roumani, M.; Salem, A. The effect of pollen storage temperatures on pollen viability, fruit set and fruit quality of six date palm cultivars. Sci. Hortic. 2018, 236, 279–283. [Google Scholar] [CrossRef]
- Beltrán, R.; Cebrián, N.; Zornoza, C.; Garmendia, A.; Merle, H.B. Effect of freezing conservation time on loquat (Eriobotrya japonica) pollen germination. Span. J. Agric. Res. 2020, 18, e0804. [Google Scholar] [CrossRef]
- Dutta, S.K.; Srivastav, M.; Chaudhary, R.; Lal, K.; Patil, P.; Singh, S.K.; Singh, A.K. Low temperature storage of mango (Mangifera indica L.) pollen. Sci. Hortic. 2013, 161, 193–197. [Google Scholar] [CrossRef]
- Zambon, C.R.; da Silva, L.F.O.; Pio, R.; Bianchini, F.G.; de Olivera, A.F. Storage of pollen and properties of olive stigma for breeding purposes. Rev. Ciênc. Agron. 2018, 49, 291–297. [Google Scholar] [CrossRef]
- Ozcan, A. Effect of low-temperature storage on sweet Cherry (Prunus avium L.) pollen quality. HortScience 2020, 55, 258–260. [Google Scholar] [CrossRef]
- Barbieri, C.R.; Nava, G.A. Production and in vitro viability of pollen of peach trees grown in subtropical climate. Rev. Bras. Frutic. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Novara, C.; Ascar, L.; La Morgia, V.; Reale, L.; Genre, A.; Siniscalco, C. Viability and germinability in long term storage of Corylus avellana pollen. Sci. Hortic. 2017, 214, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.Z.; Jin, Q.Y.; Ye, H.L.; Zhu, T.J. A novel in vitro germination method revealed the influence of environmental variance on the pecan pollen viability. Sci. Hortic. 2014, 181, 43–51. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Lombardini, L. In vitro viability and germination of Carya illinoinensis pollen under different storage conditions. Sci. Hortic. 2020, 275, 109662. [Google Scholar] [CrossRef]
- Aldahadha, A.M.; Sane, A.K.; Bataineh, A.; Alloush, A.A.; Hamouri, Z. Pollen viability and in vitro germination of six pistachio (Pistacia vera L.) cultivars grown in northern Jordan. Adv. Hortic. Sci. 2019, 33, 441–446. [Google Scholar]

| Factors Contributing to Pollination Deficiency | Benefits of Mechanical Pollination Systems |
|---|---|
| Adverse weather conditions may reduce insect activity, flowering time and flowering intensity. | Buffer against adverse weather conditions as MP can be applied day or night under sub-optimal weather conditions but not during rain events [17]. |
| Asynchrony of flowering—the overlap of pollen and stigma receptivity varies from year-to-year depending on environmental conditions [18]. For monoecious species—asynchrony of flowering of compatible cultivars. For dioecious species, asynchrony between staminate and pistillate blossoms. | Reduced reliance on pollinisers as pollination can occur when the main crop is ready. |
| Preference of honey bees for alternative nectar and/or pollen sources to that of the main crop. However, reduction in non-target forage sources for pollinators may reduce pollinator health to due reduced nutrition [19]. | Diversity of non-target forage sources can be promoted to support managed and native pollinators. |
| Crop species not being suited to honey bee pollination as flowers are not attractive to honey bees e.g., lack of nectar or poor quality nectar (e.g., pear [20]; kiwifruit [21]). | Reduce reliance on managed and native pollinators. |
| Pollinisers can produce a high percentage of sterile [22] or non-viable pollen [18]. Adverse weather conditions and agronomic practices (e.g., pesticides) can also reduce pollen viability [23]. | Pollen quality of stored pollen can be monitored and sourced from orchards producing fertile and viable pollen in a given season. |
| For established orchards, weaknesses in the pollination design, e.g., density (10–20%) and distribution (10–15 m range of compatible polliniser and ratio of male to female trees and in the appropriate wind direction for anemophilous species [24]. Little flexibility to test new pollinisers. | For established orchards, opportunity to improve pollination by applying new improved compatible pollinisers that could improve fruit size and quality (e.g., via xenia and metaxenia effects, [25]. For new orchards, reduction or elimination of polliniser that often has less commercial value of fruit than the main cultivar and can represent a risk if more prone to pest or disease pressure. |
| Crop | Type of Application | Pollen Information | Pollen Carrier | Impact | References |
|---|---|---|---|---|---|
| Cacao (Theobroma cacao) | Blower | No pollen applied | None | Increased fruit yield by ~8% compared to natural pollination | [46] |
| Date palm (Phoenix dactylifera) | Handheld sprayer | Air dried. No other details given. Concentrations (0.5, 1.0, 1.5, 2 g L−1) | Liquid carrier (water) (no benefit for 10% sucrose +1% agar) | Increased fruit set by 7% and 18% (assessed 7–8 weeks after pollination), depending on pollen concentration | [47] |
| Date palm (Phoenix dactylifera) | Handheld duster (attached to a 10 m boom) | Air dried No other details given | Dry carrier—wheat flour (1:5, 1:10, 1:15) | Manual pollination had higher fruit set (40–50%) than MP (30–40%), however yield was comparable. Fruit set was assessed 5 weeks after pollination | [48] |
| Date palm (Phoenix dactylifera) | Handheld sprayer (applied once) | Air dried and stored at 4 °C. | Liquid carrier—water (3 g L−1) | Mature fruit set was higher in MP (86%) than manual pollination (69%) | [49] |
| Date palm (Phoenix dactylifera) | Handheld ducted fan blower (attached to a ~7 m boom) | Air dried then stored at 4 °C for 6 days Concentrations (0.5, 2.5, 5.5 g) Applied up to 3 times | No carrier or wheat flour (1:10—10% pollen and 90% wheat flour) | Fruit setting efficiency (assessed 8 weeks in the Kimri stag) of pollen applied above 2.5 g was comparable to manual pollination | [50] |
| Kiwifruit (Actinidia deliciosa) | Handheld blowers, backpack sprayer, tractor-mounted sprayer and hand pollination | 600 to 1200 g ha−1 | Dry (Lycopodium) and liquid (PollenAid) carrier | Timing of pollen application more important than pollen amount. Yield increased by up to 12% | [51] |
| Kiwifruit (Actinidia deliciosa) | Handheld sprayer | Pollen dipped in 99.5% acetone and stored at 4 °C sans acetone | Liquid pollen carrier (sodium chloride, Arabic gum, PGDO) (4 g L−1) | No control Freshly made carriers increased seed number better than those stored for up to 5 h | [52] |
| Kiwifruit (Actinidia deliciosa) | Handheld pollen blower and pollen dispensers | Pollen blower: 400 to 1600 g ha−1 Pollen dispenser: 4 g per hour for 4 h on 4 days | No carrier on the day of application (but applied on the day before application) | No relationship observed between pollen concentration and seed number | [53] * |
| Kiwifruit (Actinidia deliciosa) | Handheld blowers, tractor-mounted sprayer (9 treatments) | 600 g ha−1 | Liquid and dry carriers (Lycopdium (45%:55%; pollen; Lycopdium), PollenAid (12 g L−1) | Lycopodium may have a drying effect | [54] |
| Kiwifruit (Actinidia deliciosa) | Pollen dispenser (Flying Doctor®) using Bombus terrestris (8–9 hives ha−1) | Pollen dispenser (54–60 g ha−1 day−1) Manual pollination (250 g ha−1) | No carrier | No detail of fruit set but seeds per fruit and individual fruit weight were comparable | [55] |
| Kiwifruit (Actinidia deliciosa) | Sprayer (one spray application —no details given) | Pollen vacuumed from male flowers and stored at −20 °C | Liquid carrier (water) (3 g L−1) | Bee pollination (0.93) resulted in higher yield than artificial pollination (0.65) | [21] |
| Kiwifruit (Actinidia deliciosa) | Air-liquid nozzle spraying vs. electric sprayer | Liquid carrier | Air nozzle spraying (87% fruit set #); Electric sprayer (74%) | [56] | |
| Japanese pear (Pyrus pyrifolia) | Direct hand application and electromotive-style sprayer | Refined with acetone and stored at −30 °C. Germination 35–45% | Liquid carriers (containing agar, xanthan gum, pectin methylesterase or polygalacturonase) | Variable responses however, initial fruit set (60–86%) with liquid carrier was comparable or lower than hand pollination (74–94%) | [57] |
| Olive (Olea europaea) | Mechanical blower applied twice | Stored at 4 °C for a few days. Germination 35 to 68% 2 g per plant | No carrier | Fruit set # of MP (15%) was higher than control (3.7%) | [58] |
| Olive (Olea europaea) | Powder duster (up to four applications) | Stored for 1 year at −20 °C 80 g ha−1 | No carrier | Improved yields were only observed in fruit bearing years and none in unproductive years | [59] |
| Sweet cherry (Prunus avium) | Electrostatic and airblast sprayer Two applications | 36, 72, 144 g ha−1 | Wet carrier (patented recipe) | 10 trials: highly variable results depending on cultivar and year ranging from 0% up to 20% increase in fruit set #. Commercial orchards had 4 to 5 hives per acre. | [17] * |
| Almond (Prunus dulcis) | Hand-held and backpack-mounted sprayers (two applications) | Bee-collected pollen stored at −20 °C. No other details given. | Liquid carrier (recipe in Hopping and Simpson, 1982) | No details given except that MP was not equivalent to hand pollination. | [60] |
| Almond (Prunus dulcis) | Electrostatic sprayer (two applications) | Germination > 85% No other details given. Concentrations (0, 59 and 175 g ha−1) | Liquid carrier (Pollen-tech®) | Year 2014: Applied twice (30–40% and 50–60% bloom)—nut set not improved by treatment Year 2015: Applied once at 60% bloom—MP achieved 17% nut set compared to 57% for combined bee and MP | [61] * |
| Hazelnut (Corylus avellana) | Handheld blower | Stored −12 °C for 2 weeks Germination (46–52%) Concentration—details not clear | Dry carrier−1% Lycopodium spore | increased yield (37%) than wind control | [62] |
| Hazelnut (Corylus avellana) | Backpack sprayer or direct hand application | Stored at −20 °C 30 g ha−1 Germination ranged from 7.5 to 34.5% | Liquid carrier (PollenAid or 10% sucrose solution + 0.5% xanthan gum, 0.02% boric acid) | Increased fruit set (50%) compared to hand application | [63] |
| Hazelnut (Corylus avellana) | Mist blower | Stored at −20 °C 150 g ha−1 | Liquid carrier (10% sucrose solution + 1% agar, 0.02% boric acid | Did not assess fruit set but stigma receptivity and ovule health | [64] |
| Pistachio (Pistacia vera) | Handheld sprayer | No details given on storage. | Liquid carrier | Fruit set # was generally lower when applied by MP than control | [65] |
| Pistachio (Pistacia vera) | Handheld sprayer (3 applications) | No details on storage conditions. | Liquid carrier (agar, zinc sulphate) | Variable responses but the addition of pollen did not necessarily improve mature fruit set | [66] |
| Pistachio (Pistacia vera) | Handheld sprayer (3 applications) | No details on storage conditions. | Liquid carrier (agar, boric acid) | MP decreased mature fruit set (3–7%) compared to open pollination (7–10%) | [67] |
| Delivery Method | Carrier | Comments | Reference |
|---|---|---|---|
| Manual (Hand pollination with brush, glass rod, feather etc.) | No | Time consuming and laborious | See references cited in Table S1 |
| Pollen-containing dispenser (which is located at the entrance of beehive.) | Optional (dry) | Reliance on activity of bee—if weather conditions poor than low pollinator activity. Allows targeted delivery. Commercial option. | e.g., Flying Doctors [20,55,91] |
| Hand-held sprayers/blowers | Optional (dry and wet *) | Bees can help redistribute pollen if using a dry carrier. Commercial option. | See references cited in Table 2 |
| Mobile sprayers/blowers (e.g., quadbikes) | Optional (dry and wet *) | Bees can help redistribute pollen if using a dry carrier. Commercial option. | See references cited in Table 2 |
| Pollen application sprayers with booms and pressure vessel | Optional (dry and wet *) | Does not work in rain. Viable commercial option. Most based on electrostatic spraying. | Examples: |
| Unmanned aerial vehicles (UAVs) (Drones) (aerial delivery of pollen) | Dry only if used. | Technology is new; however, this service is commercially available. Limited capacity on amount of pollen it can transport (e.g., 5 kg). Does not work in rain. Commercial option. | e.g., Dropcopter [97] No peer-reviewed paper found on pollination but see Zhang et al. (2019) [98] on UAVs in orchard management. |
| Robotics e.g., 1: platform mounted manifold spray nozzle; e.g., 2: robotic bees | Optional (dry and wet *) | Autonomous, precision operation. Technology is new. | e.g., Kiwifruit, [93,99,100] |
| Tree Crop (Blooming Period) | Pollination Biology | Pollen Type | Natural Fruit Set (%) | Stigma Receptivity Per Flower | Comment | MP Feasible? | References |
|---|---|---|---|---|---|---|---|
| Apple (Malus domestica) 14 days |
| Orthodox | Only 2–5% required for a commercial crop (crop thinning required) | King flowers—up to 2 days after anthesis; lateral flowers —up to 4 days after anthesis | 4 to 5 seeds require pollination to ensure symmetrical shape. Tendency for biennial bearing of some cultivars. | +++ | [10,115] |
| Apricots (Prunus armeniaca) 16 days |
| Orthodox (most cultivars)Recalcitrant (Iranian varieties?) | 41–77% (no pollinator required) | Variable data: 2–4 days after anthesis. Optimal at flat petal stage or exhibiting petal fall | Apricot one of the first fruit tree to flower in early spring | +++ | [71] |
| Avocado (Persea americana) 2 days |
| Recalcitrant | 0.3% | commonly 3–4 h | Not feasible (protogynous dichogamy may limit logistics) | [35] | |
| Blueberries (Vaccinium corymbosum) 14 to 21 days |
| Recalcitrant | 50–70% is considered good | 3–5 days after anthesis | Flower morphology prevents pollen from falling onto the stigma | Not feasible (style length is usually shorter than the urn-shaped Corolla, which makes it difficult to access the stigma) | [116] |
| Date Palm (Phoenix dactylifera) 14 to 21 days |
| Orthodox | 13 to 50% (in commercial practice, all palms are artificially pollination). | Variable: 1–14 days after spathe opening | Unsuccessful pollination results in parthenocarpic fruit, which are inedible | +++ | [117] |
| Kiwifruit (Actinidia deliciosa) 14 to 42 days |
| Orthodox | 90% (with managed pollination service) | Receptive for up to 7 days after anthesis but decreases after the fourth day | Flowers are nectarless. 1000 ovules per flower need to be fertilised to produce large fruit | +++ | [55] |
| Mango (Mangifera indica) Up to 25 days |
| Recalcitrant | <0.25% | 1 day prior to and 2 days after anthesis but maximum with within 3 h after anthesis | Tendency for biennial bearing | + | [115] |
| Olive (Olea europaea) 21 days |
| Orthodox | 1–2% fruit set is sufficient for commercial yield | 4 to 12 days | Tendency for alternate bearing. | +++ | [118] |
| Peach and nectarine (Prunus persica) 16 to 25 days |
| Orthodox | Up to 58% | Receptive for 3 days | Bees can improve number and size of fruit | +++ | [119] |
| Pear (Pyrus communis) Japanese pear (Pyrus pyrifolia) 10 to 25 days |
| Orthodox | Up to 30%, requires thinning | Receptivity for up to 6 days per flower but within a flower, stigmas may be immature, mature or degenerated. | Low volume of nectar (<3 μL) and its low sugar concentration (<25%). All 5 seeds require pollination to ensure symmetrical shape. | +++ | [120] |
| Plum (Prunus domestica) 40 days |
| Orthodox | Up to 40%, requires thinning | 1 to 4 days after anthesis | Tendency for biennial bearing | +++ | [121] |
| Sweet cherry (Prunis avium) 10 to 24 days |
| Orthodox | 15–80%, sometimes requires thinning | Optimal 2–3 days after anthesis | +++ | [101,122] | |
| Almond (Prunus dulcis) Up to 1 month |
| Orthodox | 30% | Optimal when flowers are past the fully open stage and not at younger stages. | Bloom in late winter and early spring when pollinators are scarce. Tendency for alternate bearing. | +++ | [71] |
| Brazil nut (Bertholletia excelsa) Up to 4 months |
| Recalcitrant? | <1% | 1 day | Requires pollinators that are sufficiently big enough to uncurl the ligule/androecial hood. Largely wild harvested. | Not feasible (flower morphology would prevent delivery of pollen | [123] |
| Cashew (Anacardium occidentale) Up to 3 months |
| Orthodox | 10–27% | Up to 1 day after anthesis and peaking between 10 to 12 am. | ++ | [124] | |
| Chestnut (Castanea sativa) 1 month |
| Orthodox | 24–66% | 1 day pre- and 2 days post-anthesis (each flower has 6–8 stigmas that are receptive one at a time) | Delayed fertilisation of ~ six weeks until ovary is mature | ++ | [125] |
| Hazelnut (Corylus avellana) Up to 3 months |
| Recalcitrant | 66–82% | Up to 3 months | Pollination occurs in winter. Delayed fertilisation of up to 3 months until ovary is mature. | ++ | [64,68] |
| Macadamia (Macadamia integrifolia) 33 days in Australia (up to 5 months in Hawaii) |
| Orthodox | 3–4% | 2–3 days after anthesis | Observed xenic effects of kernel size and mass. Bloom in late winter and early spring. | ++ | [8,111] |
| Pecan (Carya illinoinensis) Up to 28 days |
| Recalcitrant | 50% | 1 day after anthesis for up to 2 days | + | [126] | |
| Pistachio (Pistacia vera) Up to 25 days |
| Recalcitrant | <16.5% | Up to 4 days after anthesis | Tendence for alternate bearing | ++ | [127] |
| Coffee robusta (Coffea canephora) Up to 3 months |
| Orthodox | 9% | Bees boost yield by up to 40% | + | [128] | |
| Cacao (Theobroma cacao) Blooms all year |
| Recalcitrant | <5% | 2–3 days after anthesis | pollinated by specialised insects <2 to 3 mm i.e., tiny flies | ++ | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyles, A.; Close, D.C.; Quarrell, S.R.; Allen, G.R.; Spurr, C.J.; Barry, K.M.; Whiting, M.D.; Gracie, A.J. Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review. Agronomy 2022, 12, 1113. https://doi.org/10.3390/agronomy12051113
Eyles A, Close DC, Quarrell SR, Allen GR, Spurr CJ, Barry KM, Whiting MD, Gracie AJ. Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review. Agronomy. 2022; 12(5):1113. https://doi.org/10.3390/agronomy12051113
Chicago/Turabian StyleEyles, Alieta, Dugald C. Close, Steve R. Quarrell, Geoff R. Allen, Cameron J. Spurr, Kara M. Barry, Matthew D. Whiting, and Alistair J. Gracie. 2022. "Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review" Agronomy 12, no. 5: 1113. https://doi.org/10.3390/agronomy12051113





