Influence of Organomineral Fertiliser from Sewage Sludge on Soil Microbiome and Physiological Parameters of Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Soil Conditioner
2.2. Soil Characteristic
2.3. Experimental Set-Up
2.4. Microbial Analysis
2.5. Plant Analysis
2.5.1. Gas-Exchange Parameters of Plants
2.5.2. Concentrations of Assimilation Pigments in Leaves
2.5.3. Chlorophyll Fluorescence Parameters
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
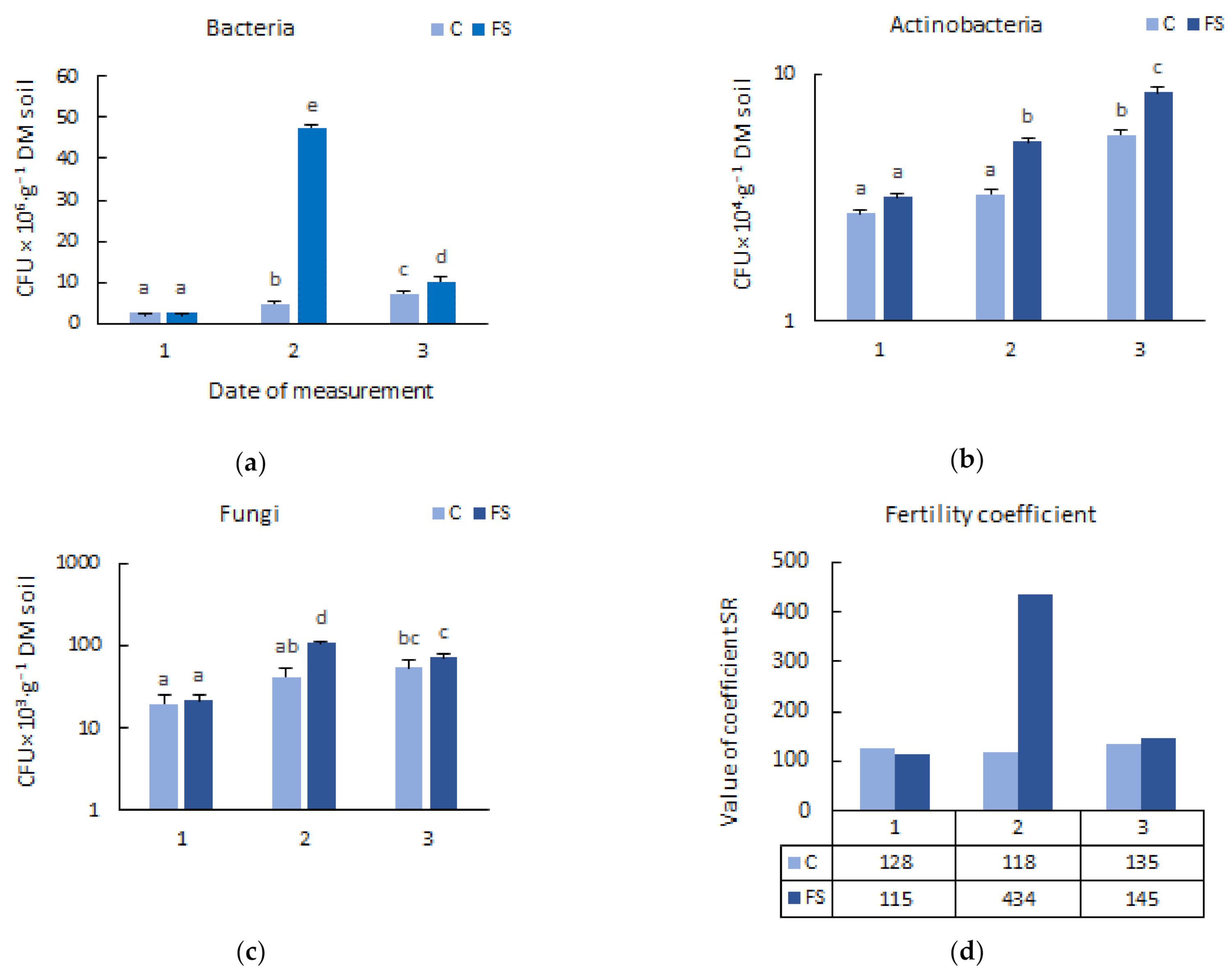
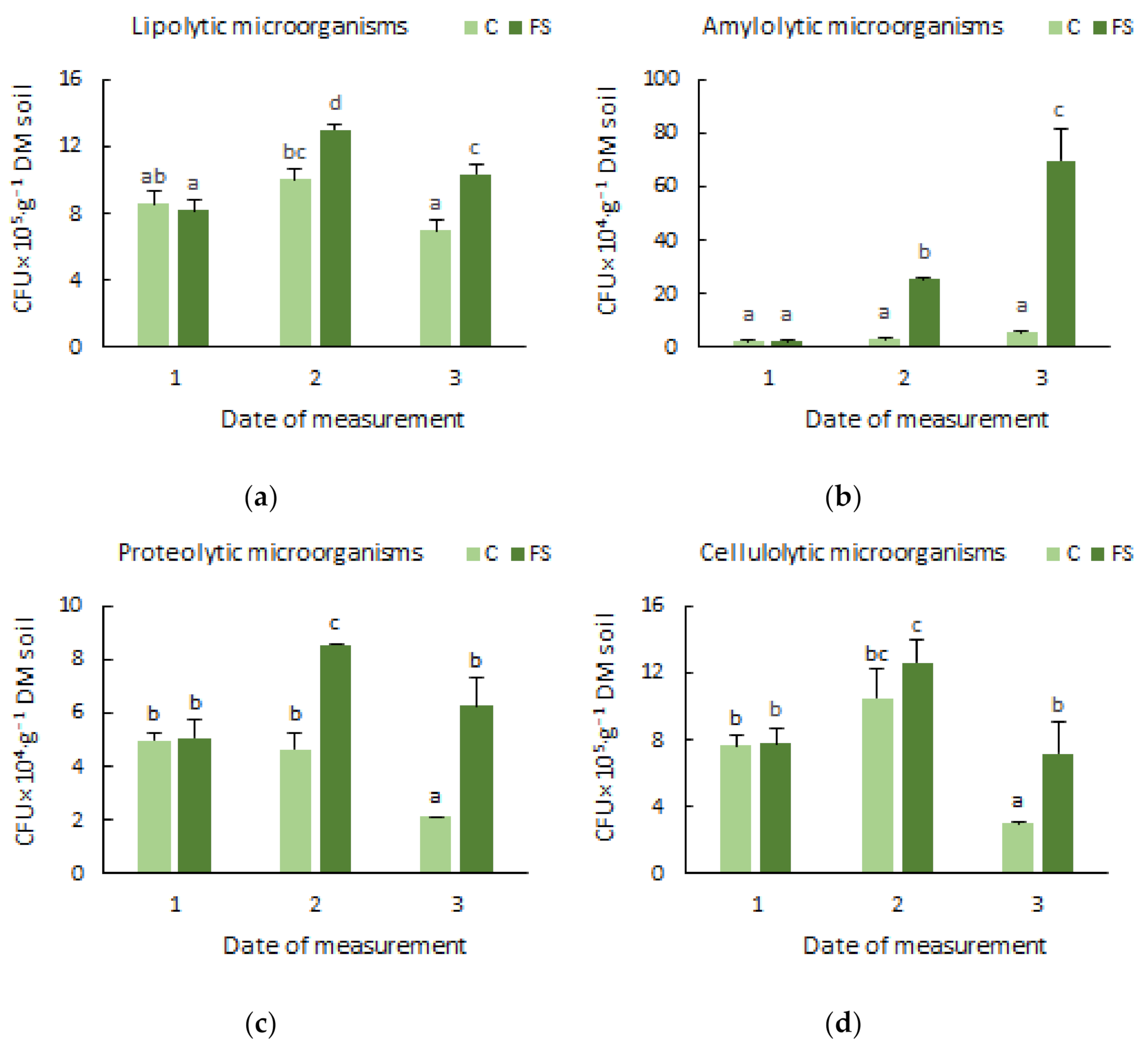
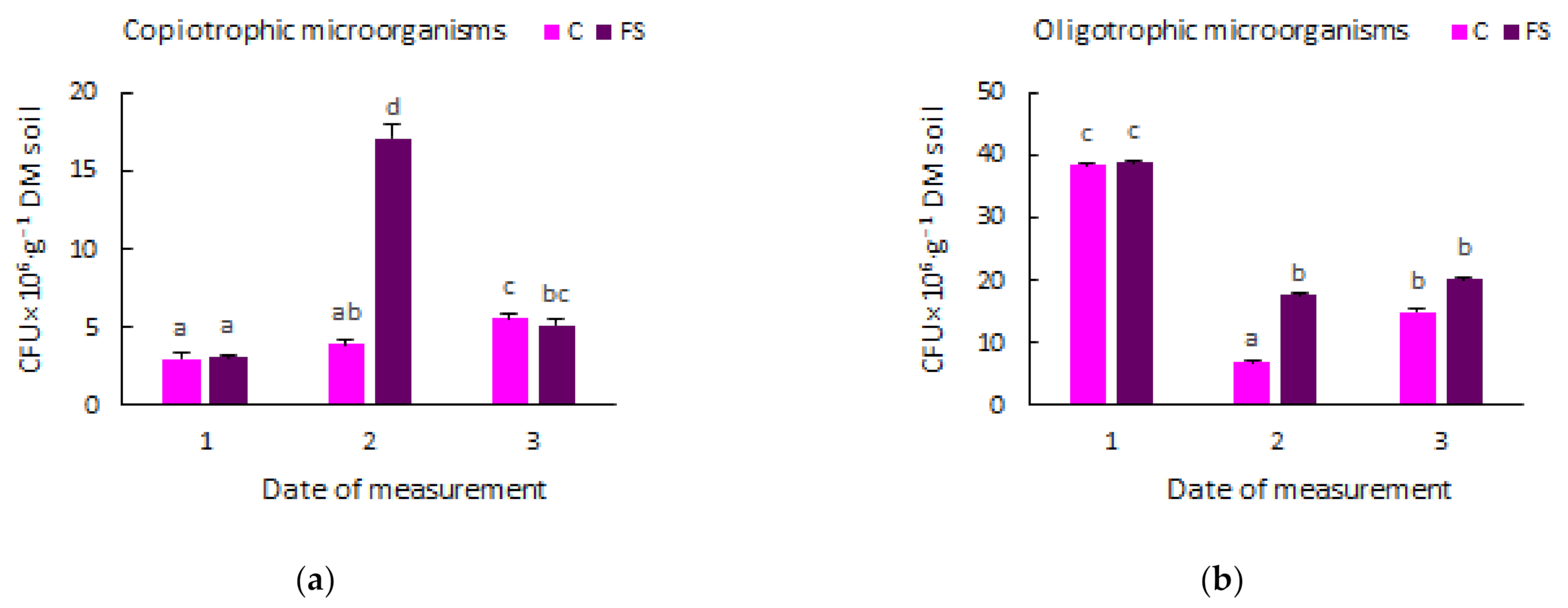
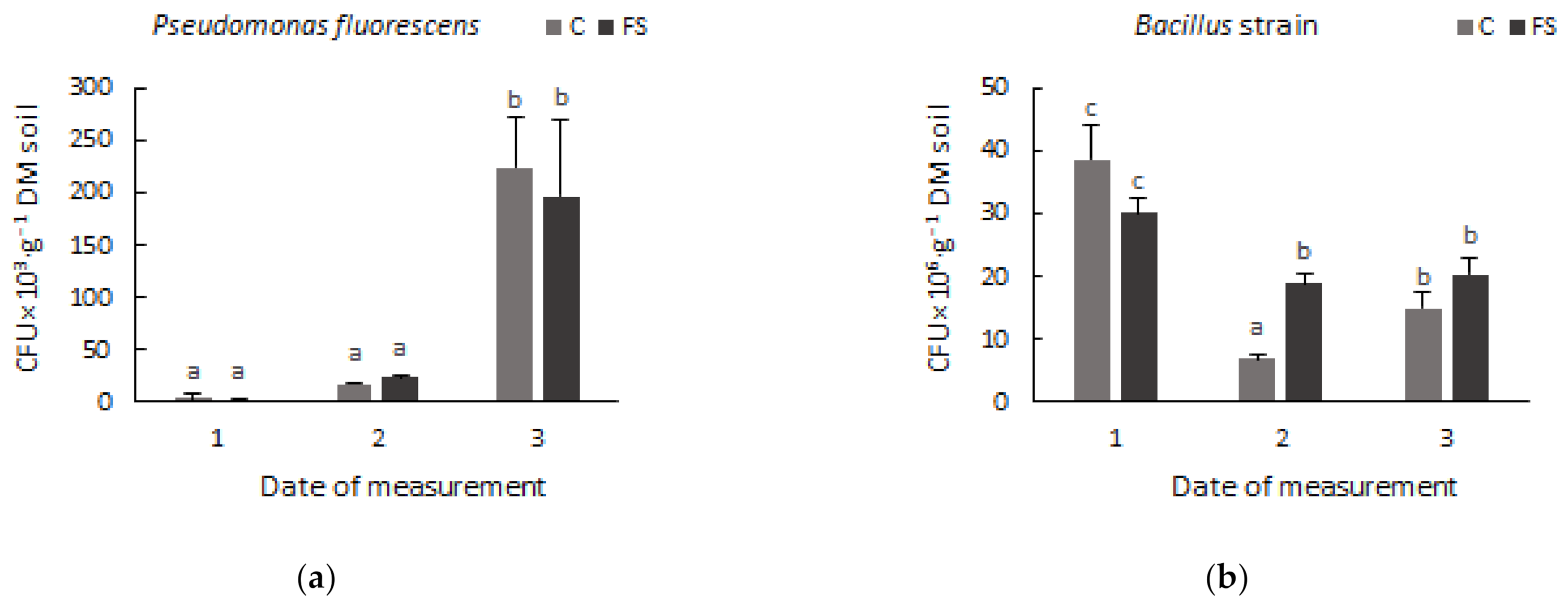
3.1. Soil Microbiome
3.2. Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erb, M.; Lu, J. Soil abiotic factors influence interactions between belowground her-bivores and plant roots. J. Exp. Bot. 2013, 64, 1295–1303. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Res. 2009, 34, 97–125. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.A.; Radovich, T.J.K.; Nguyen, H.V.; Uyeda, J.; Arakaki, A.; Cadby, J.; Paull, R.; Sugano, J.; Teves, G. Use of Organic Fertilizers to Enhance Soil Fertility, Plant Growth, and Yield in a Tropical Environment. In Organic Fertilizers-From Basic Concepts to Applied Outcomes; Larramendy, M.L., Soloneski, S., Eds.; Intech: Rijeka, Croatia, 2016; pp. 85–108. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, H.; Hechmi, S.; Khelil, M.N.; Zoghlami, I.R.; Benzarti, S.; Mokni-Tlili, S.; Hassen, A.; Jedidi, N. Repetitive land application of urban sewage sludge: Effect of amendment rates and soil texture on fertility and degradation parameters. Catena 2019, 172, 11–20. [Google Scholar] [CrossRef]
- Breda, C.C.; Soares, M.B.; Tavanti, R.F.R.; Viana, D.G.; Freddi, O.D.S.; Piedade, A.R.; Mahl, D.; Traballi, R.C.; Guerrini, I.A. Successive sewage sludge fertilization: Recycling for sustainable agriculture. Waste Manag. 2020, 109, 38–50. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B. Toxic metals in sewage sludge-amended soil: Has promotion of beneficial use discounted the risks? Adv. Environ. Res. 2003, 8, 5–19. [Google Scholar] [CrossRef]
- Kowalik, R.; Latosińska, J.; Gawdzik, J. Risk Analysis of Heavy Metal Accumulation from Sewage Sludge of Selected Wastewater Treatment Plants in Poland. Water 2021, 13, 2070. [Google Scholar] [CrossRef]
- Goberna, M.; Simón, P.; Hernández, M.T.; García, C. Prokaryotic communities and potential pathogens in sewage sludge: Response to wastewaster origin, loading rate and treatment technology. Sci. Total Environ. 2018, 615, 360–368. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Major, N.; Schierstaedt, J.; Jechalke, S.; Nesme, J.; Ban, S.G.; Černe, M.; Sørensen, S.J.; Ban, D.; Schikora, A. Composted Sewage Sludge Influences the Microbiome and Persistence of Human Pathogens in Soil. Microorganisms 2020, 8, 1020. [Google Scholar] [CrossRef]
- Hanum, F.; Yuan, L.C.; Kamahara, H.; Aziz, H.A.; Atsuta, Y.; Yamada, T.; Daimon, H. Treatment of sewage sludge using anaerobic digestion in Malaysia: Current state and challenges. Front. Energy Res. 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Dastpak, H.; Pasalari, H.; Jafari, A.J.; Gholami, M.; Farzadki, M. Improvement of Co-Composting by a combined pretreatment Ozonation/Ultrasonic process in stabilization of raw activated sludge. Sci. Rep. 2020, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Tsybina, A.; Wuensch, C. Analysis of sewage sludge thermal treatment methods in the context of circular economy. Detritus 2018, 2, 3. [Google Scholar] [CrossRef]
- Farzadkia, M.; Bazrafshan, E. Lime Stabilization of Waste Activated Sludge. Health Scope 2014, 3, e16035. [Google Scholar] [CrossRef] [Green Version]
- Rousk, J.; Brookes, P.C.; Baath, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concept. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef]
- Köberl, M.; Wagner, P.; Müller, H.; Matzer, R.; Unterfrauner, H.; Cernava, T.; Berg, G. Unraveling the Complexity of Soil Microbiomes in a Large-Scale Study Subjected to Different Agricultural Management in Styria. Front. Microbiol. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Zaccardelli, M.; De Nicola, F.; Villecco, D.; Scotti, R. The development and suppressive activity of soil microbial communities under compost amendment. J. Soil Sci. Plant. Nutr. 2013, 13, 730–742. [Google Scholar] [CrossRef] [Green Version]
- Furtak, K.; Gajda, A.M. Activity and Variety of Soil Microorganisms Depending on the Diversity of the Soil Tillage System. In Sustainability of Agroecosystems; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Rui, Y.; Murphy, D.; Wang, X.; Hoyle, F.C. Microbial respiration, but not biomass, responded linearly to increasing light fraction organic matter input: Consequences for carbon sequestration. Sci. Rep. 2016, 6, 35496. [Google Scholar] [CrossRef] [Green Version]
- Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci. Rep. 2020, 10, 16436. [Google Scholar] [CrossRef] [PubMed]
- Furey, G.N.; Tilman, D. Plant biodiversity and the regeneration of soil fertility. Proc. Natl. Acad. Sci. USA 2021, 118, e2111321118. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B.; Govindjee. Modeling Chlorophyll a Fluorescence Transient: Relation to Photosynthesis. Biochem. Mosc. 2014, 79, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, W.; Wójcik, M. The possibilities of utilization of municipal sewage sludge in selected sewage-treatment plants. Zesz. Nauk. Politech. Rzeszow. Mech. 2015, 87, 339–347. (In Polish) [Google Scholar]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Cyganov, V.A.; Žukov, R.A. Morfologobiohimičiskie osobennosti novovo vida actionomiceta. Mikrobiologiâ 1964, 33, 863–869. [Google Scholar]
- Martin, J.P. Use of acid rose bengale and streptomycin in the plate method f.or estimating soil fungi. Soil Sci. 1950, 6, 215–233. [Google Scholar] [CrossRef]
- Kędzia, W.; Konar, H. Microbiological Diagnosis; PZWL: Warszawa, Poland, 1974. (In Polish) [Google Scholar]
- Cooney, D.G.; Emerson, R. Termophilic Fungi; Freeman and Co.: London, UK, 1964. [Google Scholar]
- Burbianka, M.; Pliszka, A. Food Microbiology: Microbiological Methods for Testing Food Products; PZWL: Warszawa, Poland, 1977. [Google Scholar]
- Maliszewska, W. Proposed Detailed Methodology of Soil Microbiological Analyzes; IUNG: Puławy, Poland, 1954. (In Polish) [Google Scholar]
- Fenglerowa, W. Simple method for counting Azotobacter in soil samples. Acta Microbiol. Pol. 1965, 14, 203–206. [Google Scholar]
- Ohta, H.; Hattori, T. Bacteria sensitive to nutrient broth medium in terrestrial environments. Soil Sci. Plant Nutr. 1980, 26, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Myśków, W. Attempts to use microbial activity indicators to assess soil fertility. Postęp. Mikrobiol. 1981, 20, 173–192. (In Polish) [Google Scholar]
- Polish Standards PN-EN ISO 16072:2011. Soil Quality—Laboratory Methods for Determining the Respiration of Soil Microorganisms. Polish Committee for Standardisation. Available online: https://sklep.pkn.pl/pn-en-iso-16072-2011e.html (accessed on 11 March 2021).
- Candolfi-Vasconcelos, M.C.; Koblet, W. Influence of partial defoliation on gas exchange parameters and chlorophyll content of field-grown grapevines—Mechanisms and limitations of the compensation capacity. Vitis 1991, 30, 129–141. [Google Scholar]
- Arnon, D.J.; Allen, M.B.; Whatley, F. Photosynthesis by isolated chloroplast. Biochim. Biophys. Acta 1956, 20, 449–461. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Hager, A.; Mayer–Berthenrath, T. Die isolierung und quanttaive bestimung der carotenoide und chlorophyll von blatern, algea und isolierten chloroplasten mit hilfe dunnschichtchro-matographischer methoden. Planta 1966, 69, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Rutten, D.; Santarius, K.A. Age-related differences in frost sensitivity of the photosynthesis apparatus of two plagiomnium species. Planta 1992, 187, 224–229. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The Basic. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Bolhár- Nordenkampf, H.R.; Öquist, G. Chlorophyll fluorescence as a total in photosynthesis Research. In Photosynthesis and Production a Changing Environment; Hall, D.O., Ed.; Chapman and Hall: London, UK, 1991; pp. 193–206. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Evaluation of Soil and Plant Properties; Catalog Institute of Environmental Protection: Warsaw, Poland, 1991; p. 334. (In Polish) [Google Scholar]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Farsang, A.; Babcsányi, I.; Ladányi, Z.; Perei, K.; Bodor, A.; Csány, K.T.; Barta, K. Evaluating the effects of sewage sludge compost applications on the microbial activity, the nutrient and heavy metal content of a Chernozem soil in a field survey. Arab. J. Geosci. 2020, 13, 982. [Google Scholar] [CrossRef]
- Adessi, A.; de Carvalho, R.C.; De Philippis, R.; Branquinho, C.; da Silva, J.M. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, S.; Bais, H.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L.; Jin, Y. Plant Growth-Promoting Rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resour. Res. 2018, 178, 3673–3687. [Google Scholar] [CrossRef]
- Ondreičková, K.; Gubišová, M.; Gubiš, J.; Klčová, L.; Horník, M. Rhizosphere bacterial communities of Arundo donax grown in soil fertilised with sewage sludge and agricultural by-products. Agriculture 2019, 65, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sorensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhou, X.; Guo, D.; Zhao, J.; Yan, L.; Feng, G.; Gao, Q.; Yu, H.; Zhao, L. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Khafipour, L.R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.G.D. Pyrosequencing Reveals the Influence of Organic and Conventional Farming Systems on Bacterial Communities. PLoS ONE 2012, 7, e51897. [Google Scholar] [CrossRef] [Green Version]
- Barret, M.; Morrissey, J.P.; O’gara, F. Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol. Fertil. Soils 2011, 47, 729–743. [Google Scholar] [CrossRef]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Myśków, W.; Stachyra, A.; Zięba, S.; Masiak, D. Biological activity of soil as indicator of its fertility and fruitfulness. Rocz. Glebozn. 1996, 47, 89–99. [Google Scholar]
- Myśków, W. Methodological comments on microbiological studies on agricultural soils diversified by agrotechnical treatments. Postęp. Mikrobiol. 1986, 35, 319–331. (In Polish) [Google Scholar]
- Wolna-Maruwka, A.; Sulewska, H.; Niewiadomska, A.; Panasiewicz, K.; Borowiak, K.; Ratajczak, K. The influence of sewage sludge and a consortium of aerobic microorganisms added to the soil under a willow plantation on the biological indicators of transformation of organic nitrogen compounds. Pol. J. Environ. Stud. 2018, 27, 403–412. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobium—legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Pérez, R.; Oudot, M.; Serrano, L.; Hernández, I.; Nápoles, M.; Sosa, D.; Pérez-Martínez, S. Rhizospheric rhizobia identification in maize (Zea mays L.) plants. Agron. Colomb. 2019, 37, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, M.I.P.; Nascimento, R.C.; Rodrigues, D.R.; Escobar, I.E.C.; Fraiz, A.C.R.; Souza, A.P.; Freitas, A.D.S.; Nóbrega, R.S.A.; Fernandes-Júnior, P.I. Maize growth and yield promoting endophytes isolated into a legume root nodule by a cross-over approach. Rhizosphere 2020, 15, 100211. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Usman, K.; Khan, S.; Ghulam, S.; Khan, M.; Khan, N.; Khan, M.; Khalil, S. Sewage Sludge: An Important Biological Resource for Sustainable Agriculture and Its Environmental Implications. Am. J. Plant Sci. 2012, 3, 1708–1721. [Google Scholar] [CrossRef] [Green Version]
- Wolna-Maruwka, A.; Klama, J.; Niewiadomska, A. Effect of Fertilization Using Communal Sewage Sludge on Respiration Activity and Counts of Selected Microorganisms in the Grey Brown Podzolic Soil. Pol. J. Environ. Stud. 2007, 16, 899–907. [Google Scholar]
- Kai, M.; Effmert, U.; Berg, G.; Piechulla, B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 2007, 187, 351–360. [Google Scholar] [CrossRef]
- Weston, D.J.; Pelletier, D.A.; Morrell-Falvey, J.L.; Tschaplinski, T.J.; Jawdy, S.S.; Lu, T.-Y.; Allen, S.M.; Melton, S.J.; Martin, M.Z.; Schadt, C.W.; et al. Pseudomonas fluorescens induces strain-dependent and strain-independent host plant responses in defense networks, primary metabolism, photosynthesis, and fitness. Mol. Plant Microbe Interact. 2012, 25, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Han, J.S.; Cheng, J.H.; Yoon, T.M.; Song, J.; Rajkarnikar, A.; Kim, W.G. Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. J. Appl. Microbiol. 2005, 99, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Singh, R.K.; Srivastava, S.; Kumar, S.; Kashyap, P.L.; Srivastava, A.K. Characterization of antagonistic-potential of two Bacillus strains and their biocontrol activity against Rhizoctonia solani in tomato. J. Basic Microbiol. 2015, 55, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Akinrinlola, R.J.; Yuen, G.Y.; Drijber, R.A.; Adesemoye, A.O. Evaluation of Bacillus Strains for Plant Growth Promotion and Predictability of Efficacy by In Vitro Physiological Traits. Int. J. Microbiol. 2018, 2018, 1–11, 5686874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urra, J.; Alkorta, I.; Mijangos, I.; Epelde, L.; Garbisu, C. Application of sewage sludge to agricultural soil increases the abundance of antibiotic resistance genes without altering the composition of prokaryotic communities. Sci. Total Environ. 2019, 647, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.; Berlanga José, G.; Tormos, I.; Garcia, C. Organic versus inorganic fertilizers: Response of soil properties and crop yield. AIMS Geosci. 2021, 7, 415–439. [Google Scholar] [CrossRef]
- Fernandes, S.; Bettiol, W.; Cerri, C. Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and enzymatic activity. Appl. Soil Ecol. 2005, 30, 65–77. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Jaakkola, S.; Ekholm, P.; Hartikainen, H.; Stoddard, F.L.; Mäkelä SA, P. Biomass yield and quality of bioenergy crops grown with synthetic and organic fertilizers. Biomass 2013, 59, 477–485. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Karčauskienė, D.; Aleinikovienė, J.; Repšienė, R.; Skuodienė, R. The Effect of Mineral and Organic Fertilization on Common Osier (Salix viminalis L.) Productivity and Qualitative Parameters of Naturally Acidic Retisol. Agriculture 2021, 11, 42. [Google Scholar] [CrossRef]
- Gwenzi, W.; Muzava, M.; Mapanda, F.; Tauro, T.P. Comparative Short-Term Effects of Sewage Sludge and Its Biochar on Soil Properties, Maize Growth and Uptake of Nutrients on a Tropical Clay Soil in Zimbabwe. J. Integr. Agric. 2016, 15, 1395–1406. [Google Scholar] [CrossRef] [Green Version]
- Gondek, K. Assessment of the influence of sewage sludge fertilization on yield and content of nitrogen and sulphur in maize (Zea mays L.). J. Elementol. 2010, 15, 65–79. [Google Scholar] [CrossRef]
- Zalewska, M.; Stępień, A.; Wierzbowska, J. Agronomic evaluation of dried sewage sludge and sewage sludge ash as sources of nutrients for maize. J. Elem. 2020, 25, 771–785. [Google Scholar] [CrossRef]
- Nájera, F.; Tapia, Y.; Baginsky, C.; Figueroa, V.; Cabeza, R.; Salazar, O. Evaluation of soil fertility and fertilisation practices for irrigated maize (Zea mays L.) under Mediterranean conditions in central Chile. J. Soil Sci. Plant Nutr. 2015, 15, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Zielonka, D.; Nierebiński, M.; Kalaji, M.H.; Augustynowicz, J.; Prędecka, A.; Russel, S. Efficiency of the photosynthetic apparatus in Cannabis sativa L. fertilized with sludge from a wastewater treatment plant and with phosphogypsum. Ecol. Quest. 2017, 28, 55–61. [Google Scholar]
- Zabotto, A.R.; Longuini Gomes, L.; de Moura D’andréa Mateus, C.; Lyre Villas Boas, R.A.; Kanashiro, S.; Tavares, A.R. Nutrition and physiology of hybrid Eucalyptus urograndis in soil fertilized with sewage sludge. Emir. J. Food Agric. 2020, 32, 19–24. [Google Scholar] [CrossRef]
- Makino, A.; Sakuma, H.; Sudo, E.; Mae, T. Differences between maize and rice in Nuse efficiency for photosynthesis and protein allocation. Plant Cell Physiol. 2003, 44, 952–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuynsma, R.; Kleinert, A.; Kossman, J.; Valentine, A.J.; Hills, P.N. The effects of limiting phosphate on photosynthesis and growth of Lothus japonicus. S. Afr. J. Bot. 2016, 104, 244–248. [Google Scholar] [CrossRef]
- Mengel, K. Potassium. In Handbook of Plant Nutrition; Barker, A.V., Pilbean, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 91–120. [Google Scholar]
- Kalaji, M.H.; Pietkiewicz, S. Some physiological indices to be exploited as a crucial tool in plant breeding. Plant Breed. Seeds Sci. 2004, 49, 19–39. [Google Scholar]
- Kuckenberg, J.T.; Artachnyk, I.; Noga, G. Temporal and spatil changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves. Precis. Agric. 2009, 10, 34–44. [Google Scholar] [CrossRef]
- Baker, N.R.; Resenquist, E. Application of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.; Buschman, C.; Knapp, M. Measurement of chlorophyll fluorescence kinetics (Kautsky effect) and the chlorophyll fluorescence decrease ratio (RFd – values) with the PAM – fluorometer. In Analitycal Methods in Plant sStress Biology; Filek, M., Biesaga-Kościelniak, J., Marcińska, I., Eds.; The Franciszek Górski Institute of Plant Physiology, Polish Academy of Sciences: Krakow, Poland, 2004; pp. 93–111. [Google Scholar]
- Kalaji, M.H.; Łoboda, T. Chlorophyll Fluorescence in Studies of the Physiological State of Plants; SGGW: Warsaw, Poland, 2010; p. 55. (In Polish) [Google Scholar]
- Angelini, G.; Ragni, P.; Esposito, D.; Giardi, P.; Pompili, M.L.; Moscardelli, R.; Giardi, M.T. A device to study the effect of space radiation on photosynthetic organisms. Phys. Med. 2001, 17 (Suppl. 1), 267–268. [Google Scholar]
- Bjorkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Unit | Sewage Sludge after Hygienisation and Agglomeration |
|---|---|---|
| pH | - | 12.5 |
| Organic matter | % | high |
| Total N | % | 2.43 |
| Ca | % | 41.90 |
| Mg | % | 0.49 |
| Total P | % | 0.30 |
| Pb | mg/kg | 30 |
| Cr | mg/kg | 20 |
| Cu | mg/kg | 32 |
| Ni | mg/kg | 33 |
| Cd | mg/kg | 2.70 |
| Zn | mg/kg | 108.00 |
| Hg | mg/kg | 0.08 |
| Pathogenic bacteria Salmonella | presence of bacteria/100 g | not isolated |
| Viable parasite eggs | amount/kg | not found |
| Treatments | Content of Elements | |||
|---|---|---|---|---|
| N (%) | P (g·kg−1) | K (g·kg−1) | ||
| Soil | C | 0.07 ± 0.01a * | 1.06 ± 0.05a | 5.70 ± 0.08a |
| FS | 0.06 ± 0.00a | 0.93 ± 0.01b | 5.64 ± 0.05a | |
| Plants | C | 2.43 ± 0.07a | 4.94 ± 0.17a | 19.68 ± 0.68a |
| FS | 2.17 ± 0.03b | 3.92 ± 0.16b | 20.45 ± 0.58a | |
| Treatments | Gas Exchange Parameters | ||||
|---|---|---|---|---|---|
| A (μmol·m−2·s−1) | E (mmol·m−2·s−1) | ωW (mmol·mol−1) | gS (mol·m−2·s−1) | ci (μmol·mol−1) | |
| C | 6.16 ± 1.55a * | 0.26 ± 0.05a | 24.79 ± 8.07a | 0.05 ± 0.01a | 235.22 ± 61.44a |
| FS | 6.92 ± 0.92a | 0.33 ± 0.07a | 22.16 ± 6.56a | 0.05 ± 0.02a | 273.47 ± 24.36a |
| Treatments | Concentration of assimilation pigments | ||||
| Chlorophyll “a” (mg·g−1 FW) | Chlorophyll “b” (mg·g−1 FW) | Ratio chl “a”/chl. “b” | Total Chlorophyll | Carotenoids (mg·g−1 FW) | |
| C | 1.24 ± 0.12a | 0.35 ± 0.02a | 3.53 ± 0.1a | 1.59 ± 0.15a | 0.59 ± 0.05a |
| FS | 1.38 ± 0.12a | 0.38 ± 0.03a | 3.60 ± 0.09a | 1.76 ± 0.14a | 0.65 ± 0.05a |
| Treatments | F0 | FM | FV/FM | PI | Area (bms) | TFM (ms) |
|---|---|---|---|---|---|---|
| C | 219.34 ± 11.09a * | 1057.66 ± 65.49a | 0.78 ± 0.01a | 1.66 ± 0.38a | 35,009.62 ± 7474.56a | 740.62 ± 58.96a |
| FS | 228.43 ± 7.71a | 1114.39 ± 148.57a | 0.79 ± 0.01b | 1.82 ± 0.1a | 38,372.53 ± 2454.88a | 765.62 ± 15.72a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Mikiciuk, M.; Koniuszy, A.; Meller, E. Influence of Organomineral Fertiliser from Sewage Sludge on Soil Microbiome and Physiological Parameters of Maize (Zea mays L.). Agronomy 2022, 12, 1114. https://doi.org/10.3390/agronomy12051114
Hawrot-Paw M, Mikiciuk M, Koniuszy A, Meller E. Influence of Organomineral Fertiliser from Sewage Sludge on Soil Microbiome and Physiological Parameters of Maize (Zea mays L.). Agronomy. 2022; 12(5):1114. https://doi.org/10.3390/agronomy12051114
Chicago/Turabian StyleHawrot-Paw, Małgorzata, Małgorzata Mikiciuk, Adam Koniuszy, and Edward Meller. 2022. "Influence of Organomineral Fertiliser from Sewage Sludge on Soil Microbiome and Physiological Parameters of Maize (Zea mays L.)" Agronomy 12, no. 5: 1114. https://doi.org/10.3390/agronomy12051114






