Transfer of Durable Stripe Rust Resistance Gene Yr39 into Four Chinese Elite Wheat Cultivars Using Marker-Assisted Selection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Development of Breeding Population
2.3. Field Evaluation
2.4. DNA Extraction and MAS
2.5. Statistical Analysis
3. Results
3.1. Development of Backcross Inbred Line Populations
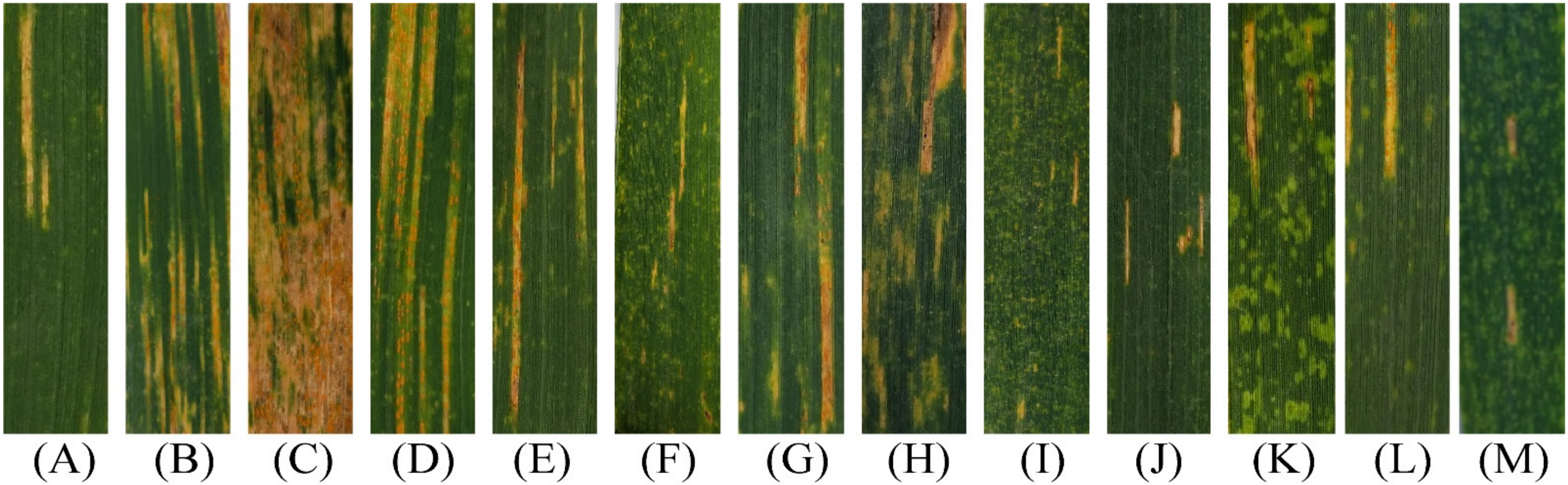
3.2. Evaluation of the Selected Lines for Resistance to Stripe Rust
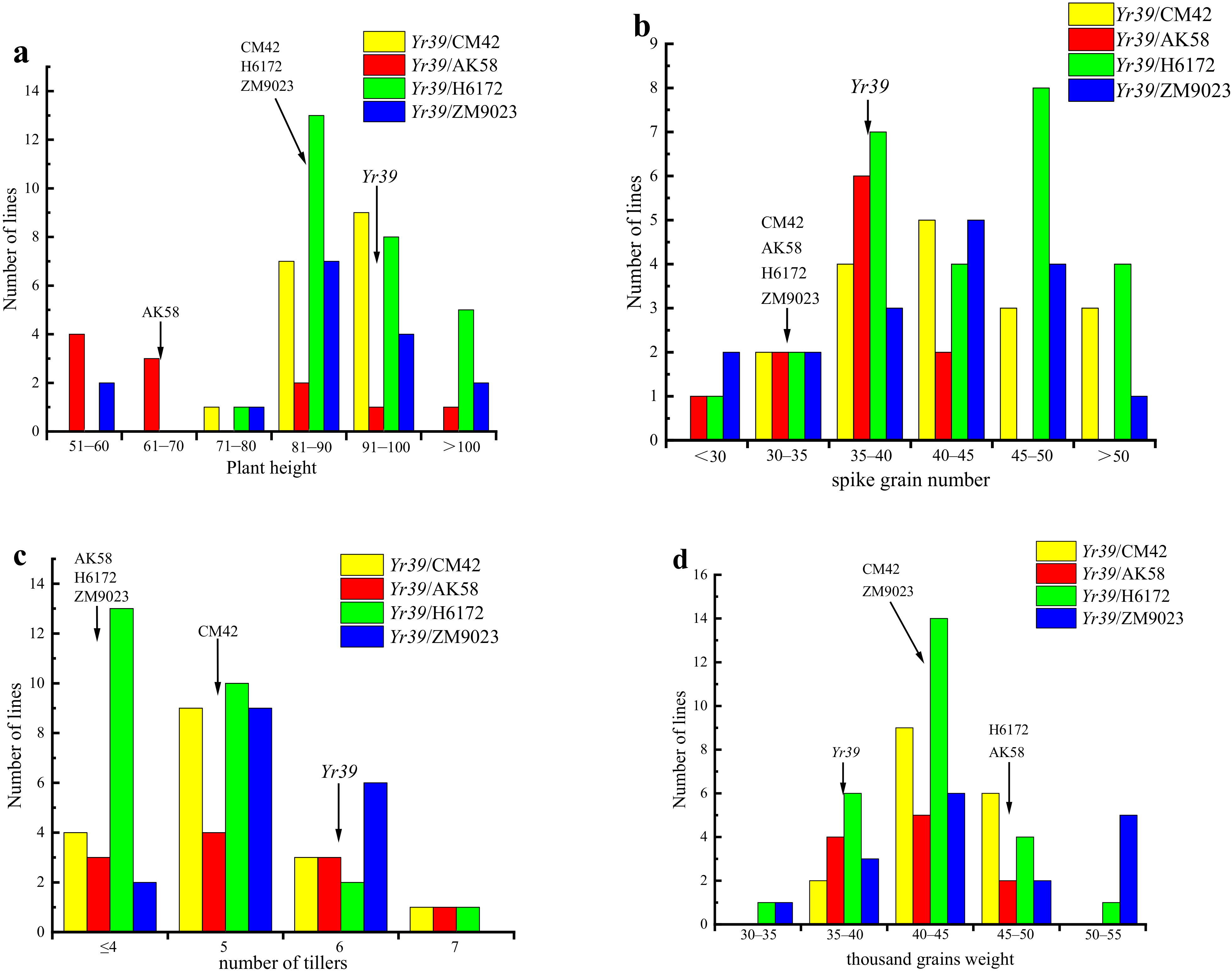
3.3. Agronomic Traits
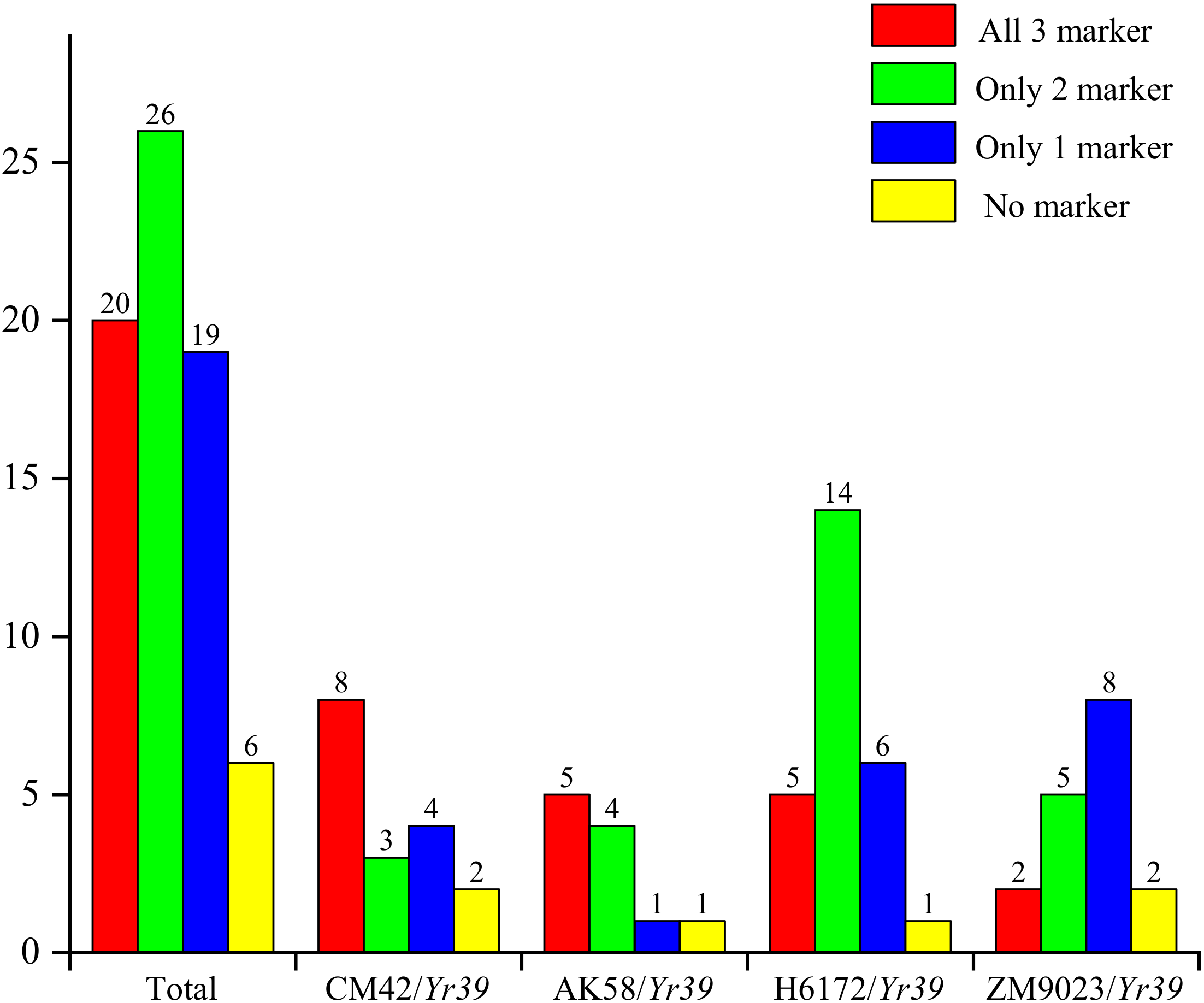
3.4. Molecular Marker Tests
4. Discussion
4.1. Effectiveness of Yr39 HTAP Resistance in China
4.2. Evaluation of Molecular Markers Linked with Yr39
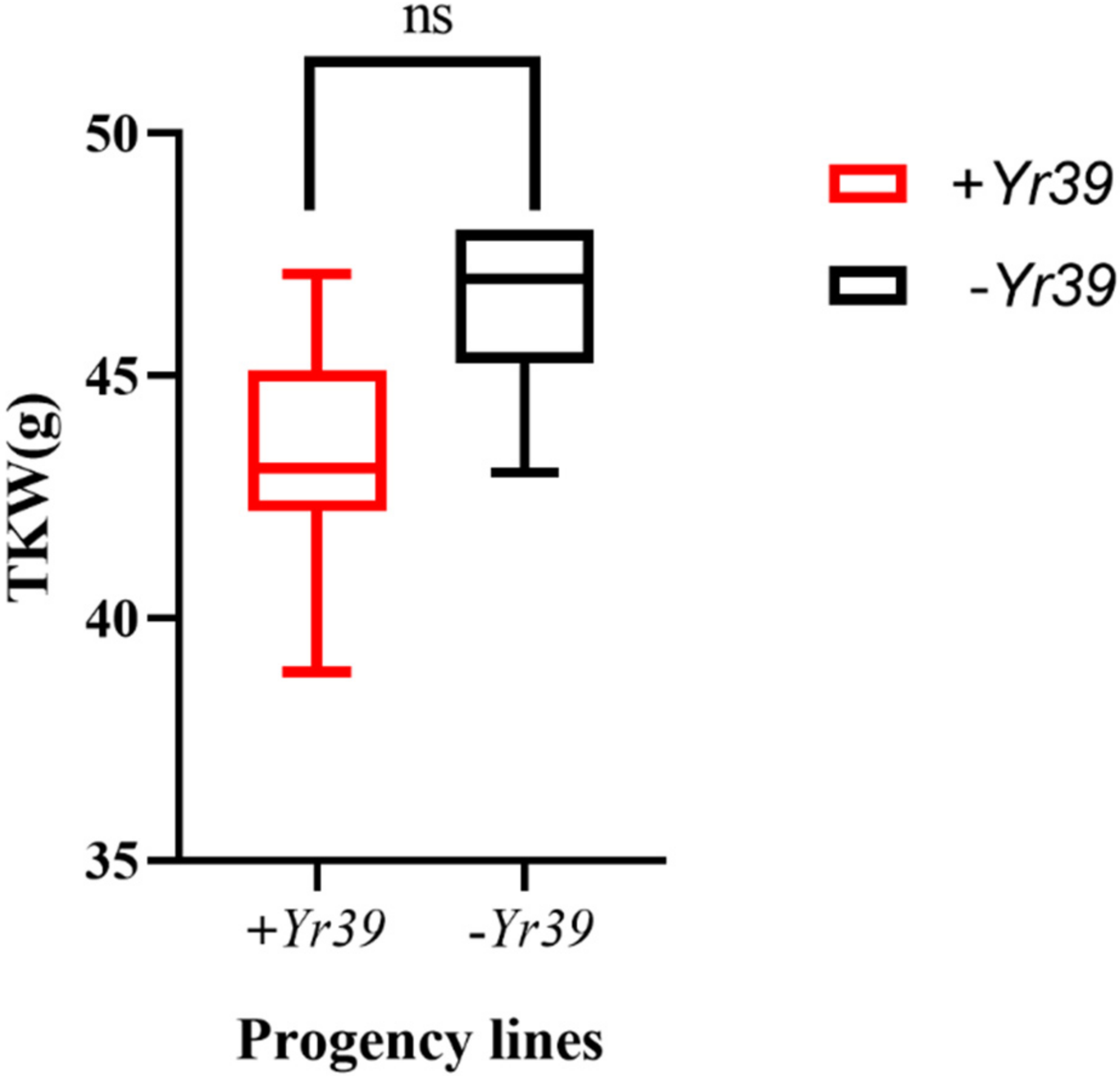
4.3. The Effect of Yr39 Transfer on Yield Components
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.M. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wu, L.R.; Liu, T.G.; Xu, S.C.; Wang, B.T. Race Dynamics, Diversity, and Virulence Evolution in Puccinia striiformis f. sp. tritici, the Causal Agent of Wheat Stripe Rust in China from 2003 to 2007. Plant Dis. 2009, 11, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, W.Y.; Peng, Y.L.; Li, J.; Zheng, Y.L. Inheritance of resistance for Chinese wheat stripe rust races in a new common wheat cultivar Chuanmai 42 derived from synthetics between Triticum durum * Aegilops tauschii. J. Triticeae Crops 2006, 33, 287–290. [Google Scholar]
- Hu, T.Z.; Li, X.J.; Dong, N.; Feng, S.W.; Li, G.; Zhang, L.L.; Ru, Z.G. Detection of Dwarfing Genes in Wheat cultivar AK58 and Its Parents. J. Triticeae Crops 2012, 41, 22–25. [Google Scholar]
- Liu, B.; Liu, T.G.; Zhang, Z.Y.; Jia, Q.Z.; Wang, B.T.; Gao, L.; Peng, Y.L.; Jin, S.L.; Chen, W.Q. Discovery and pathogenicity of CYR34, a new race of Puccinia striformis f. sp. tritici in China. J. Triticeae Crops 2017, 47, 681–687. [Google Scholar]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Görlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. J. Plant Cell 1996, 8, 629–643. [Google Scholar]
- Yang, Q.; Fang, T.H.; Li, X.; Ma, C.H.; Yang, S.Z.; Kang, Z.H.; Zhou, X.L. Improving stripe rust resistance and agronomic performance in three elite wheat cultivars using a combination of phenotypic selection and marker detection of Yr48. Crop Prot. 2021, 148, 105752. [Google Scholar] [CrossRef]
- Chen, W.Q.; Kang, Z.S.; Ma, Z.H.; Xu, S.C.; Jin, S.L.; Jiang, Y.Y. Integrated Management of Wheat Stripe Rust Caused by Puccinia striformis f. sp. tritici in China. Sci. Agric. Sin. 2013, 46, 4254–4262. [Google Scholar]
- Buerstmayr, H.; Lemmens, M.; Hartl, L.; Doldi, L.; Steiner, B.; Stierschneider, M.; Ruckenbauer, P. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theor. Appl. Genet. 2002, 104, 84–91. [Google Scholar] [CrossRef]
- Gupta, P.K.; Varshney, R.K.; Sharma, P.C.; Ramesh, B. Molecular markers and their applications in wheat breeding. Plant Breed. 1999, 118, 369–390. [Google Scholar] [CrossRef]
- Röder, M.S.; Korzun, V.; Wendehake, K.; Plaschke, J.; Tixier, M.H.; Leroy, P.; Ganal, W. A microsatellite map of wheat. Genetics 1998, 149, 2007–2023. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.C. From markers to genome-based breeding in wheat. Theor. Appl. Genet. 2019, 132, 767–784. [Google Scholar] [CrossRef]
- Akbari, M.; Wenzl, P.; Caig, V.; Carling, J.; Xia, L.; Yang, S.; Uszynski, G.; Mohler, V.; Lehmensiek, A.; Kuchel, H. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor. Appl. Genet. 2006, 113, 1409–1420. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, O.; Wang, Z.Y. Research Advance on Disease-resistant Gene of Plant by RGA Labeling. Agric. Sci. 2009, 15, 10–12. [Google Scholar]
- Yin, H.G.; Wang, J.W.; Wen, W.Y.; He, Z.H.; Li, Z.F.; Wang, H.; Xia, X.C. Mapping of Wheat Stripe Rust Resistance Gene YrZH84 with RGAP Markers and Its Application. Acta Agron. Sin. 2009, 35, 1274–1281. [Google Scholar] [CrossRef]
- Hu, T.; Zhong, X.; Yang, Q.; Zhou, X.L.; Li, X.; Yang, S.Z.; Lu, H.; Yao, Q.; Guo, Q.Y.; Kang, Z.S. Introgress of two QTL for stripe rust resistance into three Chinese wheat cultivars. Agronomy 2020, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.J.; Yang, W.J.; Chang, Z.J.; Chang, L.F.; Yan, G.Y.; Yang, S.W.; Li, X.; Qiao, L.Y.; Guo, H.J.; Lei, M.L.; et al. Evaluation on the Application of Molecular Markers Linked with the Wheat Stripe Rust Resistance Gene Yr69 in Wheat Breeding. J. Triticeae Crops 2021, 41, 417–423. [Google Scholar]
- Lin, F.; Chen, X.M. Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor. Appl. Genet. 2007, 114, 1277–1287. [Google Scholar] [CrossRef]
- Coram, T.E.; Settles, M.L.; Chen, X.M. Transcriptome analysis of high-temperature adult-plant resistance conditioned by Yr39 during the wheat Puccinia striiformis f. sp. tritici interaction. Mol. Plant Pathol. 2010, 9, 157–169. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhan, G.M.; Huang, L.L.; Han, D.J.; Kang, Z.S. Evaluation of Resistance to stripe rust in eighty abroad spring wheat germplasms. Sci. Agric. Sin. 2015, 48, 1518–1526. [Google Scholar]
- Wang, C.; Zhang, Y.; Han, D.; Kang, Z.; Li, G.; Cao, A.; Chen, P. SSR and STS markers for wheat stripe rust resistance gene Yr26. Euphytica 2008, 159, 359–366. [Google Scholar] [CrossRef]
- Li, W.; Song, G.Q.; Li, J.H.; Li, Y.L.; Zhang, S.J.; Zhang, R.Z.; Gao, J.; Gu, T.T.; Li, G.Y. Molecular Detection of Four Pleiotropic Disease Resistance Genes in Wheat. Mailei Acta Agron. Sin. 2020, 40, 638–641. [Google Scholar]
- Xue, W.B.; Xu, X.; Mu, J.M.; Wang, Q.L.; Wu, J.H.; Huang, L.L.; Kang, Z.S.; Han, D.J. Evaluation of stripe rust resistance and genes in Chinese elite wheat varieties. J. Triticeae Crops 2014, 34, 1054–1060. [Google Scholar]
- Zhao, Z.H.; Wu, L.R. Reviews of occurrence of wheat stripe rust disease in 2002 in China. Plant Port. 2003, 2, 5–8. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Line, R.F.; Qayoum, A. Virulence, aggressiveness, evolution, and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America 1968–1987. U. S. Dep. Agric. Tech. Bull. 1992, 44, 1788. [Google Scholar]
- Anderson, J.A.; Churchill, G.A.; Autrique, J.E.; Tanksley, S.D.; Sorrells, M.E. Optimizing parental selection for genetic linkage maps. Genome 1993, 36, 181–186. [Google Scholar] [CrossRef]
- Peng, J.H.; Fahima, T.; Röder, M.S.; Li, Y.C.; Grama, A.; Nevo, E. Microsatellite high-density mapping of the stripe rust resistance gene YrH52 region on chromosome 1B and evaluation of its marker-assisted selection in the F2 generation in wild emmer wheat. New Phytol. 2000, 146, 141–154. [Google Scholar] [CrossRef]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Traw, M.B.; Chen, J.Q.; Kreitman, M.; Bergelson, J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 2003, 423, 74–77. [Google Scholar] [CrossRef] [PubMed]
| Marker | Primer | Sequence (5′–3′) | Product Size (bp) | Reference |
|---|---|---|---|---|
| Xwgp36 | Pto kin1 | GCATTGGAACAAGGTGAA | 830 | Lin and Chen [19] |
| RLK For | GAYGTNAARCCIGARAA | |||
| Xwgp43 | Pto kin1 | GCATTGGAACAAGGTGAA | 820 | Lin and Chen [19] |
| PtoFen-S | ATGGGAAGCAAGTATTCAAGGC | |||
| Xwgp44 | S2-INV | CAICAIAAIGGITGIGGIGG | 875 | Lin and Chen [19] |
| NLRR-INV2 | TCAGGCCGTGAAAAATAT |
| CM42/Yr39 | AK58/Yr39 | H6172/Yr39 | ZM9023/Yr39 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Generations | Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive |
| F1 | 12 | 5 | 10 | 4 | 19 | 7 | 10 | 6 | 51 | 22 |
| BC1F1 | 17 | 8 | 13 | 5 | 22 | 16 | 15 | 6 | 67 | 35 |
| BC1F5 | 17 | 8 | 11 | 6 | 26 | 14 | 17 | 4 | 71 | 32 |
| BC1F6 | 17 | 8 | 11 | 5 | 26 | 5 | 17 | 2 | 71 | 20 |
| Cross | Resistant | Intermediate | Susceptible |
|---|---|---|---|
| (IT 1–3) | (IT 4–6) | (IT 7–9) | |
| CM42//CM42/Yr39 | 17 | 0 | 0 |
| AK58//AK58/Yr39 | 11 | 0 | 0 |
| H6172//H6172/Yr39 | 17 | 9 | 0 |
| ZM9023//ZM9023/Yr39 | 15 | 2 | 0 |
| Parent/Line | Cross | Stripe Rust | Agronomic Trait | Marker | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IT | DS (%) | PH (cm) | NT | SL (cm) | SGN | TGW (g) | Xwgp36 | Xwgp43 | Xwgp44 | ||
| Yr39 | 2 | 10 | 99.5 | 6 | 8.3 | 36 | 37.7 | + | + | + | |
| CM42 | 7 | 60 | 86.7 | 5 | 9.8 | 32 | 42.8 | − | − | − | |
| AK58 | 8 | 70 | 67 | 4 | 8.9 | 35 | 45.5 | − | − | − | |
| H6172 | 6 | 70 | 88 | 4 | 9.5 | 34 | 46.2 | − | − | − | |
| ZM9023 | 7 | 60 | 89.3 | 4 | 9.3 | 34 | 42.4 | − | − | − | |
| SWUST-3 * | CM42/Yr39 | 2 | 5 | 88.4 | 5 | 9.8 | 41 | 42.8 | + | + | + |
| SWUST-4 * | CM42/Yr39 | 2 | 5 | 88.6 | 5 | 9.2 | 36 | 42.2 | + | + | + |
| SWUST-5 * | CM42/Yr39 | 2 | 5 | 89.6 | 7 | 8.5 | 51 | 43.1 | + | + | + |
| SWUST-12 | CM42/Yr39 | 2 | 5 | 78.8 | 5 | 7.9 | 42 | 41.5 | − | − | + |
| SWUST-14 * | CM42/Yr39 | 2 | 20 | 88.2 | 5 | 10.2 | 39 | 46.3 | + | + | + |
| SWUST-15 | CM42/Yr39 | 2 | 10 | 88.4 | 5 | 10 | 40 | 44.6 | − | + | − |
| SWUST-18 | AK58/Yr39 | 2 | 20 | 81.8 | 5 | 7.9 | 39 | 40.7 | − | + | + |
| SWUST-20 * | AK58/Yr39 | 1 | 5 | 54.2 | 5 | 8.2 | 35 | 42.5 | + | + | + |
| SWUST-22 * | AK58/Yr39 | 2 | 5 | 68.4 | 6 | 8.2 | 37 | 47.1 | + | + | + |
| SWUST-24 * | AK58/Yr39 | 1 | 5 | 68 | 7 | 8.8 | 37 | 43.5 | + | + | + |
| SWUST-25 * | AK58/Yr39 | 2 | 5 | 65 | 4 | 9.9 | 39 | 42.2 | + | + | + |
| SWUST-29 | H6172/Yr39 | 2 | 5 | 80.8 | 4 | 11.1 | 42 | 43.7 | + | − | + |
| SWUST-33 | H6172/Yr39 | 4 | 30 | 75.2 | 5 | 10.1 | 44 | 41.5 | + | + | − |
| SWUST-34 | H6172/Yr39 | 3 | 5 | 88.4 | 4 | 11.2 | 53 | 41.3 | − | − | + |
| SWUST-35 | H6172/Yr39 | 4 | 10 | 90 | 5 | 8.4 | 38 | 46.7 | + | + | − |
| SWUST-36 | H6172/Yr39 | 3 | 10 | 89.5 | 7 | 9.8 | 38 | 43.4 | − | − | + |
| SWUST-44 * | H6172/Yr39 | 2 | 5 | 87.8 | 5 | 9 | 35 | 45.4 | + | + | + |
| SWUST-47 * | H6172/Yr39 | 4 | 20 | 88.6 | 4 | 8.4 | 44 | 43.9 | + | + | + |
| SWUST-52 | H6172/Yr39 | 2 | 30 | 83.6 | 5 | 10.4 | 48 | 44.2 | + | − | + |
| SWUST-53 | H6172/Yr39 | 3 | 20 | 81.8 | 4 | 9.7 | 44 | 41.8 | + | − | + |
| SWUST-55 | ZM9023/Yr39 | 4 | 30 | 83.4 | 5 | 6.9 | 35 | 41.3 | + | − | − |
| SWUST-56 | ZM9023/Yr39 | 3 | 20 | 25.7 | 4 | 7.5 | 41 | 45.8 | + | − | − |
| SWUST-58 | ZM9023/Yr39 | 2 | 5 | 88.4 | 5 | 9.9 | 45 | 41.6 | − | + | − |
| SWUST-62 | ZM9023/Yr39 | 3 | 20 | 82.6 | 5 | 9.8 | 41 | 49.7 | − | + | + |
| SWUST-63 | ZM9023/Yr39 | 2 | 10 | 82.6 | 5 | 10.9 | 59 | 50.4 | + | + | − |
| SWUST-64 | ZM9023/Yr39 | 3 | 20 | 87 | 5 | 10.1 | 48 | 52.7 | − | + | − |
| SWUST-65 | ZM9023/Yr39 | 2 | 20 | 82 | 6 | 10.1 | 48 | 52.4 | − | + | − |
| SWUST-67 * | ZM9023/Yr39 | 3 | 10 | 76 | 5 | 10.3 | 47 | 42.2 | + | + | + |
| SWUST-68 | ZM9023/Yr39 | 3 | 20 | 59 | 6 | 9 | 41 | 40 | + | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhou, J.; Zhang, M.; Tan, W.; Ma, C.; Tian, R.; Yan, Q.; Li, X.; Xia, C.; Kang, Z.; et al. Transfer of Durable Stripe Rust Resistance Gene Yr39 into Four Chinese Elite Wheat Cultivars Using Marker-Assisted Selection. Agronomy 2022, 12, 1791. https://doi.org/10.3390/agronomy12081791
Zheng X, Zhou J, Zhang M, Tan W, Ma C, Tian R, Yan Q, Li X, Xia C, Kang Z, et al. Transfer of Durable Stripe Rust Resistance Gene Yr39 into Four Chinese Elite Wheat Cultivars Using Marker-Assisted Selection. Agronomy. 2022; 12(8):1791. https://doi.org/10.3390/agronomy12081791
Chicago/Turabian StyleZheng, Xiaochen, Jianian Zhou, Min Zhang, Wenjing Tan, Chunhua Ma, Ran Tian, Qiong Yan, Xin Li, Chongjing Xia, Zhensheng Kang, and et al. 2022. "Transfer of Durable Stripe Rust Resistance Gene Yr39 into Four Chinese Elite Wheat Cultivars Using Marker-Assisted Selection" Agronomy 12, no. 8: 1791. https://doi.org/10.3390/agronomy12081791






