Soil Biological Activity, Carbon and Nitrogen Dynamics in Modified Coffee Agroforestry Systems in Mexico
Abstract
:1. Introduction
2. Materials and Methods
- -
- Renovated coffee agroforestry system (RCS). This is managed with 4000 coffee plants ha−1, newly planted, with an average height and diameter over 15 cm of 0.65 and 0.013 m, respectively, and 60 shade trees ha−1 from species of Juglans L. spp., Inga (Scop.) Mill. spp. and Grevillea robusta A. Cunn. ex R. Br., with an average height and diameter of 2.27 m and 0.985 m, respectively. Fertilization is carried out with 5 kg of compost (dry weight mass) per coffee plant. The compost is previously enriched with coffee pulp residues at a rate of 100 kg of pulp per ton of compost. Additionally, before and after renovation, 8 tons lime ha−1 is applied every three years. The system is 21 years old without any agrochemicals being added, either for soil nutrition, nor for pest or disease control. Weed control is done manually (Figure 1a).
- -
- Coffee agroforestry system with intensively pruned (IPCS). This is made up of 2400 coffee plants ha−1, having an average height and diameter of 2.85 m and 0.0251 m, respectively, 40 shade trees ha−1 of Juglans spp. and Inga spp., with an average diameter and height of 0.253 m and 11.89 m, respectively. Fertilization is carried out with 5 kg (dry weight mass) vermicompost (enriched with coffee pulp 10:1) and 1 kg of lime, annually, per coffee plant. Shade density is controlled with severe pruning. Pests and diseases are not controlled, and weed control is semi-mechanized, using a brush chopper and a hoe. This management was established 21 years ago (Figure 1b).
- -
- Coffee agroforestry system with the introduction of avocado (CAS). This is composed of 1800 coffee plants ha−1 and 100 avocado plants ha−1, with 5 shade trees per ha−1. Avocado trees average 3.0 m in height and 0.117 m in diameter; coffee plants have an average of 1.6 m in height and 1.0 cm in diameter. Fertilization consists of applying vermicompost enriched with mycorrhizae (1 kg of mycorrhizae and 500 kg of vermicompost), applied annually; the equivalent of 15 kg (dry weight mass) vermicompost and 5 kg (dry weight mass) mycorrhizae, per coffee plant. Additionally, “efficient microorganisms” ((EM) this amendment contains 4.2% humic acids and 5.0% fulvic acids) are incorporated; the additional composition is 1.3% N; 1.2% P; 2.4% K; 2.2% Ca; 1.7% Mg; 0.02% Mn; and 0.02% B. The EM is applied at the foliar level, monthly, at a rate of one liter of EM in 200 L water (this volume supplies 50 avocado plants and 900 coffee trees). The control of pests and diseases is carried out with night light traps, yellow traps, and applications of fungicide composed of Cu and hydrated lime (bordeaux broth) by 1 kg each in 100 L of water (for 100 avocado plants), and the applications are performed once a month; in coffee trees, inputs are not applied. Weed control is mechanized with a brush chopper. The management system was 8 years old (Figure 1c).
- -
- Conventional avocado orchard (CAO). This is considered as a control, made up of 210 avocado plants ha−1; the plants have an average height and diameter of 3.0 m and 0.185 m, respectively. Fertilization is carried out with agrochemicals containing N:P:K (17:17:17) and urea, 1.4 kg of each, per plant, three times a year. Control of pests and diseases is done with Metalaxy M + Mancozel (RIDONIL) and Thiamethoxan + Lambdacialothrin (ENGEO), applying 250 mL pesticides L−1 water every three months. Weed control is with a brush chopper. The management system was 8 years old (Figure 1d).
3. Results
3.1. Biological Activity
3.2. Soil Respiration (SR)
3.3. Carbon and Nitrogen Dynamics
3.3.1. Soil Organic Carbon (SOC) and Soil Total Nitrogen (STN)
3.3.2. Carbon and Nitrogen in Vegetal Biomass
3.3.3. Soil Carbon and Nitrogen Ratio (C/N)
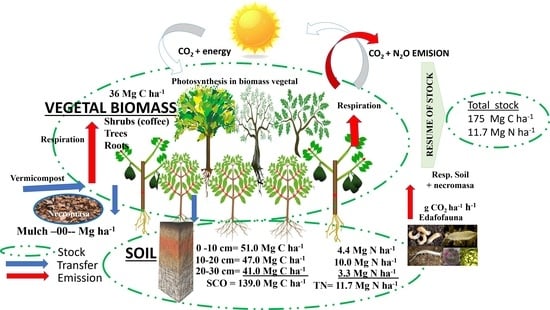
3.3.4. Carbon Stored in the AFS Studied
4. Discussion
4.1. Effect of Modified Agroforestry Systems on the Soil Biological Activity
4.1.1. Organic Residues (OR)
4.1.2. Soil Respiration (SR)
4.1.3. Soil Macrofauna Biomass (SMB)
4.1.4. Carbon and Nitrogen Dynamics
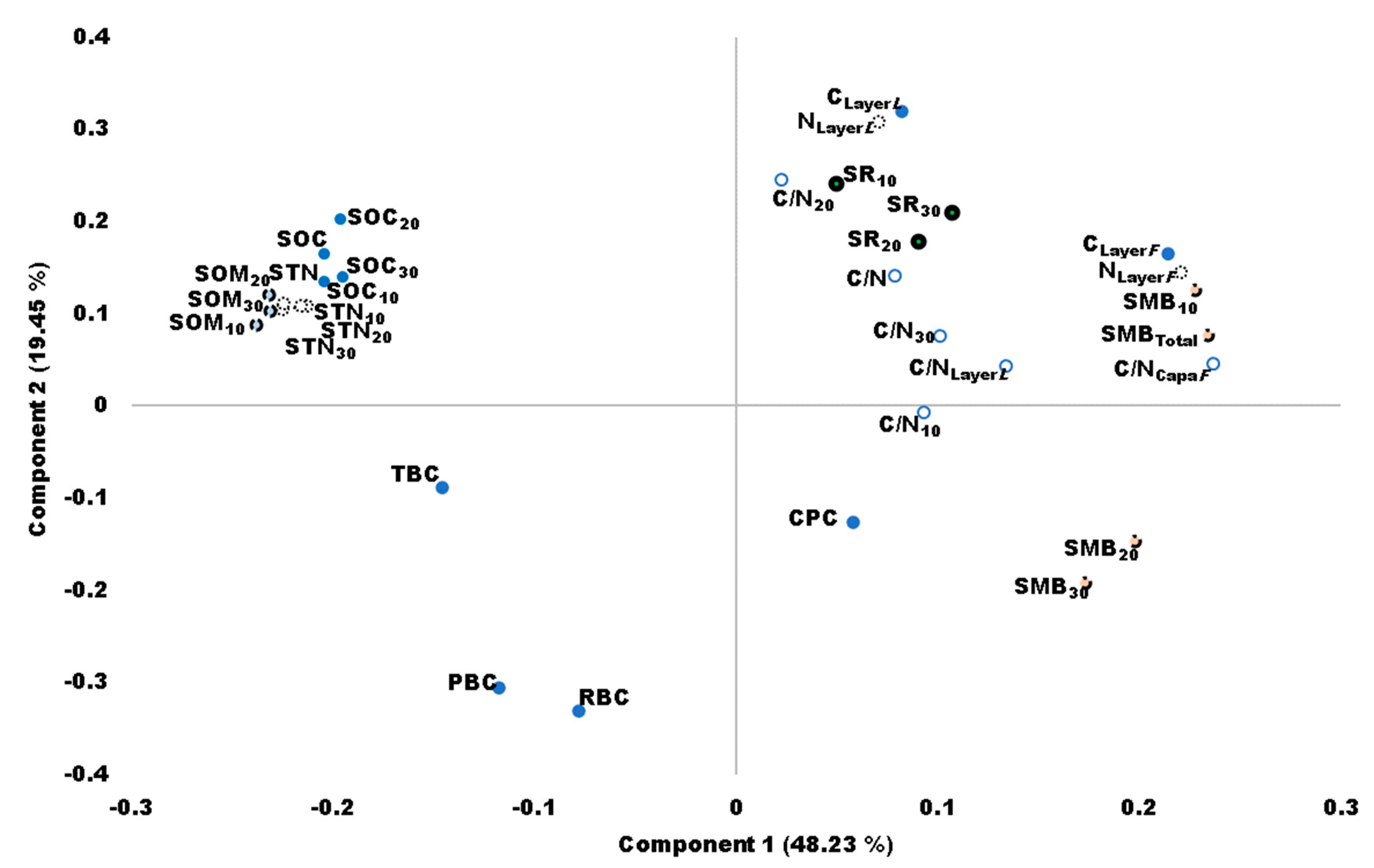
4.1.5. Principal Component Analysis (PCA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallejo-Quintero, V.E. Importance and utility of microbial elements in evaluating soil quality: Case studies in silvopastoral systems. Colomb. For. 2013, 16, 83–99, (In Spanish, with English abstract). [Google Scholar] [CrossRef]
- Jezierska-Tys, S.; Wesolowska, S.; Galazka, A.; Joniec, J.; Bednarz, J.; Cierpiaba, R. Biological activity and functional diversity in soil in different cultivation systems. Int. J. Environ. Sci. Technol. 2020, 17, 4189–4204. [Google Scholar] [CrossRef]
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Planeación Agrícola Nacional 2017–2030; SAGARPA: Mexico City, Mexico, 2017; 63p. [Google Scholar]
- Villers, L.; Arizpe, N.; Orellana, R.; Conde, C.; Hernández, J. Impacts of climatic change on coffee flowering and fruit development in Veracruz, Mexico. Interciencia 2009, 34, 322–329, (In Spanish, with English abstract). [Google Scholar]
- FIRA (Fideicomisos Instituidos en Relación con la Agricultura). Panorama Agroalimentario. Dirección de Investigación y Evaluación Económica y Sectorial. Café. Documento Técnico de Fideicomisos Instituidos en Relación con la Agricultura; Gobierno de México: Mexico City, Mexico, 2016; 37p. [Google Scholar]
- Franco Sánchez, M.A.; Leos Rodríguez, J.A.; Salas González, J.M.; Acosta Ramos, M.; García Munguía, A. Analysis of costs and competitiveness in avocado production in Michoacán, Mexico. Rev. Mex. Cienc. Agrícolas. 2018, 9, 391–403. [Google Scholar]
- Montiel, G.; Krishnamurthy, L.; Vásquez, A.; Uribe, M. Agroforestry Options for Avocado Producers. Terra Latinoam. 2008, 26, 85–90, (In Spanish, with English abstract). [Google Scholar]
- Ryan, M.; Law, B.E. Interpreting, measuring and modelling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Giardina, C.P.; Binkley, D.; Ryan, M.G.; Fownes, J.H.; Senock, R.S. Belowground carbon cycling in a humid tropical forest decreases with fertilization. Oecologia 2004, 139, 545–550. [Google Scholar] [CrossRef]
- Gallardo, J.F. La Materia Orgánica del Suelo; Residuos Orgánicos, Humus, Compostaje y Captura de Carbono; Universidad Autónoma Chapingo: Texcoco, Mexico, 2017; 424p. [Google Scholar]
- Thiele-Bruhn, S.; Bloem, J.; de Vries, F.T.; Kalbitz, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 2012, 4, 523–528. [Google Scholar] [CrossRef]
- Xu, X.; Shi, Z.; Chen, X.; Lin, Y.; Niu, S.; Jiang, L.; Luo, R.; Luo, Y. Unchanged carbon balance driven by equivalent responses of production and respiration to climate change in a mixed-grass prairie. Glob. Chang. Biol. 2016, 22, 1857–1866. [Google Scholar] [CrossRef]
- Sun, W.; Li, Z.; Lei, J.; Liu, X. Bacterial Communities of Forest Soils along Different Elevations: Diversity, Structure, and Functional Composition with Potential Impacts on CO2 Emission. Microorganisms 2022, 10, 766. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Effects of forest degradation on microbial communities and soil carbon cycling: A global meta-analysis. Glob. Ecol. Biogeogr. 2018, 27, 110–124. [Google Scholar] [CrossRef]
- Hedo, J.; Lucas-Borja, M.E.; Wic, C.; Andrés-Abellán, M.; de Las Heras, J. Soil microbiological properties and enzymatic activities of long-term post-fire recovery in dry and semiarid Aleppo pine (Pinus halepensis M.) forest stands. Solid Earth 2015, 6, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Escamilla, E.; Licona, A.L.; Díaz, S.; Sontoyo, H.V.; Sosa, R.; Rodríguez, L. Coffee production systems in central Veracruz, Mexico. A technological analysis. Rev. Hist. 1994, 30, 41–67. (In Spanish) [Google Scholar]
- Rosas, J.; Escamilla, E.; Ruiz, O. Relación de los nutrimientos del suelo con las características físicas y sensoriales del café orgánico. Terra Latinoam. 2008, 26, 375–384. [Google Scholar]
- Masuhara, A.; Valdés, E.; Pérez, J.; Gutiérrez, D.; Vázquez, J.C.; Salcedo, E.S.; Juárez, M.J.; Merino, A. Carbon stored in different coffee agroforestry systems in Huatusco, Veracruz, Mexico. Amaz. Cienc. Tecnol. 2015, 4, 66–93. [Google Scholar]
- Parkinson, K.J. An improved method for measuring soil respiration in the field. J. Appl. Ecol. 1981, 18, 221–228. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods, 2nd ed.; CAB International: Wallingford, UK, 1994; 221p. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 1. Physical Properties, 2nd ed.; American Soc. of Agronomy: Madison, WI, USA, 1982; 1159p. [Google Scholar] [CrossRef]
- Schulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight loss-on-ignition. Soil Org. Matter Anal. Interpret. 1996, 46, 21–31. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Segura, M.; Kanninen, M.; Suárez, D. Allometric models for estimating aboveground biomass of shade trees and coffee bushes grown together. Agrofor. Syst. 2006, 68, 143–150. [Google Scholar] [CrossRef]
- Begazo Curie, K.; Jave Nakayo, J. Estimation of the total aboveground biomass and by structural components in tree species from Lima, Peru. Rev. For. Perú 2018, 33, 76–90, (In Spanish, with English abstract). [Google Scholar] [CrossRef]
- Marín, Q.; Andrade, H.J.; Sandoval, A.P. Atmospheric carbon fixation in the total biomass within cocoa production systems in the department of TolimaColombia. Rev. UDCS Actual. Divulg. Científica 2016, 19, 351–360, (In Spanish, with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Strickland, M.S.; Thomason, W.E.; Avera, B.; Franklin, J.; Minick, K.; Yamada, S.; Badgley, D. Short–term effects of cover crops on soil microbial characteristics and biogeochemical processes across actively managed farms. Agrosyst. Geosci. Environ. 2019, 2, 180064. [Google Scholar] [CrossRef] [Green Version]
- Lozano-García, B.; Parras-Alcántara, L.; Brevik, E.C. Impact of topographic aspect and vegetation (native and reforested areas) on soil organic carbon and nitrogen budgets in Mediterranean natural areas. Sci. Total Environ. 2016, 544, 963–970. [Google Scholar] [CrossRef]
- Aceñolaza, P.; Gallardo, J.F. Forest age influence on the evolution of Alnus acuminata litter dry wight lost in alder forest of Tucuman province (Argentina). For. Syst. 1995, 4, 177–188, (In Spanish, with English abstract). [Google Scholar]
- Gómez, L.; Cardoso, I.M.; Mendonça, E.; Fernandes, R.B.; Lopes, V.; Oliveira, T. Trees modify the dynamics of soil CO2 efflux in coffee agroforestry systems. Agric. For. Meteorol. 2016, 224, 30–39. [Google Scholar] [CrossRef]
- Yu, H.; Sui, Y.; Chen, Y.; Bao, T.; Jiao, X. Soil Organic Carbon Mineralization and Its Temperature Sensitivity under Different Substrate Levels in the Mollisols of Northeast China. Life 2022, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Scholze, M.; Knorr, W.; Heimann, M.M. Modelling terrestrial vegetation dynamics and carbon cycling for an abrupt climatic change event. Holocene 2003, 13, 327–333. [Google Scholar] [CrossRef]
- Acosta, Y.; Paolini, J. Microbial Biomass Dynamics (C and N) in Soils on the Paraguana Peninsula Treated with Organic Residues. Multiciencias 2006, 6, 180–187, (In Spanish, with English abstract). [Google Scholar]
- Souza, H.N.; de Goede, R.G.M.; Brussaard, L.; Cardoso, I.M.; Duarte, E.M.G.; Fernandes, R.B.A.; Gomes, L.C.; Pulleman, M.M. Protective shade, tree diversity and soil properties in coffee agroforestry systems in the Atlantic Rainforest biome. Agric. Ecosyst. Environ. 2012, 146, 179–196. [Google Scholar] [CrossRef]
- Munguía, R.; Beer, J.; Harmand, J.M.; Haggar, J. Decomposition and nutrient release rates of Eucalyptus deglupta and Coffea arabica litter and Erythrina poeppigiana green leaves, alone or mixed. Agroforestería Am. 2004, 41, 62–68, (In Spanish, with English abstract). [Google Scholar]
- Medina, R.; Salazar, S.; Bonilla, J.; Herrera, J.; Ibarra, M.; Álvarez, A. Secondary Metabolites and Lignin in ‘Hass’ Avocado Fruit Skin during Fruit Development in Three Producing Regions. HortScience 2017, 52, 852–858. [Google Scholar] [CrossRef]
- Xi, X.; Wang, L.; Tang, Y.; Fu, X.; Le, Y. Response of soil microbial respiration of tidal wetlands in the Yangtze River Estuary to increasing temperature and sea level: A simulative study. Ecol. Eng. 2012, 49, 104–111. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, J.; Chen, W.; Tian, D.; Niu, S. Different responses of soil respiration and its components to nitrogen and phosphorus addition in a subtropical secondary forest. For. Ecosyst. 2021, 8, 37. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Skiba, U.; Harmand, J.M.; Hénault, C. Fluxes of greenhouse gases from Andosols under coffee in monoculture or shaded by Inga densiflora in Costa Rica. Biogeochemistry 2008, 89, 329–345. [Google Scholar] [CrossRef]
- Paolini Gómez, J.E. Microbial activity and microbial biomass in coffee soils of the Venezuelan Andes. Terra Latinoam. 2018, 36, 13–22, (In Spanish, with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Pardo, L.C.; Vélez, C.P.; Sevilla, F.; Madrid, O. Abundance and biomass of soil macroinvertabrates during the rainy season in three land use systems in the Colombian Andes. Acta Agronómica 2006, 55, 43–54, (In Spanish, with English abstract). [Google Scholar]
- Brown, G.G.; Fragoso, C.; Barois, I.; Rojas, P.; Patrón, J.C.; Bueno, J.; Moreno, G.; Lavelle, P.; Ordaz, V.; Rodríguez, C. Diversity and functional role of edaphic macrofauna in mexican tropical ecosystems. Acta Zool. Mex. 2001, 79–110, (In Spanish, with English abstract). [Google Scholar] [CrossRef]
- Cristóbal, D.; Tinoco, J.A.; Prado, J.V.; Hernández, E. Soil carbon and nitrogen in tropical montane cloud forest, agroforestry, and coffee monoculture systems. Rev. Chapingo Ser. Cienc. For. Ambiente 2019, 25, 169–184. [Google Scholar] [CrossRef]
- Vega Orozco, G.; Ordoñez Espinosa, C.M.; Suarez Salazar, J.C.; López Pantoja, C.F. Carbon storage in agroforestry arrangements associated with coffee (Coffea arabica) in the south of Colombia. Rev. Investig. Agrar. Ambient. 2014, 5, 213, (In Spanish, with English abstract). [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Byakagaba, P. Soil organic carbon stocks under coffee agroforestry systems and coffee monoculture in Uganda. Agric. Ecosyst. Environ. 2016, 216, 188–193. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 7528, 505–511. [Google Scholar] [CrossRef]
- Payán, F.; Jones, D.; Harmand, M.; Muschler, R. Soil carbon and nitrogen concentrations below Erythrina poeppigiana in organic and conventional coffee plantations. Agroforestería Am. 2002, 9, 10–15, (In Spanish, with English abstract). [Google Scholar]
- Gamarra, C.C.; Díaz, M.I.; Vera, M.P.M.; Nicolás, A.J. Carbon/nitrogen ratio in soils of silvopastoral systems in the Paraguayan Chaco. Rev. Mex. Cienc. Forestales. 2018, 9, 4–26. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Barthés, B.G.; Saby, N.P.A.; Parent, T.; Dupraz, C.; Bernoux, M.; Chenu, C. Impact of alley cropping agroforestry on stocks, forms, and spatial distribution of soil organic carbon: A case study in a Mediterranean context. Geoderma 2015, 259–260, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Cheng, R.; Shi, Z.; Wang, W. Decomposition of Leaves and Fine Roots in Three Subtropical Plantations in China Affected by Litter Substrate Quality and Soil Microbial Community. Forests 2017, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Okur, N.; Kayikcioglu, H.H.; Ates, F.; Yagmur, B. A comparison of soil quality and yield parameters under organic and conventional vineyard systems in Mediterranean conditions (West Turkey). Biol. Agric. Hortic. 2015, 32, 73–84. [Google Scholar] [CrossRef]
- Iqbal, J.; Hu, R.; Feng, M.; Lin, S.; Malghani, S.; Ali, I.M. Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: A case study at Three Gorges Reservoir Area, South China. Agric. Ecosyst. Environ. 2010, 137, 294–307. [Google Scholar] [CrossRef]
- Holatko, J.; Bielska, L.; Hammerschmiedt, T.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Baltazar, T.; Latal, O.; Brtnicky, M. Cattle manure fermented with biochar and humic substances improve the crop biomass, microbiological properties and nutrient status of soil. Agronomy 2022, 12, 368. [Google Scholar] [CrossRef]

| Forest Type | Allometric Equation | |
|---|---|---|
| AB of Juglans spp. | Log10Y = −0.834 + 2.223 × Log10 (dbh) | [24] |
| AB of Inga spp. | Log10Y = −0.889 + 2.317 × Log10 (d15) | [24] |
| AB of Grevillea robusta | LnY = −2.0082 + 2.3293 × Ln(dbh) | [25] |
| AB of coffee plants | Log10Y = −1.113 + 1.578 × Log10(d15) + 0.581 × Log10(h) | [24] |
| AB of avocado plants | [26] |
| System | Layer L | Layer F | Soil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 20–30 cm | ||||||||
| RCS | 383 ± 22 | A | 653 ± 43 | A | 179 ± 7.7 | B | 180 ± 9.3 | A | 171 ± 3.9 | B |
| IPCS | 382 ± 22 | A | 589 ± 43 | A | 230 ± 7.7 | A | 205 ± 9.3 | A | 202 ± 3.9 | A |
| CAS | 219 ± 22 | B | Inexistent | C | 193 ± 7.7 | B | 183 ± 9.3 | A | 172 ± 3.9 | B |
| CAO | 253 ± 38 | AB | Inexistent | C | 212 ± 7.7 | AB | 183 ± 9.3 | A | 186 ± 3.9 | AB |
| p-value | 0.0045 | 0.0001 | 0.0081 | 0.2800 | 0.0016 | |||||
| LSD | 131.48 | 257.55 | 34.77 | 42.26 | 0.1769 | |||||
| System | SMB 0–10 cm Mg DM ha−1% | SMB 10–20 cm Mg DM ha−1% | SMB 20–30 cm Mg DM ha−1% | Total SMB Mg DM ha−1 | ||||
|---|---|---|---|---|---|---|---|---|
| RCS | 0.88 ± 0.07 | B 71.5 | 0.30 ± 0.01 | A 24.4 | 0.044 ± 0.004 | A 3.2 | 1.23 ± 0.07 | B |
| IPCS | 1.46 ± 0.07 | A 90.1 | 0.14 ± 0.01 | A 8.6 | 0.017 ± 0.004 | B 1.03 | 1.62 ± 0.07 | A |
| CAS | 0.02 ± 0.11 | C 100.0 | Non- existent | Non- existent | 0.02 ± 0.12 | C | ||
| CAO | 0.01 ± 0.11 | C 100.0 | Non- existent | Non- existent | 0.01 ± 0.12 | C | ||
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||||
| LSD | 0.364 | 0.0714 | 0.0222 | 0.3919 | ||||
| System | SOC | Soil | STN | Soil | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 0–20 cm | 0–30 cm | 0–10 cm | 0–20 cm | 0–30 cm | |||||||||||
| RCS | 60 ± 3.9 | B | 28 ± 2.0 | B | 17 ± 1.5 | B | 15 ± 1.6 | B | 4.9 ±0.37 | C | 2.0 ± 0.15 | B | 1.8 ± 0.18 | B | 1.1 ± 0.13 | C |
| IPCS | 71 ± 3.9 | B | 29 ± 2.0 | B | 25 ± 1.5 | B | 17 ± 1.6 | B | 5.6 ±0.37 | BC | 2.3 ± 0.15 | B | 2.1 ± 0.18 | B | 1.3 ± 0.13 | BC |
| CAS | 139 ± 6.1 | A | 51 ± 3.4 | A | 47 ± 2.6 | A | 41 ± 2.8 | A | 11.7 ± 0.65 | A | 4.4 ± 0.26 | A | 4.0 ± 0.32 | A | 3.3 ± 0.22 | A |
| CAO | 74 ± 6.8 | B | 32 ± 3.4 | B | 24 ± 2.6 | B | 18 ± 2.8 | B | 7.2 ±0.65 | B | 2.7 ± 0.26 | B | 2.5 ± 0.32 | B | 2.0 ± 0.22 | B |
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||||||||
| LSD | 22.020 | 10.9649 | 8.5201 | 9.0768 | 2.0950 | 0.8249 | 1.0208 | 0.7187 | ||||||||
| System | Carbon | Nitrogen | ||||||
|---|---|---|---|---|---|---|---|---|
| Layer L | Layer F | Layer L | Layer F | |||||
| RCS | 0.19 ± 0.06 | A | 0.57 ± 0.09 | B | 0.007 ± 0.003 | A | 0.026 ± 0.004 | B |
| IPCS | 0.37 ± 0.06 | A | 1.12 ± 0.09 | A | 0.014 ± 0.01 | A | 0.047 ± 0.004 | A |
| CAS | 0.26 ± 0.11 | A | Inexistent | C | 0.010 ± 0.01 | A | Inexistent | C |
| CAO | 0.18 ± 0.11 | A | Inexistent | C | 0.007 ± 0.003 | A | Inexistent | C |
| p-value | 0.2466 | 0.0001 | 0.3762 | 0.0016 | ||||
| LSD | 0.36378 | 0.46832 | 0.0117 | 0.8249 | ||||
| System | C/N Layer L | C/N Layer F | C/N 0–10 cm | C/N 10–20 cm | C/N 20–30 cm | C/N (SOC/STN) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCS | 27 ± 1.5 | A | 21.0 ± 0.84 | B | 14 ± 1.2 | A | 9 ± 1.2 | A | 15 ± 2.0 | A | 12 ± 1.1 | A |
| IPCS | 27 ± 1.5 | A | 23.8 ±0.84 | A | 14 ± 1.2 | A | 13 ± 1.2 | A | 15 ± 2.0 | A | 14 ± 1.1 | A |
| CAS | 25 ± 2.6 | A | Inexistent | C | 12 ± 2.1 | A | 12 ± 2.1 | A | 13 ± 3.4 | A | 12 ± 2.0 | A |
| CAO | 22 ± 2.6 | A | Inexistent | C | 13 ± 2.1 | A | 10 ± 2.1 | A | 10 ± 3.4 | A | 11 ± 2.0 | A |
| p-value | 0.5192 | 0.0287 | 0.7511 | 0.1524 | 0.5639 | 0.6268 | ||||||
| LSD | 10.548 | 2.518 | 6.819 | 6.773 | 11.113 | 6.346 | ||||||
| System | Trees | Shrubs | Roots | PBC | ||||
|---|---|---|---|---|---|---|---|---|
| RCS | 0.8 ± 1.6 | C | 32 ± 1.7 | A | 6.7 ± 0.4 | A | 40 ± 2.6 | A |
| IPCS | 2.3 ± 1.6 | C | 11 ± 1.7 | C | 3.1 ± 0.4 | B | 16 ± 2.6 | B |
| CAS | 11 ± 1.6 | B | 19 ± 1.7 | B | 5.4 ± 0.4 | A | 36 ± 2.6 | A |
| CAO | 31 ± 1.6 | A | Inexistent | D | 6.1 ± 0.4 | A | 37 ± 2.6 | A |
| p-value | 0.0001 | 0.0001 | 0.0001 | 0.0007 | ||||
| LSD | 7.41 | 7.67 | 1.90 | 11.68 | ||||
| System | Aboveground (Trees + Shrubs) + Roots | Stand-Litter (Litter + Mulch) | SOC | Total C | ||||
|---|---|---|---|---|---|---|---|---|
| RCS | 40 ± 2.6 | A | 0.76 ± 0.14 | B | 60 ± 3.9 | B | 100.07 ± 6 | B |
| IPCS | 16 ± 2.6 | B | 1.51 ± 0.14 | A | 71 ± 3.9 | B | 88.64 ± 6 | B |
| CAS | 36 ± 2.6 | A | 0.26 ± 0.14 | B | 139 ± 6.1 | A | 175.06 ± 6 | A |
| CAO | 37 ± 2.6 | A | 0.17 ± 0.14 | B | 74 ± 6.8 | B | 111.38 ± 6 | B |
| p-value | 0.0007 | 0.0004 | 0.0001 | 0.0001 | ||||
| LSD | 11.68 | 0.615 | 22.020 | 27.172 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Montejo, D.; Valdés-Velarde, E.; Benedicto-Valdés, G.S.; Escamilla-Prado, E.; Sánchez-Hernández, R.; Gallardo, J.F.; Martínez-Zurimendi, P. Soil Biological Activity, Carbon and Nitrogen Dynamics in Modified Coffee Agroforestry Systems in Mexico. Agronomy 2022, 12, 1794. https://doi.org/10.3390/agronomy12081794
Ayala-Montejo D, Valdés-Velarde E, Benedicto-Valdés GS, Escamilla-Prado E, Sánchez-Hernández R, Gallardo JF, Martínez-Zurimendi P. Soil Biological Activity, Carbon and Nitrogen Dynamics in Modified Coffee Agroforestry Systems in Mexico. Agronomy. 2022; 12(8):1794. https://doi.org/10.3390/agronomy12081794
Chicago/Turabian StyleAyala-Montejo, Diana, Eduardo Valdés-Velarde, Gerardo Sergio Benedicto-Valdés, Esteban Escamilla-Prado, Rufo Sánchez-Hernández, Juan Fernando Gallardo, and Pablo Martínez-Zurimendi. 2022. "Soil Biological Activity, Carbon and Nitrogen Dynamics in Modified Coffee Agroforestry Systems in Mexico" Agronomy 12, no. 8: 1794. https://doi.org/10.3390/agronomy12081794