A CRISPR/Cas9-Based Study of CgloRPCYG, a Gene That Regulates Pathogenicity, Conidial Yield, and Germination in Colletotrichum gloeosporioides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain, Growth Conditions, and Plant Fruits
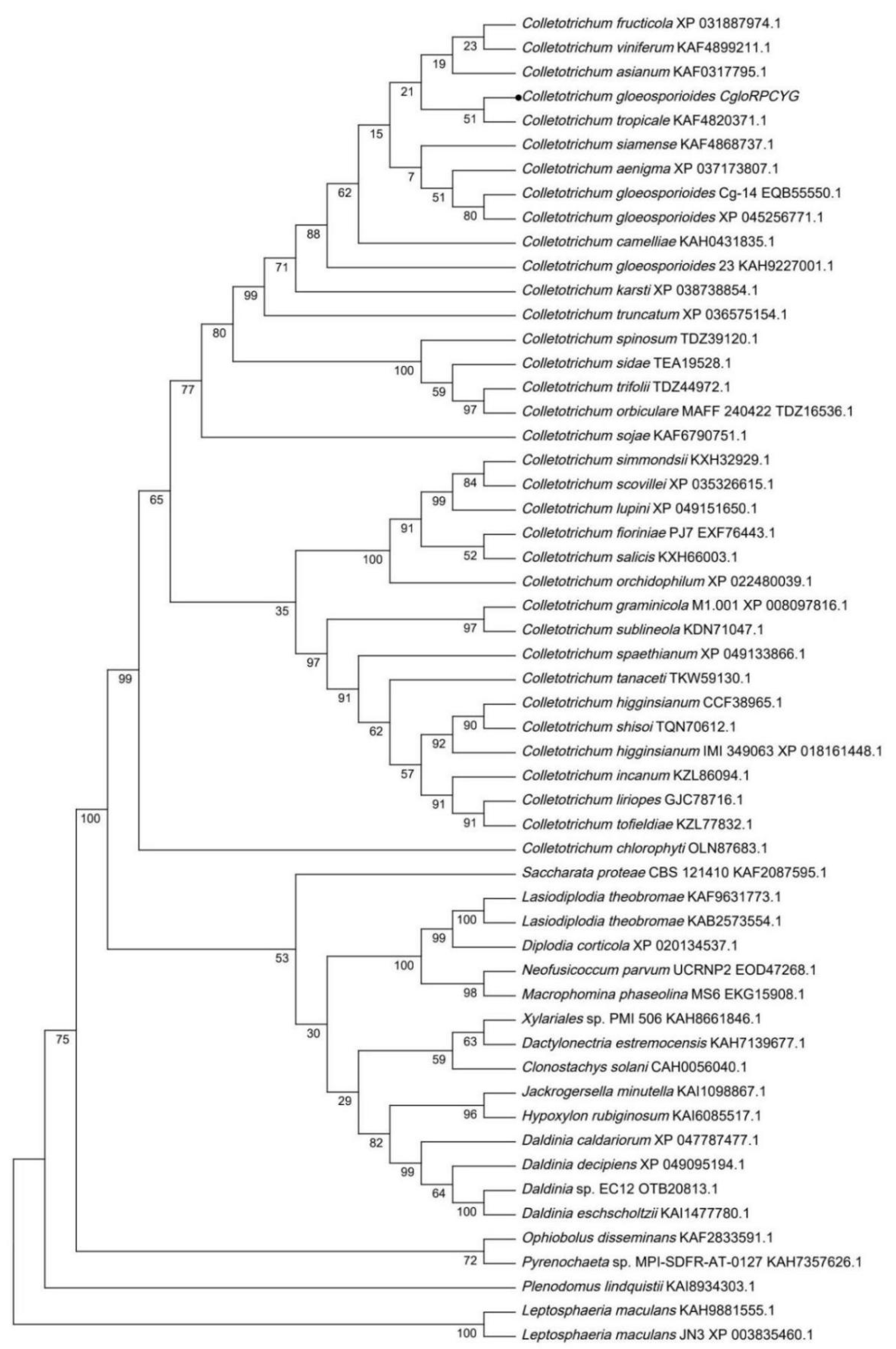
2.2. Sequence Alignment and Phylogenetic Analysis
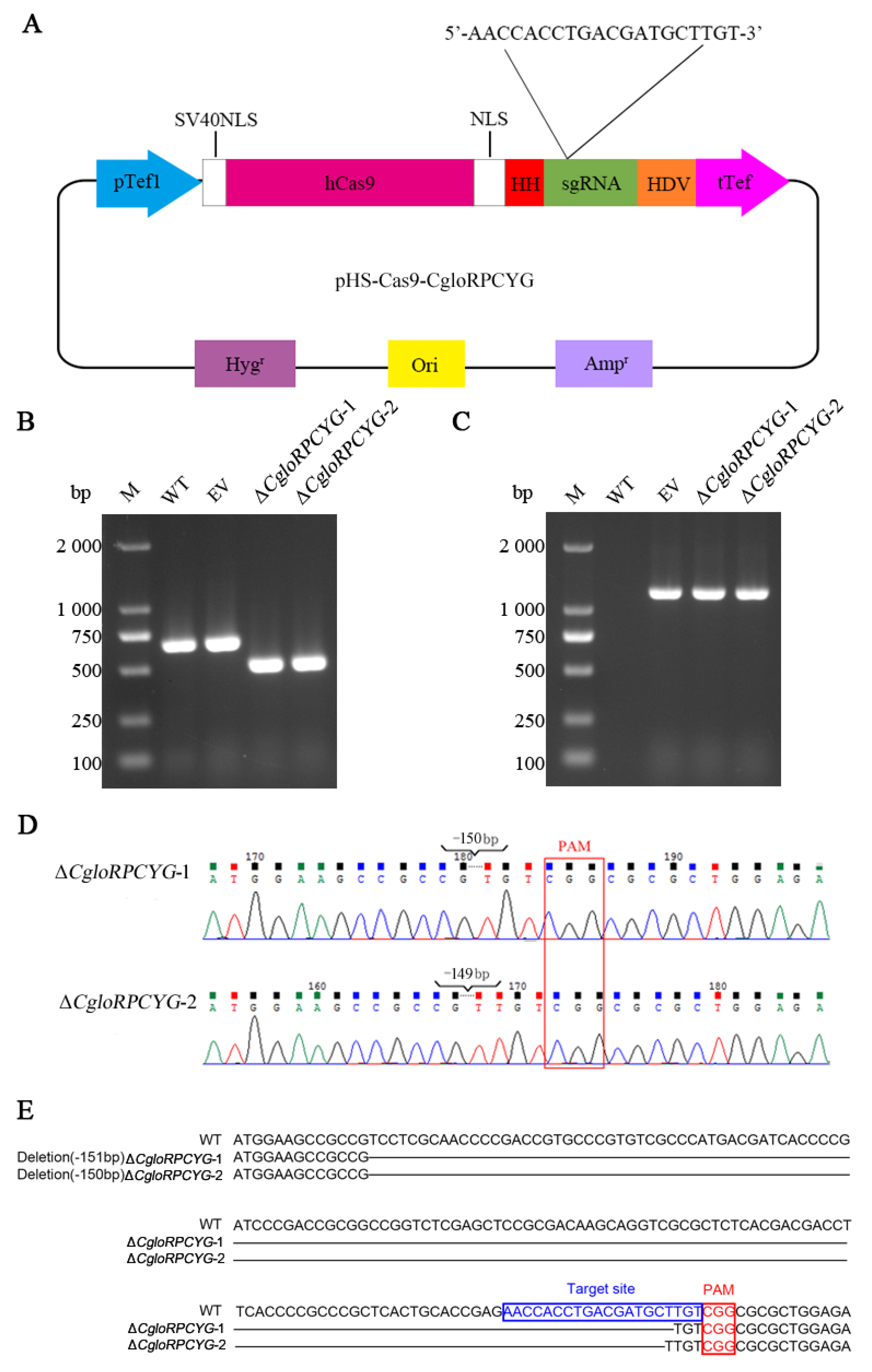
2.3. Plasmid Construction
2.4. Polyethylene Glycol-Mediated Protoplast Transformation
2.5. Extraction of DNA and Verification of Transformants
2.6. Characterization of C. gloeosporioides Mutants
2.7. Pathogenicity Assay
2.8. Statistical Analysis
3. Results
3.1. Bioinformatics and Phylogenetic Analyses of the CgloRPCYG Gene
3.2. Successful Creation of Fusion Plasmid, Knockout, and Complementary Mutants
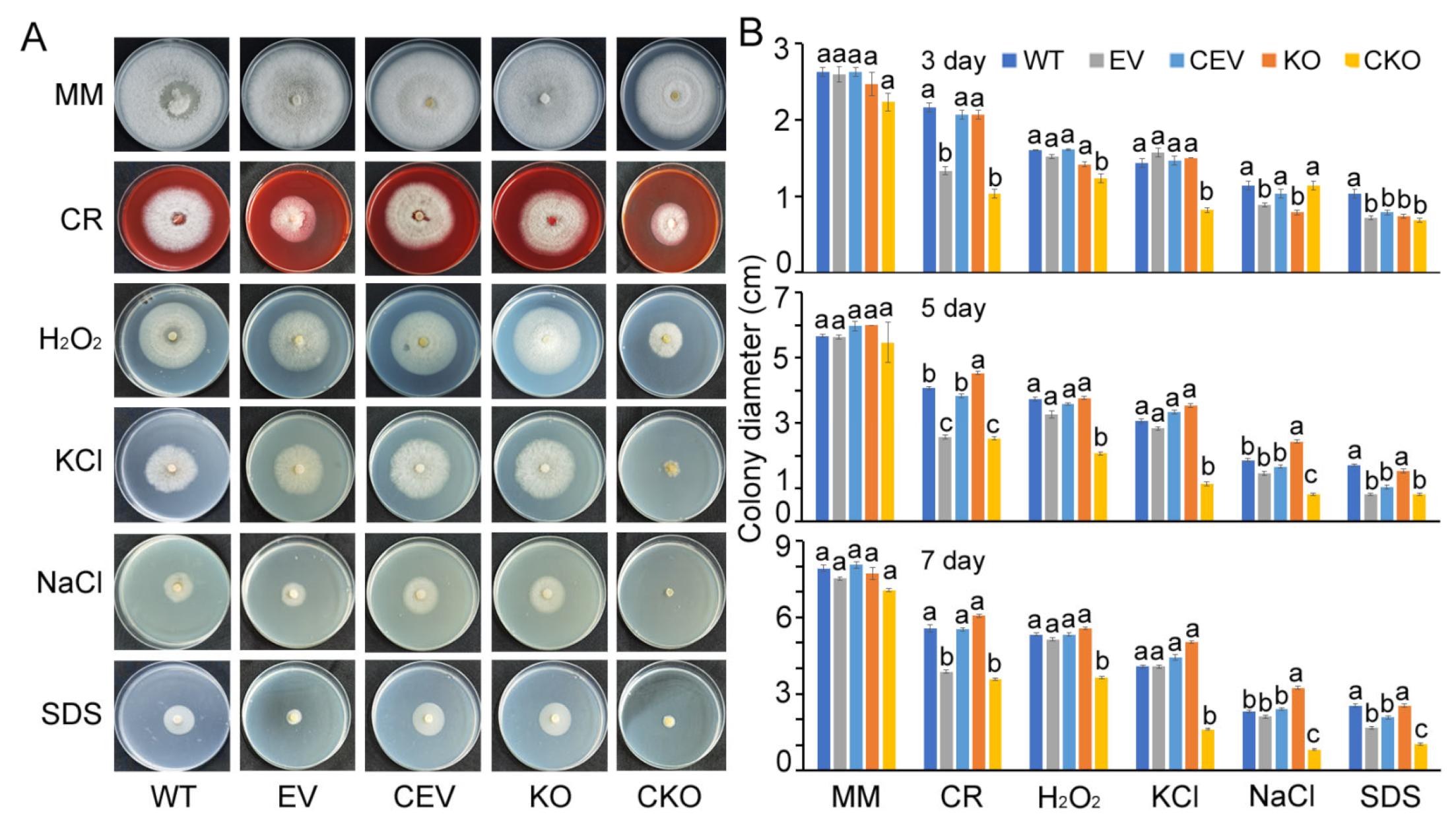
3.3. Role of CgloRPCYG in Mycelial Growth and Stress Tolerance
3.4. CgloRPCYG Plays a Crucial Role in Pathogenicity, Conidial Yield, and Germination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, B.; Zehr, E.I.; Dean, R.A.; Shabi, E. Characteristics of Colletotrichum from peach, apple, pecan, and other hosts. Plant Dis. 1995, 79, 478–482. [Google Scholar] [CrossRef]
- Yakoby, N.; Beno-Moualem, D.; Keen, N.T.; Dinoor, A.; Pines, O.; Prusky, D. Colletotrichum gloeosporioides pelB is an important virulence factor in avocado fruit-fungus interaction. Mol. Plant Microbe Interact. 2001, 14, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.L.; MacKenzie, S.J.; Legard, D.E. Genetic and pathogenic analyses of Colletotrichum gloeosporioides isolates from strawberry and noncultivated hosts. Phytopathology 2004, 94, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Kefialew, Y.; Ayalew, A. Postharvest biological control of anthracnose (Colletotrichum gloeosporioides) on mango (Mangifera indica). Postharvest Biol. Technol. 2008, 50, 8–11. [Google Scholar] [CrossRef]
- Lima, W.G.; Spósito, M.B.; Amorim, L.; Gonçalves, F.P.; de Filho, P. Colletotrichum gloeosporioides, a new causal agent of citrus post-bloom fruit drop. Eur. J. Plant Pathol. 2011, 131, 157–165. [Google Scholar] [CrossRef]
- González, E.; Sutton, T.B.; Correll, J.C. Clarification of the etiology of glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular, and pathogenicity tests. Phytopathology 2006, 96, 982–992. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, J.B.; Hartman, G.L.; Wang, T.C. Anthracnose development on pepper fruits inoculated with Colletotrichum gloeosporioides. Plant Dis. 1995, 79, 380–383. [Google Scholar] [CrossRef]
- Liu, F.; Tang, G.; Zheng, X.; Li, Y.; Sun, X.; Qi, X.; Zhou, Y.; Xu, J.; Chen, H.; Chang, X.; et al. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci. Rep. 2016, 6, 32761. [Google Scholar] [CrossRef] [Green Version]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2015, 205, 801–815. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Yin, J.; Yang, Q.; Sheng, O.; Deng, G.; Li, C.; Hu, C.; Dong, T.; Dou, T.; et al. CgGCS, encoding a glucosylceramide synthase, is required for growth conidiation and pathofenicity in Colletotrichum gloeosporioides. Front. Microbiol. 2019, 10, 1016. [Google Scholar] [CrossRef] [Green Version]
- Hubballi, M.; Nakkeeran, S.; Raguchander, T. First report of anthracnose on noni caused by Colletotrichum gloeosporioides in India. Arch. Phytopathol. Plant Prot. 2012, 45, 276–279. [Google Scholar] [CrossRef]
- Song, R.; Zhai, Q.; Sun, L.; Huang, E.; Zhang, Y.; Zhu, Y.; Guo, Q.; Tian, Y.; Zhao, B.; Lu, H. CRISPR/Cas9 genome editing technology in filamentous fungi: Progress and perspective. Appl. Microbiol. Biotechnol. 2019, 103, 6919–6932. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Lu, M.; Yang, D. CRISPR/Cas9-based tools for targeted genome editing and replication control of HBV. Virol. Sin. 2015, 30, 317–325. [Google Scholar] [CrossRef]
- Cobb, R.E.; Wang, Y.; Zhao, H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth. Biol. 2015, 4, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Zhang, Q.; Yang, H.; Zou, Q.; Tang, C.; Fan, N.; Lai, L. Generation of multi-gene knockout rabbits using the Cas9/gRNA system. Cell Regener. 2014, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Tong, Y.; Wen, Z.; Zhu, L.; Ge, M.; Chen, D.; Jiang, Y.; Yang, S. Multiplex gene editing of the Yarrowia lipolytica genome using the CRISPR-Cas9 system. J. Ind. Microbiol. Biotechnol. 2016, 43, 1085–1093. [Google Scholar] [CrossRef]
- Hruscha, A.; Krawitz, P.; Rechenberg, A.; Heinrich, V.; Hecht, J.; Haass, C.; Schmid, B. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 2013, 140, 4982–4987. [Google Scholar] [CrossRef] [Green Version]
- Platt, R.J.; Chen, S.; Zhou, Y.; Yim, M.J.; Swiech, L.; Kempton, H.R.; Dahlman, J.E.; Parnas, O.; Eisenhaure, T.M.; Jovanovic, M.; et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cao, C.; Huang, J.; Yao, J.; Hai, T.; Zheng, Q.; Wang, X.; Zhang, H.; Qin, G.; Cheng, J.; et al. One-step generation of triple gene-targeted pigs using CRISPR/Cas9 system. Sci. Rep. 2016, 6, 20620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Patron, N.J.; Nekrasov, V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015, 32, 76–84. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, Q.; Chen, W.; Zhang, X.; Mei, F.; Zhang, P.; Zhao, M.; Wang, X.; Shi, N.; Jackson, S.; et al. Multigene editing via CRISPR/Cas9 guided by a single-sgRNA seed in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Rosas-Diaz, T.; Caceres-Moreno, C.; Lozano-Duran, R.; Macho, A.P. The IMMUNE-ASSOCIATED NUCLEOTIDE-BINDING 9 protein is a regulator of basal immunity in Arabidopsis thaliana. Mol. Plant Microbe In. 2019, 32, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Geng, L.; Yuan, M.; Wei, J.; Jin, C.; Li, M.; Yu, K.; Zhang, Y.; Jin, H.; Wang, E.; et al. Deletion of a target gene in Indica rice via CRISPR/Cas9. Plant Cell Rep. 2017, 36, 1333–1343. [Google Scholar] [CrossRef]
- Gumtow, R.; Wu, D.; Uchida, J.; Tian, M. A Phytophthora palmivora extracellular cystatin-like protease inhibitor targets papain to contribute to virulence on papaya. Mol. Plant. Microbe In. 2018, 31, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Katayama, T.; Tanaka, Y.; Okabe, T.; Nakamura, H.; Fujii, W.; Kitamoto, K.; Maruyama, J. Development of a genome editing technique using the CRISPR/Cas9 system in the industrial filamentous fungus Aspergillus oryzae. Biotechnol. Lett. 2015, 38, 637–642. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Jiang, Y.; Zhou, Z.; Zou, G. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov. 2015, 1, 15077. [Google Scholar] [CrossRef] [Green Version]
- Foster, A.J.; Martin-Urdiroz, M.; Yan, X.; Wright, H.S.; Soanes, D.M.; Talbot, N.J. CRISPR-Cas9 ribonucleoprotein-mediated co-editing and counterselection in the rice blast fungus. Sci. Rep. 2018, 8, 14355. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Chi, Y.; Lin, D.; Tyler, B.M.; Liu, X. Mutations in ORP1 conferring oxathiapiprolin resistance confirmed by genome editing using CRISPR/Cas9 in Phytophthora capsici and P. sojae. Phytopathology 2018, 108, 1412–1419. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Wang, Z.; Fang, Y.; Wang, W.; Cheng, X.; Liu, X. Point mutations in the β-tubulin of Phytophthora sojae confer resistance to ethaboxam. Phytopathology 2019, 109, 2096–2106. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Li, H.; Zhang, Z.; Sheng, G.; Li, Y.; Xing, Y.; Huang, S.; Tao, H.; Kuan, T.; et al. The RXLR effector PcAvh1 is required for full virulence of Phytophthora capsici. Mol. Plant Microbe Interact. 2019, 32, 986–1000. [Google Scholar] [CrossRef]
- Situ, J.; Jiang, L.; Fan, X.; Yang, W.; Li, W.; Xi, P.; Deng, Y.; Kong, G.; Jiang, Z. An RXLR effector PlAvh142 from Peronophythora litchii triggers plant cell death and contributes to virulence. Mol. Plant Pathol. 2020, 21, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2015; pp. 571–607. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Tarleton, R. EuPaGDT: A web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb. Genom. 2015, 1, e000033. [Google Scholar] [CrossRef] [Green Version]
- Turgeon, B.G.; Garber, R.C.; Yoder, O.C. Transformation of the fungal maize pathogen Cochliobolus heterostrophus using the Aspergillus nidulans amdS gene. Mol. Gen. Genet. 1985, 201, 450–453. [Google Scholar] [CrossRef]
- Wei, Y.; Pu, J.; Zhang, H.; Liu, Y.; Zhou, F.; Zhang, K.; Liu, X. The laccase gene (LAC1) is essential for Colletotrichum gloeosporioides development and virulence on mango leaves and fruits. Physiol. Mol. Plant Pathol. 2017, 99, 55–64. [Google Scholar] [CrossRef]
- Liu, J.K.; Chang, H.W.; Liu, Y.; Qin, Y.H.; Ding, Y.H.; Wang, L.; Zhao, Y.; Zhang, M.Z.; Cao, S.N.; Li, L.T.; et al. The key gluconeogenic gene PCK1 is crucial for virulence of Botrytis cinerea via initiating its conidial germination and host penetration. Environ. Microbiol. 2018, 20, 1794–1814. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Y.; Wang, C.; Jiang, C.; Xu, J.R. Targeted deletion of the USTA and UvSLT2 genes efficiently in Ustilaginoidea virens with the CRISPR-Cas9 system. Front. Plant Sci. 2018, 9, 699. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, S.; Chui, C.; Ma, T.; Jiang, H.; Hahn, M.; Ma, Z. The MAPK kinase BcMkk1 suppresses oxalic acid biosynthesis via impeding phosphorylation of BcRim15 by BcSch9 in Botrytis cinerea. PLoS Pathog. 2018, 14, e1007285. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Classification and nomenclature of CRISPR-Cas systems: Where from here? CRISPR J. 2018, 1, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas systems towards nextgeneration biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef]
- Jiang, C.; Lv, G.; Tu, Y.; Cheng, X.; Duan, Y.; Zeng, B.; He, B. Applications of CRISPR/Cas9 in the synthesis of secondary metabolites in filamentous fungi. Front. Microbiol. 2021, 12, 638096. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Batta, A.; Singh, H.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Bioengineering of fungal endophytes through the CRISPR/Cas9 system. Front. Microbiol. 2023, 14, 1146650. [Google Scholar] [CrossRef] [PubMed]
- Králová, M.; Bergougnoux, V.; Frébort, I. CRISPR/Cas9 genome editing in ergot fungus Claviceps purpurea. J. Biotechnol. 2021, 325, 341–354. [Google Scholar] [CrossRef]
- Fuller, K.K.; Chen, S.; Loros, J.J.; Dunlap, J.C. Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus. Eukaryotic Cell 2015, 14, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Gao, R.; Li, J.; Lin, L.; Zhao, J.; Sun, W.; Tian, C. Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol. Biofuels 2017, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.; Schweizer, G.; Reissmann, S.; Kahmann, R. Genome editing in Ustilago maydis using the CRISPR–Cas system. Fungal Genet. Biol. 2016, 89, 3–9. [Google Scholar] [CrossRef]
- Qiu, M.; Li, Y.; Ye, W.; Zheng, X.; Wang, Y. A CRISPR/Cas9-mediated in situ complementation method for Phytophthora sojae mutants. Mol. Plant Pathol. 2021, 22, 373–381. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Liu, N.; Luo, H.; He, C.; An, B. The histone deacetylase HOS2 controls pathogenicity through regulation of melanin biosynthesis and appressorium formation in Colletotrichum gloeosporioides. Phytopathol. Res. 2022, 4, 21. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Q.; Feng, Q.; Zhang, B.; He, C.; Luo, H.; An, B. Heat shock transcription factor CgHSF1 is required for melanin biosynthesis, appressorium formation, and pathogenicity in Colletotrichum gloeosporioides. J. Fungi 2022, 8, 175. [Google Scholar] [CrossRef]
- Li, X.; Ke, Z.; Xu, S.; Tang, W.; Liu, Z. The G-protein alpha subunit CgGa1 mediates growth, sporulation, penetration and pathogenicity in Colletotrichum gloeosporioides. Microb. Pathog. 2021, 161, 105254. [Google Scholar] [CrossRef]
- Mushtaq, A.; Tariq, M.; Ahmed, M.; Zhou, Z.; Ali, I.; Mahmood, R.T. Carbamoyl phosphate synthase subunit CgCPS1 is necessary for virulence and to regulate stress tolerance in Colletotrichum gloeosporioides. Plant Pathol. J. 2021, 37, 232–242. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Zhang, N.; Liu, Z. Function and transcriptome analysis of an oligopeptide transporter CgOPT2 in the rubber anthracnose fungus Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 2021, 115, 101661. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Tian, C. CgEnd3 regulates endocytosis, appressorium formation, and virulence in the poplar anthracnose fungus Colletotrichum gloeosporioides. Int. J. Mol. Sci. 2021, 22, 4029. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, W.; Wu, Y.; Zhou, Y.; Peng, F.; Zhang, S.; Chen, P.; Xu, X. BcCFEM1, a CFEM domain-containing protein with putative GPI-anchored site, is involved in pathogenicity, conidial production, and stress tolerance in Botrytis cinerea. Front. Microbiol. 2017, 8, 1807. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xia, Y.-Q.; Xia, Y.; Zhang, M.-T.; Ye, Z.; Sun, R.-Q.; Liu, X.-M.; Pu, J.-J. A CRISPR/Cas9-Based Study of CgloRPCYG, a Gene That Regulates Pathogenicity, Conidial Yield, and Germination in Colletotrichum gloeosporioides. Agronomy 2023, 13, 1681. https://doi.org/10.3390/agronomy13071681
Zhang H, Xia Y-Q, Xia Y, Zhang M-T, Ye Z, Sun R-Q, Liu X-M, Pu J-J. A CRISPR/Cas9-Based Study of CgloRPCYG, a Gene That Regulates Pathogenicity, Conidial Yield, and Germination in Colletotrichum gloeosporioides. Agronomy. 2023; 13(7):1681. https://doi.org/10.3390/agronomy13071681
Chicago/Turabian StyleZhang, He, Yu-Qi Xia, Yang Xia, Meng-Ting Zhang, Zi Ye, Rui-Qing Sun, Xiao-Mei Liu, and Jin-Ji Pu. 2023. "A CRISPR/Cas9-Based Study of CgloRPCYG, a Gene That Regulates Pathogenicity, Conidial Yield, and Germination in Colletotrichum gloeosporioides" Agronomy 13, no. 7: 1681. https://doi.org/10.3390/agronomy13071681





