Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico
Abstract
:1. Introduction
2. Materials and Methods
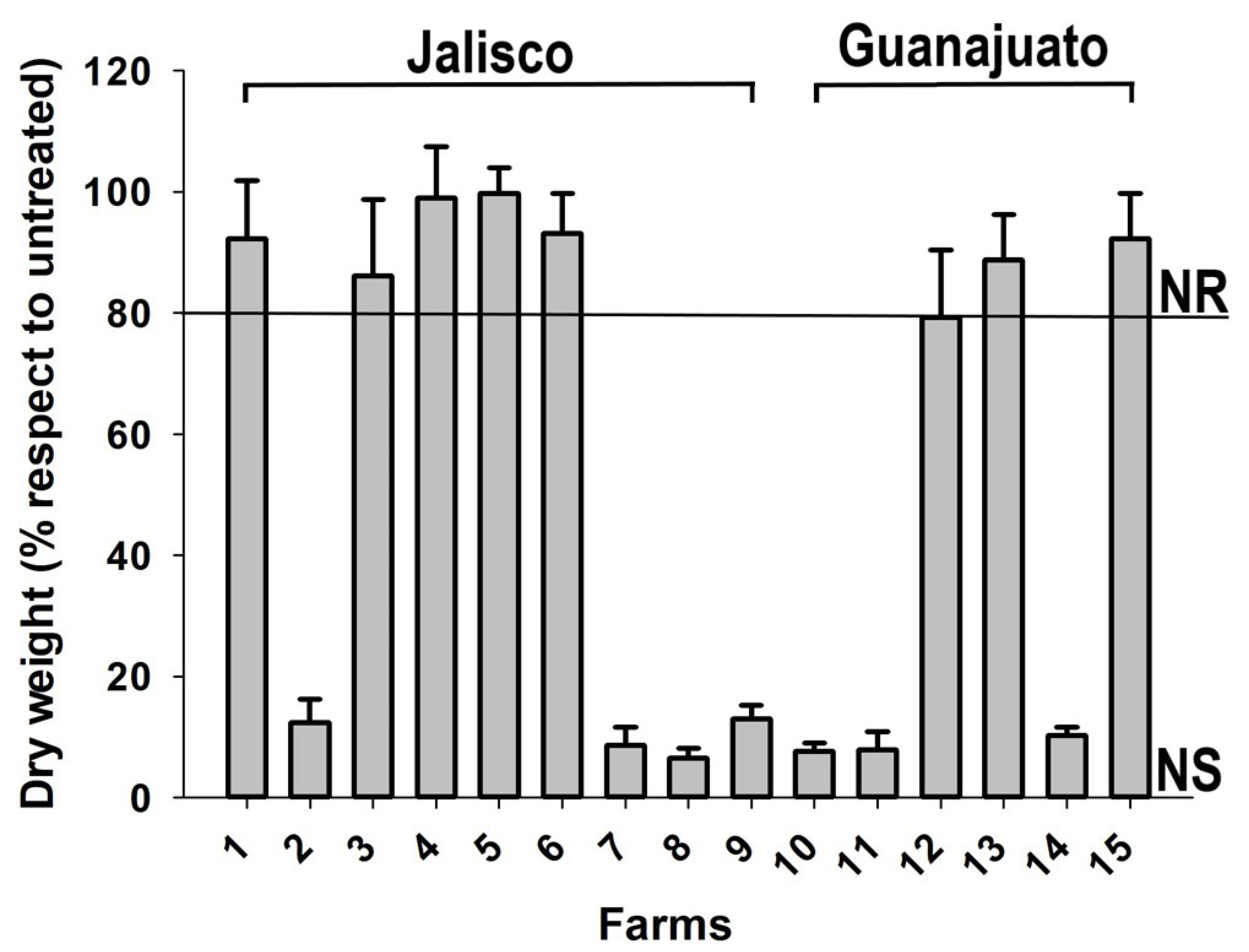
2.1. Resistance Screening in Corn Fields
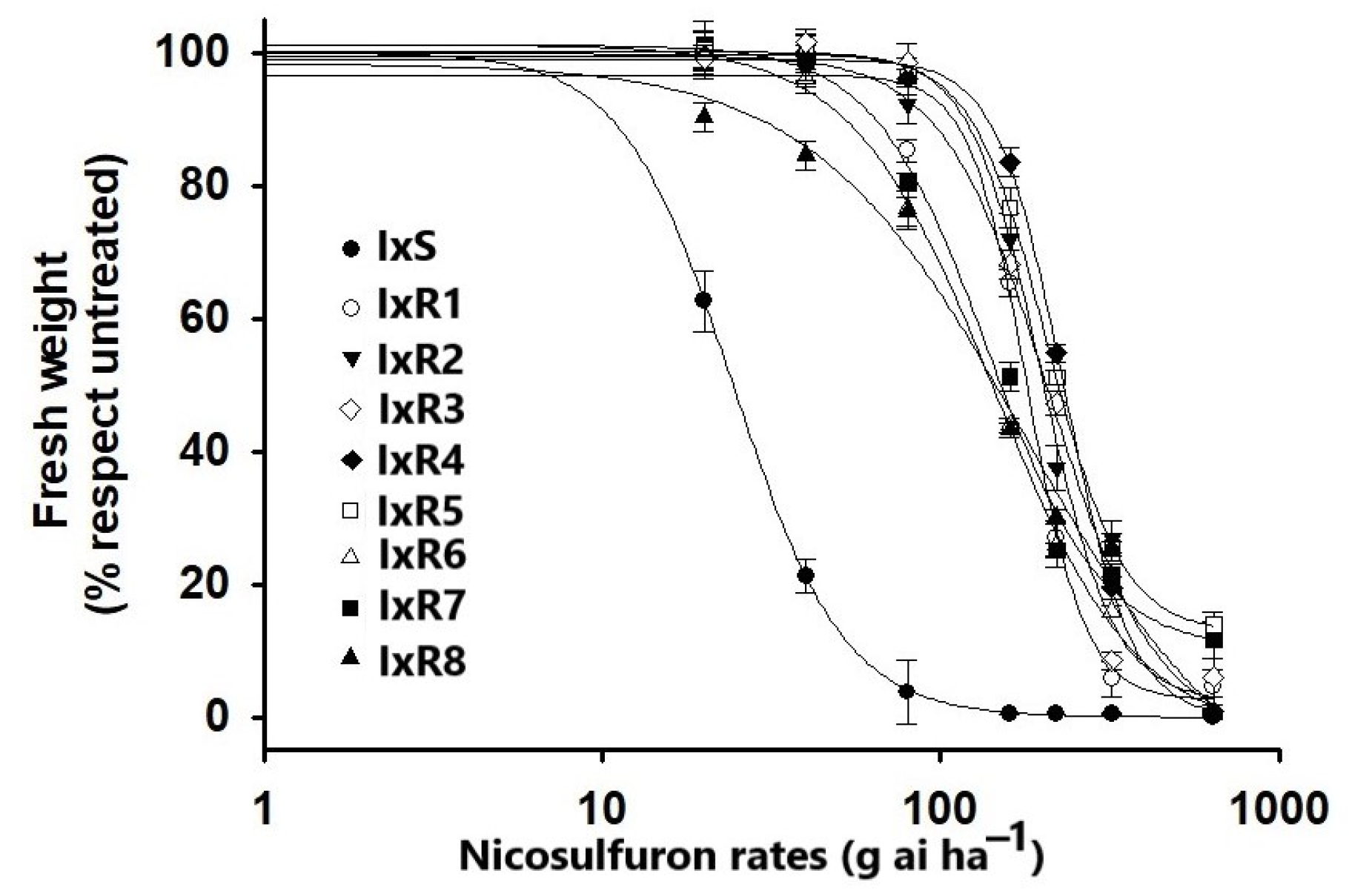
2.2. Dose–Response Curves
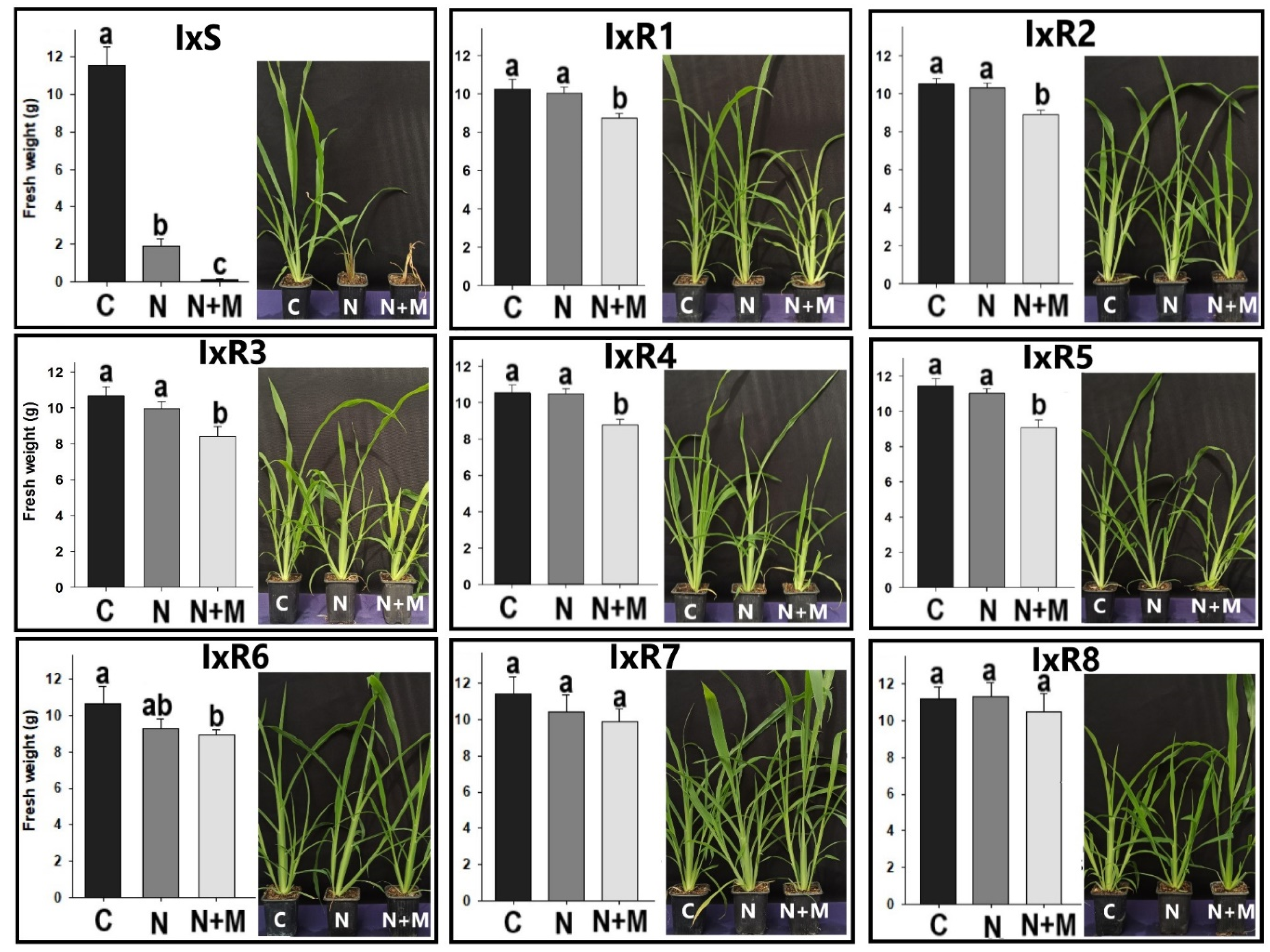
2.3. Synergism of Herbicide plus Malathion
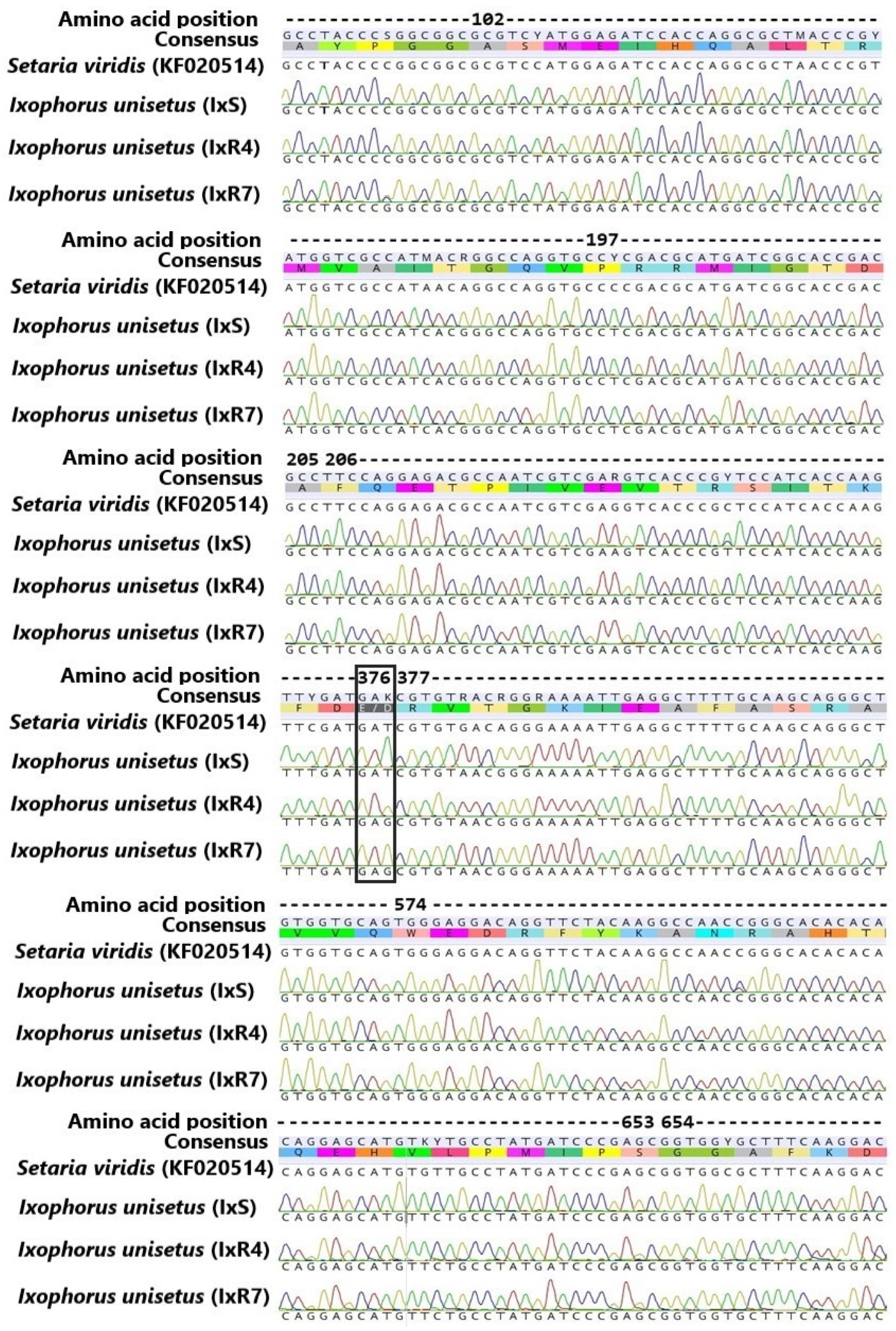
2.4. Molecular Analyses
DNA Isolation and PCR Amplification
2.5. Cross-Resistance In Vitro Assay (ALS Activity)
2.6. Cross-Resistance In Vivo Assay
2.7. Multiple-Resistance Screening
2.8. Data Analysis
2.9. Chemicals
3. Results
3.1. Herbicide-Resistance Field Screening
3.2. Dose–Response Curves
3.3. Nicosulfuron and Malathion Synergism
3.4. Partial ALS Gene Sequencing
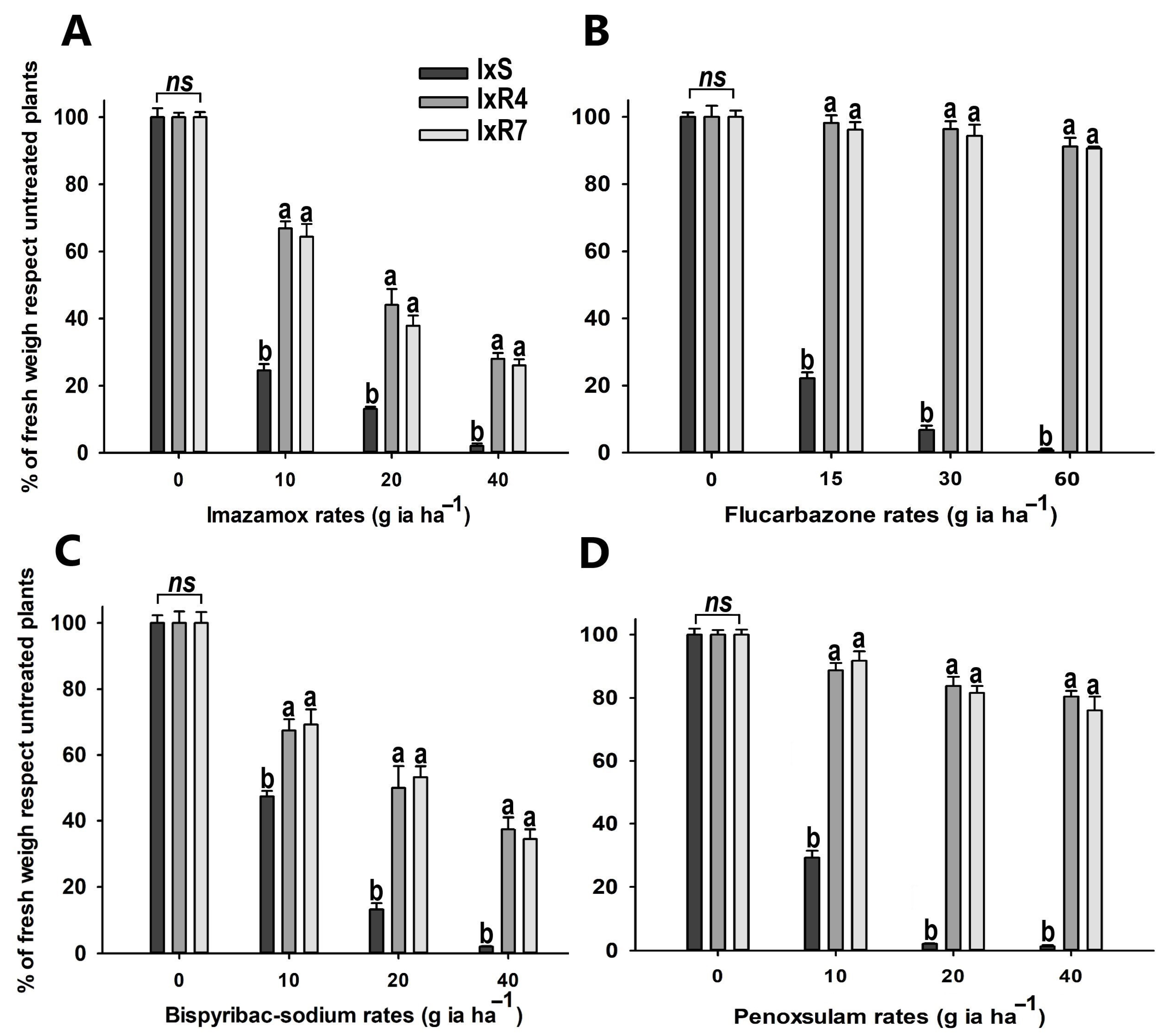
3.5. Cross-Resistance In Vivo Assay
3.6. Cross-Resistance In Vitro Assay
3.7. Multiple-Resistance Screening Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vibrans, H. Malezas de México: Ixophorus unisetus (J. Presl) Schltdl. Available online: http://www.conabio.gob.mx/malezasdemexico/poaceae/ixophorus-unisetus/fichas/ficha.htm (accessed on 29 January 2023).
- Sánchez, J.G.; Zita-Padilla, G.A.; Mendoza-Cruz, M. Catálogo de las Gramíneas Malezas Nativas e Introducidas de México; CONACOFI: Montecillo, Mexico, 2012. [Google Scholar]
- Esqueda, V.A.; Tosquy, O.H. Efecto del volumen y el pH del agua en el control de Ixophorus unisetus (J. Presl) Schltdl. con glifosato. Rev. Mex. De Cienc. Agrícolas 2015, 6, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Cano, O.; López, E. Control preemergente y postemergente de malezas en frijol de humedad residual en Veracruz, Mexico. Agron. Mesoam. 1996, 7, 42–49. [Google Scholar] [CrossRef]
- Herrera, F. Efecto de rastrojos de malezas y herbicidas pre-emergentes en el control de malezas en frijol. Agron. Mesoam. 2000, 11, 63–71. [Google Scholar] [CrossRef]
- Portillo, H.; Pitre, H.N.; Meckenstock, D.H.; Andrews, K.L. Oviposition preference of Spodoptera latifascia (Lepidoptera:Noctuidae) for sorghum, maize and non-crop vegetation. Fla. Entomol. 1996, 79, 552–562. [Google Scholar] [CrossRef]
- Pleasant, J.M.T.; Burt, J.; Frisch, J.C. Integrating mechanical and chemical weed management in corn (Zea mays). Weed Technol. 1994, 8, 217–223. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: http://weedscience.org (accessed on 25 January 2023).
- HRAC. Herbicide Resistance Action Comitte: Herbicide Resistance. Available online: https://hracglobal.com/herbicide-resistance/overview (accessed on 23 January 2023).
- Preston, C.; Powles, S.B. Evolution of herbicide resistance in weeds: Initial frequency of target site-based resistance to acetolactate synthase-inhibiting herbicides in Lolium rigidum. Heredity 2002, 88, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef]
- Panozzo, S.; Mascanzoni, E.; Scarabel, L.; Milani, A.; Dalazen, G.; Merotto, A.J.; Tranel, P.J.; Sattin, M. Target-site mutations and expression of ALS gene copies vary according to Echinochloa species. Genes 2021, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-García, J.G.; De Portugal, J.; Torra, J.; Osuna, M.D.; Palma-Bautista, C.; Cruz-Hipolito, H.E.; De Prado, R. Comparison between the mechanisms of Clearfield® wheat and Lolium rigidum multiple resistant to acetyl CoA carboxylase and acetolactate synthase inhibitors. Environ. Pollut. 2022, 306, 119438. [Google Scholar] [CrossRef]
- Fang, J.; Yang, D.; Zhao, Z.; Chen, J.; Dong, L. A novel Phe-206-Leu mutation in acetolactate synthase confers resistance to penoxsulam in barnyardgrass (Echinochloa crus-galli (L.) P. Beauv). Pest Manag. Sci. 2022, 78, 2560–2570. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Y.; Liu, W.; Wang, J. Cytochrome P450 CYP709C56 metabolizing mesosulfuron-methyl confers herbicide resistance in Alopecurus aequalis. Cell. Mol. Life Sci. 2022, 75, 205. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Laplante, J.; Rajcan, I.; Tardif, F.J. Multiple allelic forms of acetohydroxyacid synthase are responsible for herbicide resistance in Setaria viridis. Theor. Appl. Genet. 2009, 119, 577–585. [Google Scholar] [CrossRef]
- Amaro-Blanco, I.; Romano, Y.; Palmerin, J.A.; Gordo, R.; Palma-Bautista, C.; De Prado, R.; Osuna, M.D. Different mutations providing target site resistance to ALS- ACCase-inhibiting herbicides in Echinochloa spp. from rice fields. Agriculture 2021, 11, 382. [Google Scholar] [CrossRef]
- Panozzo, S.; Scarabel, L.; Tranel, P.J.; Sattin, M. Target-site resistance to ALS inhibitors in polyploid species Echinochloa crus-galli. Pest. Biochem. Physiol. 2013, 105, 93–101. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; De Prado, R. Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response análisis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Alcántara-de la Cruz, R.; da Silva Amaral, G.; Ferreira Mendes, K.; Rojano-Delgado, A.M.; De Prado, R.; das Graças Fernandes da Silva, M.F. Absorption, translocation, and metabolism studies of herbicides in weeds and crops. In Radioisotopes Weed Research; Ferreira Mendes, K., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 127–154. [Google Scholar]
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An ecological future for weed science to sustain crop production and the environment. A review. Agron. Sustain. Dev. 2020, 40, 24. [Google Scholar] [CrossRef]
- Ramiz, Z.; Malone, J.; Preston, C.; Gurjeet, G. Genetic control of seed dormancy in Lolium rigidum and its association with GA20oX and ABA1 expression. Crop Pasture Sci. 2022, 73, 1406–1415. [Google Scholar] [CrossRef]
- Christopher, J.T.; Preston, C.; Powles, S.B. Malathion antagonizes metabolism-based chlorsulfuron resistance in Lolium rigidum. Pest. Biochem. Physiol. 1994, 49, 172–182. [Google Scholar] [CrossRef]
- Mei, Y.; Si, C.; Liu, M.; Qiu, L.; Zheng, M. Investigation of resistance levels and mechanisms to nicosulfuron conferred by non-target-site mechanisms in large crabgrass (Digitaria sanguinalis L.) from China. Pest. Biochem. Physiol. 2017, 141, 84–89. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, Z.; Huang, H.; Li, W.; Cao, Y.; Wei, S. Target site mutations and cytochrome P450s-involved metabolism confer resistance to nicosulfuron in green foxtail (Setaria viridis). Pest. Biochem. Physiol. 2021, 179, 104956. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, Y.; Zhang, Y.; Dong, L.; Li, J. Mechanisms of resistance to pyroxsulam and ACCase inhibitors in Japanase foxtail (Alopecurus japonicus). Weed Sci. 2016, 64, 695–704. [Google Scholar] [CrossRef]
- Menegat, A.; Bailly, G.C.; Aponte, R.; Heinrich, G.M.T.; Sievernich, B.; Gerhards, R. Acetohydroxyacid synthase (AHAS) amino acid substitution Asp376Glu in Lolium perenne: Effect on herbicide efficacy and plant growth. J. Plant Dis. Prot. 2016, 123, 145–153. [Google Scholar] [CrossRef]
- Panozzo, S.; Milani, A.; Scarabel, L.; Balogh, A.; Dancza, I.; Sattin, M. Ocurrence of different resistance mechanisms to acetolactate synthase inhibitors in European Sorghum halepense. J. Agric. Food Chem. 2017, 65, 7320–7327. [Google Scholar] [CrossRef]
- McCourt, J.A.; Pang, S.S.; King-Scott, J.; Guddat, L.W.; Duggleby, R.G. Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc. Natl. Acad. Sci. USA 2006, 103, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Shuang, L.; Hao, L.; Li, X.; Zheng, M. Tribenuron-methyl-resistant Descurainia sophia L. exhibits negative cross-resistance to imazethapyr conferred by a Pro197Ser mutation in acetolactate synthase and reduced metabolism. Pest Manag. Sci. 2021, 78, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Carbonari, C.A.; Duke, S.O. The potential influence of hormesis on evolution of resistance to herbicides. Curr. Opin. Environ. Sci. Health 2022, 27, 100360. [Google Scholar] [CrossRef]


| Tradename | Active Ingredient | MoA WSSA/HRAC a | Timing b | Field Dose (g ai ha−1) |
|---|---|---|---|---|
| Herbicide-resistance field screening and dose–response curves | ||||
| SANSON® 4 SC | Nicosulfuron | 2 | Post | 40 |
| Herbicide and Cyt-P450-inhibitor synergism | ||||
| SANSON® 4 SC | Nicosulfuron | 2 | Post | 40 |
| INMAR 50 | Malathion | - | - | 1000 |
| Cross-resistance in vivo assay | ||||
| Everest 70 | Flucarbazone | 2 | Post | 25 |
| Pulsar® | Imazamox | 2 | Post | 40 |
| Nomine® | Bispyribac-Na | 2 | Post | 40 |
| Viper | Penoxsulam | 2 | Post | 20 |
| Multiple-resistance screening | ||||
| Asgard® | Petoxamida | 15 | Pre | 1800 |
| Anthem-Maxx 518 | Piroxasulfone + Fluthiacet | 15 + 14 | Pre | 376.7 + 9.8 |
| Roundup | Glyphosate | 9 | Post | 720 |
| Leopard 5% | Quizalofop | 1 | Post | 100 |
| Laudis 20% | Tembotrione | 27 | Post | 120 |
| Callisto 100 | Mesotrione | 27 | Post | 150 |
| Convey® | Topramezone | 27 | Post | 26.9 |
| Raker PRO® | Tolpyralate | 27 | Post | 40 |
| Elumis | Nicosulfuron + Mesotrione | 2 + 27 | Post | 60 + 150 |
| Population Code | Place | b | d | GR50 | 95% CI | RI a | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| IxS | Jalisco | 2.6 | 99.9 | 24.3 | 22.2 | 26.4 | -- |
| IxR1 | Jalisco | 3.98 | 97.2 | 186.6 | 176.5 | 196.7 | 7.6 |
| IxR2 | Jalisco | 2.9 | 98.9 | 214.4 | 201.9 | 226.8 | 8.8 |
| IxR3 | Jalisco | 2.7 | 100.7 | 237.2 | 223.6 | 250.8 | 9.7 |
| IxR4 | Jalisco | 4.6 | 98.3 | 245.5 | 235.0 | 255.8 | 10.1 |
| IxR5 | Jalisco | 3.61 | 100.1 | 209.5 | 198.6 | 220.3 | 8.6 |
| IxR6 | Guanajuato | 2.4 | 99.9 | 140.3 | 128.4 | 152.1 | 5.7 |
| IxR7 | Guanajuato | 2.0 | 102.5 | 155.0 | 141.8 | 168.1 | 6.3 |
| IxR8 | Guanajuato | 1.7 | 96.1 | 151.9 | 135.1 | 168.5 | 6.2 |
| Treatment | Plant Mortality (%) a | |||||
|---|---|---|---|---|---|---|
| IxS | IxR4 | IxR7 | ||||
| Petoxamide | 100 ± 0 | a | 100 ± 0 | b | 100 ± 0 | a |
| Pyroxasulfone + Fluthiacet | 100 ± 0 | a | 90 ± 0 | b | 95 ± 7 | ab |
| Glyphosate | 100 ± 0 | a | 100 ± 0 | a | 100 | a |
| Quizalofop | 100 ± 0 | a | 100 ± 0 | a | 100 ± 0 | a |
| Tembotrione | 85 ± 7 | bc | 85 ± 0 | b | 75 ± 7 | c |
| Mesotrione | 85 ± 7 | bc | 90 ± 0 | b | 85 ± 7 | bc |
| Topramezone | 100 ± 0 | a | 90 ± 14 | ab | 100 ± 0 | a |
| Tolpyralate | 100 ± 0 | a | 95 ± 7 | ab | 100 ± 0 | a |
| Nicosulfuron + Mesotrione | 100 ± 0 | a | 100 ± 0 | a | 100 ± 0 | a |
| Species a | Herbicide Family Abbreviations b,c | ||||
|---|---|---|---|---|---|
| SU | IMI | SCT | PTB | TP | |
| Ixophorus unisetus (studied here) (SW) | R | r | R | r | R |
| Sorghum halepense (SW) | R | S | R | R | ND |
| Alopecurus japonicus (WW) | R | R | R | r | R |
| Setaria viridis (SW) | R | R | ND | ND | ND |
| Lolium perenne (WW) | R | r | R | ND | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Valenzuela, J.A.; Vázquez-García, J.G.; Castro, P.; Palma-Bautista, C.; Cruz-Hipólito, H.E.; Rey, M.-D.; De Prado, R.; Portugal, J. Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico. Agronomy 2023, 13, 1682. https://doi.org/10.3390/agronomy13071682
Domínguez-Valenzuela JA, Vázquez-García JG, Castro P, Palma-Bautista C, Cruz-Hipólito HE, Rey M-D, De Prado R, Portugal J. Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico. Agronomy. 2023; 13(7):1682. https://doi.org/10.3390/agronomy13071682
Chicago/Turabian StyleDomínguez-Valenzuela, José Alfredo, José G. Vázquez-García, Patricia Castro, Candelario Palma-Bautista, Hugo E. Cruz-Hipólito, Maria-Dolores Rey, Rafael De Prado, and João Portugal. 2023. "Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico" Agronomy 13, no. 7: 1682. https://doi.org/10.3390/agronomy13071682






