Abstract
The global use of pesticides, exceeding 4 million tons annually, poses substantial threats to public health and the environment. Numerous studies emphasize the correlation between pesticide exposure, genotoxicity, and neurobehavioral effects, which particularly impact children and agricultural workers. Recent research underscores the health risks associated with moderately toxic pesticides like chlorpyrifos. Chlorpyrifos contamination in crops, a result of extensive pesticide use since the 1960s, raises significant concerns regarding human health and the environment. Given its potential atmospheric volatilization from crops, alternative detection methods are imperative. This study employs gas chromatography coupled with mass spectrometry (GC/MS) in MS/MS mode, focusing on detecting chlorpyrifos in Chilean lettuce, using insects as indicators. Two extraction methods, micro-QuEChERS and HEADSPACE-SPME, were compared, with HEADSPACE-SPME demonstrating superior sensitivity (6.77 ng/mg per sample vs. 3.99 ng/mg per sample) and offering time- and solvent-saving advantages. Additionally, HEADSPACE-SPME preserves samples for future research, enhancing its utility. The study confirms the presence of pesticide residues in insects from lettuce crops, with 52.3% displaying chlorpyrifos when the SPME method was used. Predatory and parasitoid insect families like Braconidae and Ichneumonidae show high pesticide loads. The findings of this study emphasize the widespread contamination of chlorpyrifos in lettuce crop insects and highlight the efficacy of SPME for detection, suggesting its broader applicability for evaluating pesticide residues in horticultural systems. This optimized method holds promise for advancing chlorpyrifos contamination detection in agroecosystems, contributing to environmental monitoring and food security.
Keywords:
agriculture; agronomy; agroecosystem; entomology; horticulture; integrated pest management 1. Introduction
Pesticides are extensively employed worldwide for pest control, primarily within the agricultural sector [1]. Annually, more than 4 million tons of active ingredients are used in commercially available pesticides [2]. Individuals face direct exposure to pesticides in occupational, agricultural, and domestic settings, as well as indirect exposure through environmental matrices such as air, water, soil, and food [3].
Contamination from pesticides is a widespread issue with far-reaching consequences for both public health and the environment. Human exposure to these chemicals primarily occurs through dermal contact, oral ingestion, and inhalation. Individuals who encounter pesticides in substantial amounts, either directly or indirectly, are at risk of experiencing acute poisoning symptoms such as nausea, headaches, muscle weakness, cramps, vomiting, and, in severe cases, loss of consciousness, cardiac arrest, and even death. Furthermore, prolonged exposure to lower-to-moderate pesticide doses can lead to the development of chronic diseases, significantly impacting individuals’ overall quality of life and well-being [4,5,6].
In Latin America, as evidenced by several studies [7], a link has been observed between exposure to organophosphate and carbamate pesticides, as well as various classes of pesticides, and the presence of genotoxicity biomarkers and adverse neurobehavioral effects. This phenomenon is particularly notable among children and agricultural workers.
Recent research examining the health risk index associated with pesticide exposure [2] revealed that certain low-toxicity pesticides produce hazardous burdens comparable to those of high-toxicity pesticides due to their extensive use. Approximately 24% of the global population resides in areas near locations with pesticide applications that exceed acceptable levels, and 32% of the population could surpass acceptable intake limits for these chemicals.
An additional, an indirect consequence of intensive pesticide management is local environmental contamination, which can have significant repercussions on agricultural production [8]. While a majority of studies on this subject concentrate on its implications for human health or crop vitality, there exists a less explored yet equally consequential dimension to consider: the influence of high-intensity pesticide management on the composition and functionality of the insect community surrounding economically vital crops. These agricultural systems are critically dependent on ecosystem services encompassing pollination, nutrient cycling, soil enhancement, and pest control [9]. Intensive agriculture, characterized by its heightened reliance on agrochemical inputs, has been demonstrated to markedly erode these indispensable services [10].
One of the most frequently utilized and controversial organophosphorus agrochemicals is chlorpyrifos [6]. Its extensive application in agriculture has led to cases of poisoning, potentially resulting in the death of non-target species upon exposure. Furthermore, it contaminates and exerts adverse effects on various aspects, including plant development, aquatic life, soil quality, livestock, wildlife, and human health [11,12].
In response to this issue, several strategies have been explored to monitor its presence in the environment, regulate its use, and explore alternative options. It is important to note, however, that these initiatives are still in their early stages or may prove to be economically burdensome, particularly in countries with moderate-to-low incomes [13,14].
In Chile, chlorpyrifos ranks among the top-selling insecticides [15]. Its presence has been identified through comprehensive monitoring efforts, encompassing air monitoring [16,17,18], water quality assessments [19], honeybee investigations [20], and residue analyses in food products [21,22,23,24,25,26,27], and even in the urinary biomarkers of children [28,29].
The results of all these studies point to high levels of chlorpyrifos residues in the air, vegetables, and fruits, along with elevated concentrations of chlorpyrifos metabolites in urine.
In Chile, the exempt Resolution 5810/2022 prohibits specific pesticide brands containing chlorpyrifos either within a two-year timeframe or until the depletion of existing pesticide stocks. Additionally, Resolution 7128/2022 outlines Ministry of Agriculture guidelines for the 2022–2026 period, focusing on sustainable forestry and agricultural development, food security, and sovereignty [30,31]. Consequently, the development of an efficient technique for promptly detecting chlorpyrifos residues in agricultural areas exposed to these pesticides is essential.
These findings highlight the importance of conducting research that evaluates both direct and indirect pesticide exposure, employing efficient and expeditious methods on a broader scale.
The employment of solid-phase microextraction (SPME) stands out as a swifter approach that eliminates the need for solvents when compared to other techniques, such as solid-phase extraction (SPE), which has been reported to be more efficient for pesticide extraction [32].
The solid-phase microextraction (SPME) technique, combined with mass spectrometry (GC/MS), has been employed for the analysis of volatile components in Tenebrio molitor (Coleoptera: Tenebrionidae) and Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) larvae. These studies have revealed the presence of aldehydes, alkaloids, esters, ketones, aromatic ketones, fatty acids, and terpenes which were detected 24 and 48 h after the application of the insecticides fluthrin, thiamethoxam, and acetamiprid [14].
Nevertheless, there is a dearth of information regarding the application of analytical techniques, such as chromatography coupled with mass spectrometry, for assessing pesticides in insects. Hence, we assert that this study, presented below, is innovative in addressing this research gap.
The present study aimed to identify and quantify traces of pesticides, including chlorpyrifos, absorbed by insects in lettuce crops managed using both traditional and integrated pest management practices in the Coquimbo Region of Chile. Moreover, the study sought to assess the efficiency of detecting pesticide residue in these insect samples using a new SMPE injection technique in comparison to the conventional micro-QuEChERS method. These samples were subsequently analyzed using GC/MS/MS.
2. Materials and Methods
2.1. Study Area
The research was conducted within the Coquimbo Region, Chile, which is one of the primary regions for leafy vegetable production in the country, alongside the Valparaíso and Metropolitan Regions. This region is distinguished by its balanced distribution of integrated pest management (approximately 55%) and traditional management practices (approximately 45%) [33].
This balance makes it an ideal setting for conducting a comparative analysis. Seven specific sampling sites were selected within the Coquimbo Region of Chile. Detailed data are provided in Table 1.

Table 1.
Locations and characteristics of sampling points.
Due to legal constraints, the actual names of companies that apply traditional management practices were replaced with designations such as “high pesticide load 1–4”.
2.2. Insect Collection
Insect sampling was conducted for 14 months, from February 2021 to March 2022, using yellow pans. The sampling method was adapted from Bellamy et al. [9] to suit the lettuce crops under study. Two sites were sampled within each of the seven lettuce crop fields. The sites were selected along a transect running from (1) the middle of the farm, referred to as the “inside site”, and (2) 30 m from the edge of the lettuce crop. At each site, five replicate yellow pan traps were placed, spaced at least 5 m apart, in accordance with Sutherland [34] and Brown and Matthews [35]. The selection of a site 30 m from the crop edge was made to minimize potential variations due to edge effects in fields of different sizes, thus serving as a standardized point of comparison across the selected lettuce crop fields. The “inside” point was approximately 100 m from the farm’s edge. Traps were left in position at each site for 24 h periods.
Yellow pan traps are passive collection tools that operate on a premise that leverages the attractive properties of the color as an attractant for flying insects, especially Hymenoptera, Coleoptera, Lepidoptera, Diptera, and Hemiptera [36]. The yellow color is known to be highly effective at attracting a wide range of insect groups, including herbivorous pests [37], parasitoid wasps [38], and pollinators [39]. The pan traps used were bright yellow plastic bowls, measuring 20 cm in diameter and 3 cm deep, positioned just above ground level. Within each trap was 300 mL of a solution composed of 1/3 propylene glycol and 2/3 water.
2.3. Sample Distribution for Analysis
The insects’ masses ranged from 20 to 200 mg depending on the available sample. In all cases, the sample was evenly divided for both the QuEChERS and HEADSPACE-SPME analyses. HPLC-grade ethyl acetate and standard chlorpyrifos were purchased from MERCK; the QuEChERS kit was purchased from Agilent.
Solid-phase microextraction (SPME): To extract volatile compounds from the insect (Table 1), every sample was weighed before the extraction method (direct SPME or micro-QuEChERS). The extraction of volatile compounds from the free space of the samples was performed using polydimethylsiloxane/carboxen/divinylbenzene (PDMS/CAR/DVB) at a temperature of 105 °C. The extraction time for the fiber was 40 min, based on reported work related to pesticide detection in larvae [14].
Micro-QuEChERS: A QuEChERS method was adapted [40] at the microgram level. The protocol was as follows: The sample was dried at 50 °C for 12 h and subsequently homogenized using a mortar. The weighed content was deposited in an Eppendorf tube to which 1 mL of ethyl acetate was added and shaken for one minute. QuEChERS Final Polish EMR-Lipid® (25–250 mg) was introduced and stirred for 1 min. The tube was centrifuged at 12,000 rpm for 4 min at room temperature. Subsequently, 600 uL of the supernatant was extracted and transferred to a new Eppendorf tube containing anhydrous MgSO4: PSA (12–120 mg). The contents were agitated for 1 min and then centrifuged at 12,000 rpm for 4 min at room temperature. The resulting supernatant was filtered for a GC/MS/MS analysis.
GC/MS/MS Chromatographic Conditions: Thermo TSQ DUO equipment (Thermo Fischer Scientific, Waltham, MA, USA) was used in Selective Reaction Monitoring (SRM) mode. Helium served as the mobile phase with a flow rate of 1 mL/min, and the injector temperature was maintained at 230 °C. The RTX5-ms column was utilized under the following conditions: an initial temperature of 60 °C for 2 min followed by a temperature increase to 180 °C at a rate of 20 °C/min which was held for 2 min, and a further temperature increase to 270 °C at a rate of 10 °C/min which was held for 2 min. The mass spectrometer system was operated in electron ionization (EI) mode at 70 eV. The ion source and transfer line temperatures were both 280 °C.
For quantifying the pesticide chlorpyrifos, the SRM method was optimized using the “AUTO-SRM” function, allowing for the selection of appropriate energies for the fragmentation of “parent” ions and monitoring “daughter” ions. Specifically, the 169 m/z ion was used for qualification, while the 79 m/z ion was selected for quantification (Table 2). The qualitative/quantitative data were processed using Chromeleon software version 7.2.10.

Table 2.
Energy conditions optimized for SRM.
The QuEChERS extraction was standardized with the pesticide chlorpyrifos (1 ng/mg of insect) using the spiked method, achieving an average recovery of 95.5% in pesticide-free insects with an RSD of less than 2% (Table 3).

Table 3.
Recovery of chlorpyrifos using QuEChERS.
The HEADSPACE-SPME extraction technique is based on the release of the pesticide from the surface of the insect rather than the entire insect as in a “bulk” method (such as QuEChERS). Therefore, it depends on the adsorption and desorption relationship of these compounds on the insect’s surface. We propose using different masses of chlorpyrifos in the HEADSPACE-SPME vial and constructing a calibration curve that allows for a good correlation as the volatile pesticide will be analyzed under the same incubation conditions as the insects themselves.
In the case of the micro-QuEChERS technique, the limit of detection (LOD) was 0.9 ng/g, and the limit of quantification (LOQ) was 2.3 ng/g, whereas for HEADSPACE-SPME, the LOD was 2.6 ng/g, and the LOQ was 12 ng/g.
For statistical comparison between the two pesticide detection methods used in this research, a paired t-test was performed with a statistical significance level set at 99%. This method of analysis is suitable for experiments in which both methods are applied to the same materials. The analysis focused on comparing the following parameters: the percentage of samples in which pesticides were detected (detection efficiency), the total number of pesticides found in all samples (including those without detection), and the number of pesticides in samples in which detections were made. The analyses were performed using “Past 4.09” software, and bar graphs were generated to visualize mean values and standard deviation variations for each analyzed variable. It is important to note that the quantity of pesticide detected per entomological family was not subjected to a statistical comparison due to the limited number of sample repetitions per group for meaningful comparisons.
3. Results
A total of 68 out of 130 samples were detected using SPME-HEADSPACE, while 64 samples were detected using the micro-quEChERS method.
A comprehensive analysis was conducted on 130 samples of insects captured and separated according to family level and examined through GC/MS, using both the HEASPACE-SPME and micro-QuEChERS techniques. The results of the sample analysis by SPME extraction are shown in Table 4. In this analysis, it was observed that chlorpyrifos was detected in 48.46% of the sample population when employing the HEADSPACE-SPME method, whereas it was identified in 43.84% when using the Micro-QuEChERS technique. Three ranges of chlorpyrifos content were established: low (<0.1 ng chlorpyrifos/mg insect), medium (0.1–1 ng chlorpyrifos/mg insect), and high (>1 ng chlorpyrifos/mg insect).

Table 4.
Comparison of chlorpyrifos levels in samples analyzed through SPME and QuEChERS extraction techniques at high, medium, and low concentrations.
The pesticide detection methods exhibited variations in both the percentage of samples containing detected pesticides and the quantity of pesticide residues within the insects. The SMPE method detected pesticide residues in 49.34% of the samples, while the QuEChERS method detected them in 42.10% of the samples (p > 0.001).
Regarding the number of residues found in the samples, the SMPE method yielded an average of 3.56 ng/mg across all the analyzed samples. In contrast, the QuEChERS method produced an average of 2.10 ng/mg per sample, including those without pesticide residues (p > 0.001).
When considering only the samples with pesticide residues, the SMPE method found an average of 6.77 ng/mg per sample, while the QuEChERS method identified only 3.99 ng/mg per sample (p > 0.001). A visual representation of these numerical differences can be observed in Figure 1.
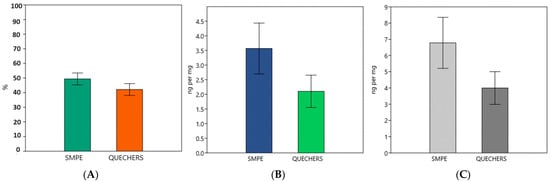
Figure 1.
(A) Percentage of samples with detected pesticides; (B) pesticide quantity in all samples (including non-detected samples) in ng/mg of sample; (C) pesticide amount in samples with pesticide detection (ng/mg of sample).
In different locations characterized by varying pesticide application loads, the detection of chlorpyrifos exhibited discrepancies among environments and between methods. In high-pesticide-load environments, the SMPE method detected 15.03 ng/mg of insects, followed by 12.71 ng/mg of insects for the QuEChERS method. In contrast, in the environment with a medium pesticide load, the values were 0.42 ng/mg insect for SMPE and 0.46 ng/mg insect for QuEChERS. In low-pesticide-load environments, the values were 0.04 ng/mg insect for SMPE and 0.03 ng/mg insect for QuEChERS.
The detection values of chlorpyrifos varied among entomological families. For example, Psyllidae had 56.71 ng of chlorpyrifos per mg of insect, Hemerobiidae had 49.16 ng of chlorpyrifos per mg of insect, and Cicadellidae had 23.91 ng of chlorpyrifos per mg of insects. Values for all entomological groups are provided in Table 4.
Usually, the SMPE method also presents different values from the QuEChERS method among entomological families but does not necessarily present higher values. Due to the low number of samples per entomological family, it was not possible to make a statistical comparison between the chlorpyrifos detection values among the groups. However, in absolute terms, some families had higher detection values, particularly in locations characterized by high pesticide application loads.
4. Discussion
Pesticide toxicity tests on insects are typically conducted by quantifying the pesticide before exposure without confirming the actual dose reached or absorbed by the insect. These tests are typically confined to controlled laboratory settings [41]. The purpose of detecting pesticides in insects is to assess the efficacy of pesticides concerning both target and non-target insects serving as a bioindicator. This approach aims to ascertain the actual quantity of pesticide reaching insects and to elucidate the responses of various insect groups to pesticide applications in crop environments.
The use of different entomological groups for pesticide detection has proven to be a valuable strategy for verifying how pesticides affect the environment [42]. Tison et al. [43], while looking for pesticide residues in Vespa velutina nests, found contamination in at least 53% of the nests, with detection ranging from 0.5 to 19.5 ng/g (using the QuEChERS extraction method coupled with HPLC-MS/MS). The authors noted that wasps, being predators of honeybees (Apis mellifera), are at a higher risk of pesticide exposure due to their predatory feeding habits.
Regarding the residual dynamics of pesticide residues in the environment, it is anticipated that insect groups more exposed to pesticide applications would have a higher residual load of pesticides. This perspective aligns with existing knowledge, such as the accumulation of DDT in food webs [44], underlining the potential for predatory insects and parasitoids to carry a significant pesticide burden.
The outcomes of this study affirm the presence of pesticide residues in insects collected from lettuce crops, indicating their potential exposure to an array of agricultural chemicals employed in these fields. A significant finding of this investigation is that 52.3% of the insect samples displayed the presence of chlorpyrifos when the SPME method was used. In this work, several entomological families such as Braconidae, Hemerobiidae, Ichneumonidae, and Pteromalidae also showed high residual loads of pesticides. These families consist of numerous predatory and parasitoid insect species. The strategy of employing functional or phylogenetic groups of insects to understand the pathways of pesticides in the environment is already a strategy employed, particularly for aquatic insects and bees [45]. With the approach presented here, other groups of insects can also be considered for the evaluation of pesticide residues in horticultural systems.
This result underscores the prevalence of chlorpyrifos contamination within the insect community inhabiting lettuce crops. Moreover, the effective application of the SPME technique in detecting chlorpyrifos is evident.
Additionally, the comparison between the SPME and QuEChERS methods (Table 4) illustrates the distribution of the chlorpyrifos content in the samples. Categorizing the chlorpyrifos content into low, medium, and high ranges provides a comprehensive understanding of contamination levels within the insect population. This differentiation helps assess the potential risks associated with the accumulation of chlorpyrifos in these insects. Furthermore, it is revealed that other pesticide residues detected using the SPME method exhibit higher detection proportions compared to those detected using the QuEChERS method, with statistically significant differences (over 17% higher; p > 0.001). When examining the quantity of residues found in the samples, the SMPE method yielded a higher average across all analyzed samples than the QuEChERS method, even in cases in which no pesticide residues were present (almost 70% higher; p > 0.001).
These findings underscore the SMPE method’s capability to detect and quantify pesticide residues with enhanced precision in terms of both detection and concentration. The disparity in results between the two methods could be attributed to the intrinsic characteristics of each extraction and analysis technique [32,40]. These results also underscore the importance of carefully selecting the extraction method based on the study’s objectives and the requirements for detection and quantification.
Ultimately, the choice of an appropriate extraction method can have significant implications for the accuracy and reliability of results in large-scale environmental pesticide residue monitoring studies [12,13].
The assessment of multiple extraction and analysis methods, as conducted in this study, contributes to the advancement of scientific knowledge and the enhancement of techniques employed in determining pesticide presence and concentration in the environment. This, in turn, aims to develop more efficient and sustainable measures for insect pest control in agricultural production [6].
Due to the toxicity of chlorpyrifos to humans, its detection is carried out in vegetables or samples of water and soil [46]. Among methods, the most widely used is the QuEChERS method; however, like any method, it has advantages and disadvantages. This method requires larger quantities of substrate for pesticide detection within the method’s detection limits.
A way of testing the toxicity of pesticides to insects is to quantify the pesticide before exposure without verifying the actual dose the insect received or absorbed [41], and another alternative is to measure LD50 [47]; however, this requires an isolated population of insects which does not represent a real environment of insect communities. Detecting pesticides within insects themselves serves multiple purposes, including verifying a pesticide’s effectiveness against both target and non-target insects, acting as a bioindicator to ascertain the actual amount of pesticide reaching insects, and shedding light on how different insect groups respond to pesticide applications in crops. While these assessments are more commonly conducted on foods intended for human consumption due to methodological considerations, it is noteworthy to emphasize that there exists a specific number of studies directly measuring the detection of pesticide levels in insects [48]. Therefore, this approach presents several challenges, including determining the detectable concentration of pesticides within an insect’s body and establishing the required sample size for effective detection.
The results obtained through the SMPE extraction method provide a significant contribution to compliance with global regulations regarding pesticide prohibition and restriction, especially in middle- and low-income countries [3]. The accurate detection and quantification of pesticide residues, such as chlorpyrifos, is essential to ensuring food safety, protecting human health, and preserving the environment. It is recognized as a relevant proposition to reduce the health risk index due to direct and/or indirect exposure to pesticides [2].
In particular, the capability of the SMPE method to detect and quantify pesticide residues, as demonstrated in previous studies [32], could support the efforts of the Chilean Agricultural and Livestock Service (SAG) in monitoring and regulating the presence of chlorpyrifos and other banned pesticides in agricultural products and the environment [30,31]. Moreover, the potential to employ more efficient and sensitive extraction methods, like SMPE, could streamline the processes of detection and analysis, allowing for a timelier and more precise response to non-compliance situations [13].
On the other hand, these results have significant implications for both agricultural practices and environmental health. The detection of multiple pesticide residues indicates a potential challenge in the management of pest control strategies [26] while simultaneously minimizing the exposure of non-target species [2,30]. The prevalence of chlorpyrifos residues in insects raises concerns about the possible transfer of these chemicals through the food chain, which could impact both human and ecological health [20,22,23,27].
It is also relevant to underscore the significance of the SMPE method due to its capacity to maintain sample integrity, a feature that sharply contrasts with the QuEChERS method, which frequently leads to sample degradation. The preservation of sample integrity assumes paramount importance, particularly when dealing with entomological specimens, as it facilitates the replication of methodologies and supports a range of taxonomic and ecological investigations. By preserving the structural integrity of insects, researchers can accurately identify species, assess ecological interactions, and investigate the details of their biology, thereby contributing substantially to the advancement of our understanding of the natural world.
In summary, the findings of this study have the potential to significantly contribute to the implementation and enforcement of regulations for the prohibition and restriction of pesticide use on a global scale. Specifically, in the case of Chile, they could enhance the capacity of the SAG to oversee exempt regulation 5810 [31]. This technological proposal offers a new alternative to ensure proper pesticide management and to safeguard the health and well-being of communities and the environment.
5. Conclusions
Based on the obtained results, we can infer that the SPME technique exhibits a greater extraction capacity according to the outlined procedures. It is noteworthy that the SPME technique holds a distinct advantage due to its lack of sample treatment procedures, a step required in micro-QuEChERS, which necessitates a processing time of 2 to 3 h. The utilization of automated SPME extraction leads to a reduction in instrumental error associated with sample manipulation. Additionally, it allows for the potential reuse of the sample for morphological analyses and insect characterization, if necessary, as the applied heating temperature is insufficient to generate significant structural alterations in the visible morphology of the insect. This preservation not only facilitates method replication but also permits future taxonomic and ecological investigations regarding these specimens.
The identification of pesticide residues in insect samples from lettuce crops underscores the complexity of pesticide exposure in agricultural systems. The significant presence of chlorpyrifos, along with its distribution across various concentration ranges, as revealed by the SPME method, emphasizes the ongoing need for the vigilant monitoring and management of pesticide usage to mitigate its ecological and human health repercussions.
Author Contributions
Conceptualization, C.V., C.M.S.-N., C.S. and R.O.A.; methodology, C.V. and R.O.A.; formal analysis, C.V.; investigation, C.V., C.M.S.-N., C.S., M.T.M.-Q., M.M. and R.O.A.; resources, C.V. and R.O.A.; data curation, C.V., M.M. and R.O.A.; writing—original draft preparation, C.V., M.T.M.-Q. and R.O.A.; writing—review and editing, C.V., C.M.S.-N., C.S., M.T.M.-Q. and R.O.A.; project administration, R.O.A.; funding acquisition, R.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “Agencia Nacional de Investigación y Desarrollo (ANID)”, Chile, through the project “FONDECYT Iniciación” n° 11200014; the “Subsecretaría de Agricultura”, Chile, and through the project “Programa de reducción de uso y riesgo de plaguicidas en la producción comercial de hortalizas para la pequeña y mediana agricultura”, código INIA n° 502453-70.
Data Availability Statement
The data used in this research are not available for ethical reasons; any requests for data availability should be directed to the author via correspondence.
Acknowledgments
The authors express their gratitude to Carlos Astudillo Oreste, Alejandro Andrés Layana Salinas, Monika Victoria Maltés Ruiz, and Anibal Alberto Valencia Venegas for their contributions to various field activities related to this investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and Soil Invertebrates: A Hazard Assessment. Front. Environ. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H.M.; Black, A.J.; Marks, G.B.; McBratney, A. The pesticide health risk index—An application to the world’s countries. Sci. Total Environ. 2021, 801, 149731. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Lerro, C.C.; Koutros, S.; Andreotti, G.; Friesen, M.C.; Alavanja, M.C.; Blair, A.; Hoppin, J.A.; Sandler, D.P.; Lubin, J.H.; Ma, X.; et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2015, 72, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Mrema, E.J.; Rubino, F.M.; Brambilla, G.; Moretto, A.; Tsatsakis, A.M.; Colosio, C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013, 307, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Wolejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A.; Pietruszyńska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef]
- Zúñiga-Venegas, L.A.; Hyland, C.; Muñoz-Quezada, M.T.; Quirós-Alcalá, L.; Butinof, M.; Buralli, R.; Cardenas, A.; Fernandez, R.A.; Foerster, C.; Gouveia, N.; et al. Health Effects of Pesticide Exposure in Latin American and the Caribbean Populations: A Scoping Review. Environ. Health Perspect. 2022, 130, 96002. [Google Scholar] [CrossRef]
- Mahdjoub, H.; Blanckenhorn, W.U.; Lüpold, S.; Roy, J.; Gourgoulianni, N.; Khelifa, R. Fitness consequences of the combined effects of veterinary and agricultural pesticides on a non-target insect. Chemosphere 2020, 250, 126271. [Google Scholar] [CrossRef]
- Bellamy, A.S.; Svensson, O.; van Den Brink, P.J.; Gunnarsson, J.; Tedengren, M. Insect community composition and functional roles along a tropical agricultural production gradient. Environ. Sci. Pollut. Res. 2018, 25, 13426–13438. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Dar, M.A.; Kaushik, G.; Villarreal-Chiu, J.F. Pollution status and bioremediation of chlorpyrifos in environmental matrices by the application of bacterial communities: A review. J. Environ. Manag. 2019, 239, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Phys. 2022, 185, 105138. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, A.R.; Harshiny, M.; Gummadi, S.N. Chlorpyrifos in environment and food: A critical review of detection methods and degradation pathways. Environ. Sci. Process Impacts 2021, 23, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, M.; Gołębiowski, M. SPME-GC/MS Analysis of Volatile Compounds Contained in the Insect Larvae of Tenebrio molitor and Leptinotarsa decemlineata before and after Using Insecticides. Chem. Biodivers. 2020, 17, e1900743. [Google Scholar] [CrossRef] [PubMed]
- Servicio Agrícola Ganadero-SAG. Sales Declaration of Pesticides for Agricultural Use 2019. 2019. Available online: https://www.sag.gob.cl/content/declaracion-de-ventas-de-plaguicidas-de-uso-agricola-2019 (accessed on 20 July 2023).
- Climent, M.J.; Coscollà, C.; López, A.; Barra, R.; Urrutia, R. Legacy and current-use pesticides (CUPs) in the atmosphere of a rural area in central Chile, using passive air samplers. Sci. Total Environ. 2019, 662, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Cortés, S.; Pozo, K.; Llanos, Y.; Martinez, N.; Foerster, C.; Leiva, C.; Ustáriz, J.; Přibylová, P.; Klánová, J.; Jorquera, H. First measurement of human exposure to current use pesticides (CUPs) in the atmosphere of central Chile: The case study of Mauco cohort. Atmos. Pollut. Res. 2020, 11, 776–784. [Google Scholar] [CrossRef]
- Pozo, K.; Llanos, Y.; Estellano, V.; Cortés, S.; Jorquera, H.; Gerli, L.; Pozo, K.; Encina, F.; Palma, R.; Focardi, F. Occurrence of chlorpyrifos in the atmosphere of the Araucanía Region in Chile using polyurethane foam-based passive air samplers. Atmos. Pollut. Res. 2016, 7, 706–710. [Google Scholar] [CrossRef]
- Climent, M.J.; Herrero-Hernández, E.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Pedreros, P.; Urrutia, R. Residues of pesticides and some metabolites in dissolved and particulate phase in surface stream water of Cachapoal River basin, central Chile. Enviro. Pollut. 2019, 251, 90–101. [Google Scholar] [CrossRef]
- Balsebre, A.; Báez, M.E.; Martínez, J.; Fuentes, E. Matrix solid-phase dispersion associated to gas chromatography for the assessment in honey bee of a group of pesticides of concern in the apicultural field. J. Chromatogr. 2018, 1567, 47–54. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Grandon, S.; Sepúlveda, G.; Diaz, R.; Yuri, J.A.; Torres, C. Pesticide residues quantification in frozen fruit and vegetables in Chilean domestic market using QuEChERS extraction with ultra-high-performance liquid chromatography electrospray ionization Orbitrap mass spectrometry. Food Chem. 2019, 295, 64–71. [Google Scholar] [CrossRef]
- Elgueta, S.; Moyano, S.; Sepúlveda, P.; Quiroz, C.; Correa, A. Pesticide residues in leafy vegetables and human health risk assessment in North Central agricultural areas of Chile. Food Addit. Contam. Part B Surveill. 2017, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, S.; Fuentes, M.; Valenzuela, M.; Zhao, G.; Liu, S.; Lu, H.; Correa, A. Pesticide residues in ready-to-eat leafy vegetables from markets of Santiago, Chile, and consumer’s risk. Food Addit. Contam. Part B Surveill. 2019, 12, 259–267. [Google Scholar] [CrossRef]
- Elgueta, S.; Valenzuela, M.; Fuentes, M.; Ulloa, P.E.; Ramos, C.; Correa, A.; Molinett, S. Analysis of Multi-Pesticide Residues and Dietary Risk Assessment in Fresh Tomatoes (Lycopersicum esculentum) from Local Supermarkets of the Metropolitan Region, Chile. Toxics 2021, 9, 249. [Google Scholar] [CrossRef]
- Fuentes, E.; Báez, M.E.; Díaz, J. Survey of organophosphorus pesticide residues in virgin olive oils produced in Chile. Food Addit. Contam. Part B Surveill. 2010, 3, 101–107. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.; Iglesias, V.; Muñoz, M.P. Exposure pathways to pesticides in schoolchildren in the Province of Talca, Chile. Gac. Sanit. 2014, 28, 190–195. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Burgos-Díaz, C.; Soto-Cerda, B.; Barahona, T.; Anguita-Barrales, F.; Mosi-Roa, Y. Assessment of the Nutritional Value of Traditional Vegetables from Southern Chile as Potential Sources of Natural Ingredients. Plant Foods Hum. Nutr. 2021, 76, 523–532. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.; Bradman, A.; Steenland, K.; Zúñiga, L.; Calafat, A.M.; Ospina, M.; Iglesias, V.; Muñoz, M.P.; Buralli, R.J.; et al. An educational intervention on the risk perception of pesticides exposure and organophosphate metabolites urinary concentrations in rural schoolchildren in Maule Region, Chile. Environ. Res. 2019, 176, 108554. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Gutiérrez-Jara, J.P.; Buralli, R.J.; Zúñiga-Venegas, L.; Muñoz, M.P.; Ponce, K.V.; Iglesias, V. Longitudinal exposure to pyrethroids (3-PBA and trans-DCCA) and 2,4-D herbicide in rural schoolchildren of Maule region, Chile. Sci. Total Environ. 2020, 749, 141512. [Google Scholar] [CrossRef]
- Ministerio de Agricultura. Resolution 4245 Exempt. Cancels the Current Authorizations of Pesticides Formulated Based on Methamidophos and Prohibits Pesticides Based on Azinphos Methyl, Carbofuran and Methamidophos as of the Date Indicated. Resolución 4245 Exenta 2019. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1132625 (accessed on 20 July 2023).
- Ministerio de Agricultura. Resolution 5810 Exempt. Bans pesticides Based on Chlorpyrifos (Chlorpyrifos-Ethyl), Chlorpyrifos-Methyl, Paraquat and Methomyl Dichloride and Cancels Current Authorizations for Pesticides that Contain Them. Resolución 5810 Exenta 2022. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1182686&idParte=0 (accessed on 20 July 2023).
- Milhome, M.A.L.; Sousa, P.L.R.; De Keukeleire, D.; Nascimento, R.F. Multiresidue methods for determination of pesticides using SPME and SPE followed by GC-NPD system: A comparative study. J. Braz. Chem. Soc. 2011, 22, 2048–2055. [Google Scholar] [CrossRef]
- Correa, A.B.; Elgueta, P.S.; Sepúlveda, R.P.; Quiroz, E.C. Analysis of primary information related to the production of leafy vegetables in Chile (lettuce, spinach and chard). INIA Bull. 2017, 343, 1–70. Available online: https://biblioteca.inia.cl/handle/20.500.14001/6569 (accessed on 20 July 2023).
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006; p. 450. [Google Scholar] [CrossRef]
- Brown, G.R.; Matthews, I.M. A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol. Evol. 2016, 6, 3953–3964. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, S.M.; Samways, M.J. Optimising colored pan traps to survey flower visiting insects. J. Insect Conserv. 2012, 16, 345–354. [Google Scholar] [CrossRef]
- Francese, J.A.; Crook, D.J.; Fraser, I.; Lance, D.R.; Sawyer, A.J.; Mastro, V.C. Optimization of trap color for emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2010, 103, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Parisio, M.S.; Gould, J.R.; Vandenberg, J.D.; Bauer, L.S.; Fierke, M.K. Evaluation of recovery and monitoring methods for parasitoids released against emerald ash borer. Biol. Control 2017, 106, 45–53. [Google Scholar] [CrossRef]
- Wilson, J.S.; Griswold, T.; Messinger, O.J. Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: Are pan traps sufficient? J. Kans. Entomol. Soc. 2008, 81, 288–300. [Google Scholar] [CrossRef]
- Stoeckelhuber, M.; Müller, C.; Vetter, F.; Mingo, V.; Lötters, S.; Wagner, N.; Bracher, F. Determinatioíof Pesticides Adsorbed on Arthropods and Gastropods by a Micro-QuEChERS Approach and GC–MS/MS. Chromatographia 2017, 80, 825–829. [Google Scholar] [CrossRef]
- Fernandes, M.E.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as bioindicator: A hidden gem for environmental monitoring. Front. Environ. Sci. 2023, 11, 273. [Google Scholar] [CrossRef]
- Tison, L.; Franc, C.; Burkart, L.; Jactel, H.; Monceau, K.; de Revel, G.; Thiéry, D. Pesticide contamination in an intensive insect predator of honey bees. Environm. Int. 2023, 176, 107975. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- Uhl, P.; Brühl, C.A. The impact of pesticides on flower-visiting insects: A review with regard to European risk assessment. Environ. Toxicol. Chem. 2019, 38, 2355–2370. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.; Yaqub, G.; Ayub, M.; Naeem, M. Determination of malathion, chlorpyrifos, λ-cyhalothrin and arsenic in rice. Food Sci. Technol. 2020, 41, 461–466. [Google Scholar] [CrossRef]
- Dallaire, F.; Cusson, M. Comparative toxicity of the nonsteroidal ecdysone agonists tebufenozide and methoxyfenozide to early and late larval instars of the whitemarked tussock moth, Orgyia leucostigma. J. Entomol. Soc. Ont. 2017, 148, 6–12. [Google Scholar]
- Poma, G.; Yin, S.; Folarin, B.T.; Schönleben, A.M.; Bombeke, J.; Altamirano, J.C.; Ssepuuya, G.; Nakimbugwe, D.; Oluseyi, T.; Covaci, A. First insights into the occurrence of pesticide residues in edible insects from sub-Saharan African countries. J. Expo. Sci. Environ. Epidemiol. 2022, 1, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).