Weed Species’ Diversity and Composition as Shaped by the Interaction of Management, Site, and Soil Variables in Olive Groves of Southern Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Data Collection
- (a)
- Untreated olive groves or no treatment (used as the control in the current study)
- (b)
- Mowing: This involved 1–5 passes (average: 1.26; median: 1) from March to July (primarily in May) to trim weeds to a height of approximately 5 cm above the ground.
- (c)
- Rotary tiller: Farmers conducted 1–2 passes (with an average of 1.2 and a median of 1) from March to July, mainly in April, at a depth of ≤10 cm.
- (d)
- Animal grazing: Sheep and goats were used for grazing.
- (e)
- Herbicide application (glyphosate): This method involved 1–2 sprays (average: 1.2; median: 1) with glyphosate. The application rate of glyphosate varied among the areas due to differences in the targeted weeds and local practicalities [35]. In Chora and Peza, glyphosate was applied in the surveyed sites after the onset of rainfall between February and early April (primarily in March), while in Meramvello province, there was no use of herbicides for weed control.
2.2. Data Analysis
3. Results
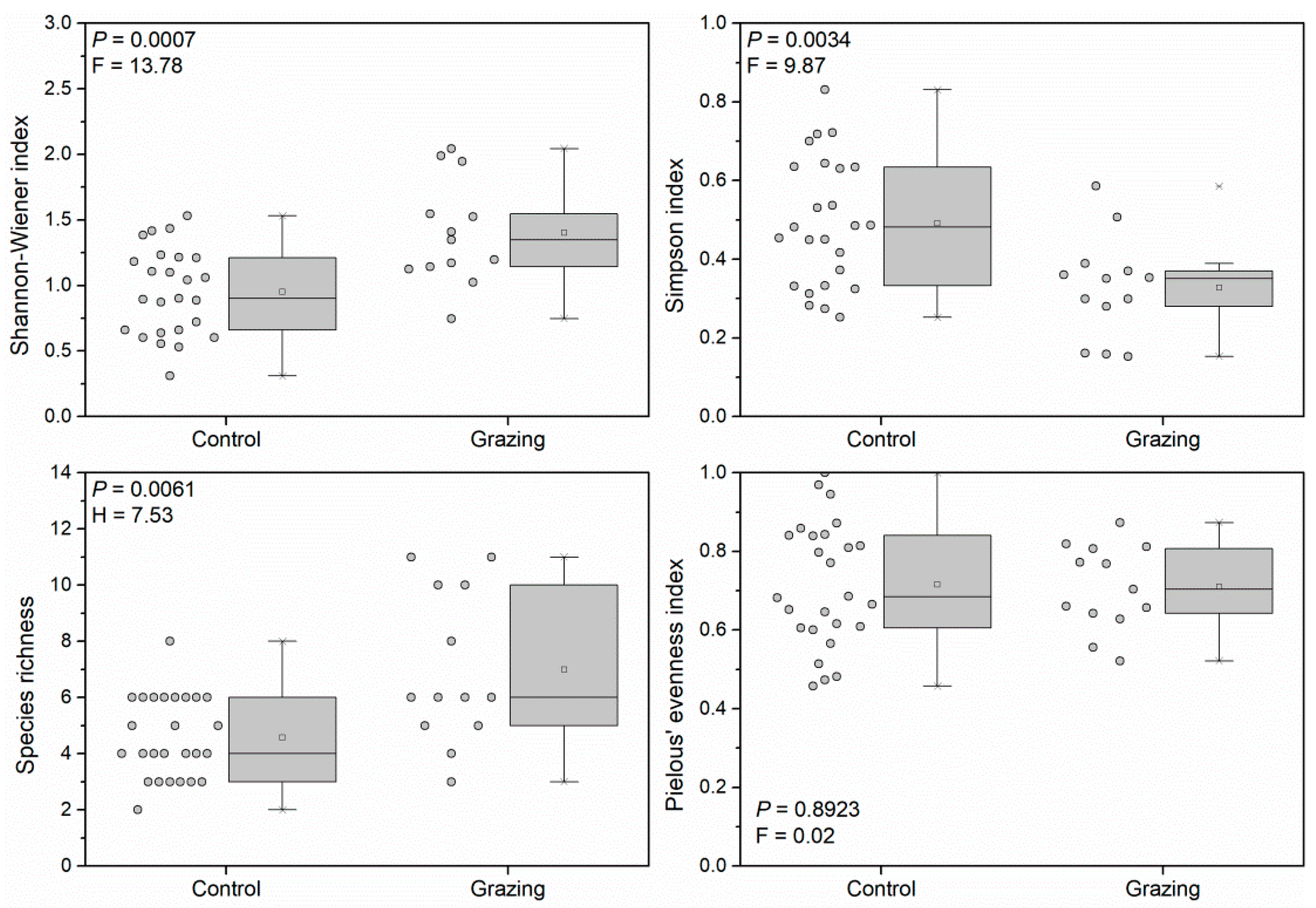
3.1. Responses of Plant Diversity to Weed Management Practices
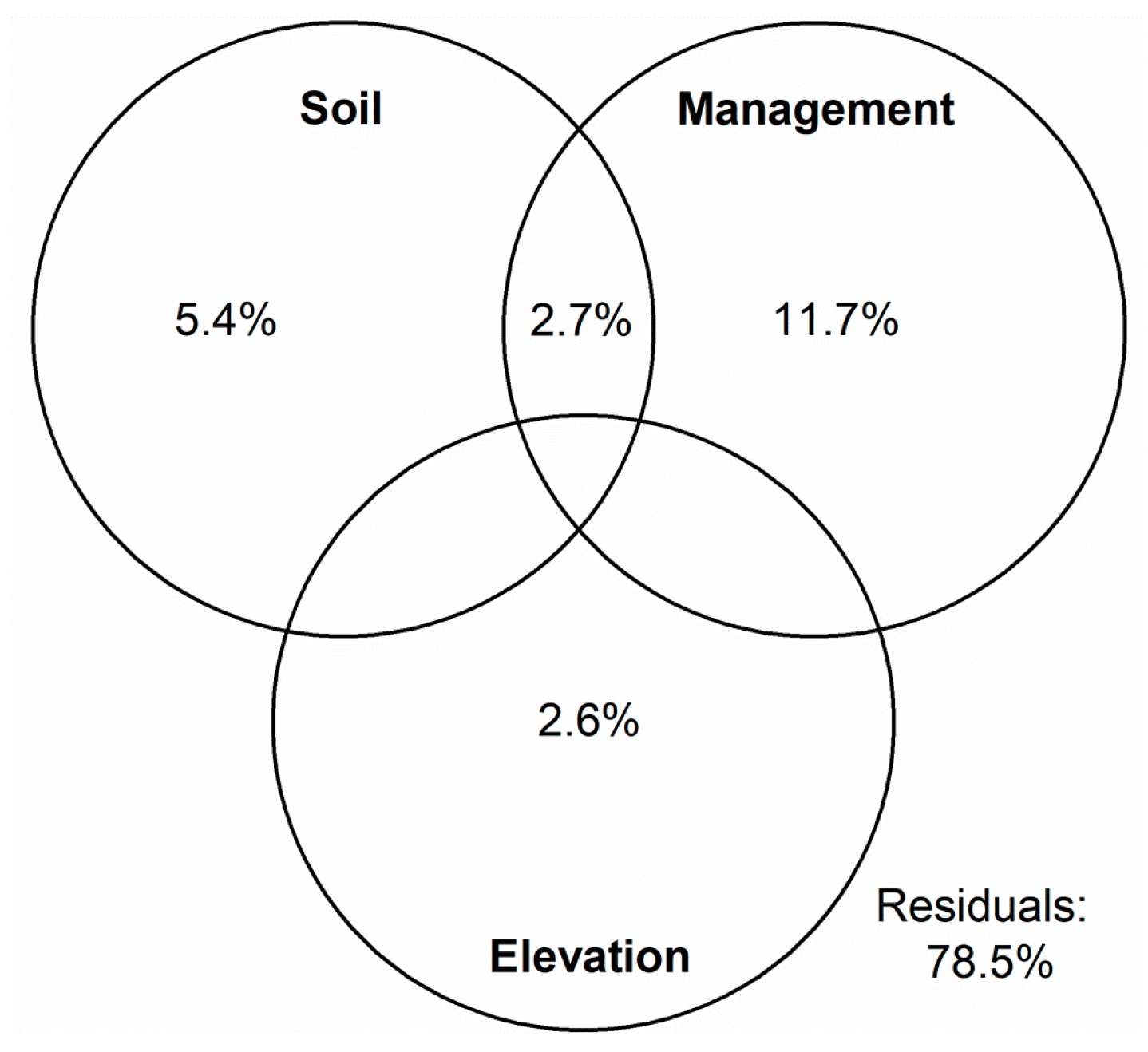
3.2. Factors Affecting the Weed Community’s Composition
3.3. Weed Species’ Composition and Plant Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumacher, M.; Dieterich, M.; Gerhards, R. Effects of weed biodiversity on the ecosystem service of weed seed predation along a farming intensity gradient. Glob. Ecol. Conserv. 2020, 24, e01316. [Google Scholar] [CrossRef]
- Gerowitt, B.; Bàrberi, P.; Darmency, H.; Petit, S.; Storkey, J.; Westerman, P. Weeds and Biodiversity. In Weed Research; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 115–147. [Google Scholar]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Loumou, A.; Giourga, C. Olive groves: “The life and identity of the Mediterranean”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Fernández-Zamudio, M.A.; De Miguel, M.D. Sustainable management for woody crops in Mediterranean dry-lands. Span. J. Agric. Res. 2006, 4, 111. [Google Scholar] [CrossRef]
- Allen, H.D.; Randall, R.E.; Amable, G.S.; Devereux, B.J. The impact of changing olive cultivation practices on the ground flora of olive groves in the Messara and Psiloritis regions, Crete, Greece. Land Degrad. Dev. 2006, 17, 249–273. [Google Scholar] [CrossRef]
- Morgado, R.; Ribeiro, P.F.; Santos, J.L.; Rego, F.; Beja, P.; Moreira, F. Drivers of irrigated olive grove expansion in Mediterranean landscapes and associated biodiversity impacts. Landsc. Urban Plan. 2022, 225, 104429. [Google Scholar] [CrossRef]
- Huqi, B.; Dhima, K.; Vasilakoglou, I.; Keco, R.; Salaku, F. Weed flora and weed management in established olive groves in Albania. Weed Biol. Manag. 2009, 9, 276–285. [Google Scholar] [CrossRef]
- Nagy, K.; Lengyel, A.; Kovács, A.; Türei, D.; Csergő, A.M.; Pinke, G. Weed species composition of small-scale farmlands bears a strong crop-related and environmental signature. Weed Res. 2018, 58, 46–56. [Google Scholar] [CrossRef]
- Bopp, M.-C.; Kazakou, E.; Metay, A.; Fried, G. Relative importance of region, seasonality and weed management practice effects on the functional structure of weed communities in French vineyards. Agric. Ecosyst. Environ. 2022, 330, 107892. [Google Scholar] [CrossRef]
- Solomou, A.D.; Sfougaris, A.I.; Kalburtji, K.L.; Nanos, G.D. Effects of Organic Farming on Winter Plant Composition, Cover and Diversity in Olive Grove Ecosystems in Central Greece. Commun. Soil Sci. Plant Anal. 2013, 44, 312–319. [Google Scholar] [CrossRef]
- Simoes, M.P.; Belo, A.F.; Pinto-Cruz, C.; Pinheiro, A.C. Natural vegetation management to conserve biodiversity and soil water in olive orchards. Span. J. Agric. Res. 2014, 12, 633. [Google Scholar] [CrossRef]
- Solomou, A.D.; Sfougaris, A. Contribution of Agro-Environmental Factors to Yield and Plant Diversity of Olive Grove Ecosystems (Olea europaea L.) in the Mediterranean Landscape. Agronomy 2021, 11, 161. [Google Scholar] [CrossRef]
- Terzi, M.; Barca, E.; Cazzato, E.; D’amico, F.S.; Lasorella, C.; Fracchiolla, M. Effects of Weed Control Practices on Plant Diversity in a Homogenous Olive-Dominated Landscape (South-East of Italy). Plants 2021, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Fried, G.; Cordeau, S.; Metay, A.; Kazakou, E. Relative importance of environmental factors and farming practices in shaping weed communities structure and composition in French vineyards. Agric. Ecosyst. Environ. 2019, 275, 1–13. [Google Scholar] [CrossRef]
- Fried, G.; Blanchet, C.; Cazenave, L.; Bopp, M.-C.; Kazakou, E.; Metay, A.; Christen, M.; Alard, D.; Cordeau, S. Consistent response of weeds according to Grime’s CSR strategies along disturbance and resource gradients in Bordeaux vineyards. Weed Res. 2022, 62, 347–359. [Google Scholar] [CrossRef]
- Popolizio, S.; Vivaldi, G.A.; Camposeo, S. Different Weed Managements Influence the Seasonal Floristic Composition in a Super High-Density Olive Orchard. Plants 2023, 12, 2921. [Google Scholar] [CrossRef] [PubMed]
- Carpio, A.J.; Lora, A.; Martin-Consuegra, E.; Sanchez-Cuesta, R.; Tortosa, F.S.; Castro, J. The influence of the soil management systems on aboveground and seed bank weed communities in olive orchards. Weed Biol. Manag. 2020, 20, 12–23. [Google Scholar] [CrossRef]
- Pereira, A.J.; Porto, M.; Correia, O.; Beja, P. Traditional ploughing is critical to the conservation of threatened plants in Mediterranean olive groves. Agric. Ecosyst. Environ. 2024, 359, 108775. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Castro, J.; Alonso-Prados, E.; Alonso-Prados, J.L.; García-Baudín, J.M.; Talavera, M.; Durán-Zuazo, V.H. Tillage and herbicide decrease soil biodiversity in olive orchards. Agron. Sustain. Dev. 2015, 35, 691–700. [Google Scholar] [CrossRef]
- Lisek, J. Diversity of Summer Weed Communities in Response to Different Plum Orchard Floor Management in-Row. Agronomy 2023, 13, 1421. [Google Scholar] [CrossRef]
- De Mol, F.; von Redwitz, C.; Gerowitt, B. Weed species composition of maize fields in Germany is influenced by site and crop sequence. Weed Res. 2015, 55, 574–585. [Google Scholar] [CrossRef]
- Pinke, G.; Blazsek, K.; Magyar, L.; Nagy, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed species composition of conventional soyabean crops in Hungary is determined by environmental, cultural, weed management and site variables. Weed Res. 2016, 56, 470–481. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. When herbicides don’t really matter: Weed species composition of oil pumpkin (Cucurbita pepo L.) fields in Hungary. Crop. Prot. 2018, 110, 236–244. [Google Scholar] [CrossRef]
- Emberger, F. Bioclomatic Map of the Mediterranean Zone; UNESCO-FAO: Paris, France, 1963. [Google Scholar]
- Karyotis, T.; Arampatzis, G.; Panagopoulos, A.; Hatzigiannakis, E.; Tziritis, E.; Karyoti, K.; Vrouchakis, J. Nutrients, trace elements and water deficit in Greek soils cultivated with olive trees. EQA Int. J. Environ. Qual. 2014, 13, 9–20. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle Size Analysis by Hydrometer: A Simplified Method for Routine Textural Analysis and a Sensitivity Test of Measurement Parameters. Soil Sci. Soc. Am. J. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Allison, L.E.; Moodie, C.D. Carbonate. In Methods of Soil Analysis, Part 2; Black, C.A., Angle, J.S., Weaver, R.W., Eds.; Monograph 9; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1379–1400. [Google Scholar]
- Thomas, G. Exchangeable Cations. In Methods of Soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Book Series 9; American Society of Agronomy: Madison, WI, USA, 1982; pp. 159–164. [Google Scholar]
- Rhoades, J.D. Cation Exchange Capacity. In Methods of Soil Analysis; Agronomy Monographs; Wiley: Hoboken, NJ, USA, 2015; pp. 149–157. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3; SSSA Book Series; Wiley: Hoboken, NJ, USA, 2018; pp. 961–1010. [Google Scholar]
- Olsen, K.M.; Gould, S.A.; Patience, J.F. A comparison of the release of phosphorus by a phytase enzyme in pigs fed diets deficient or adequate in phosphorus content. J. Anim. Sci. 2021, 99, skab001. [Google Scholar] [CrossRef]
- Keren, R. Boron: Soil Contaminant. In Managing Global Resources and Universal Processes; CRC Press: Boca Raton, FL, USA, 2020; pp. 175–180. [Google Scholar]
- Lindsay, W.; Norvell, W. Development of a DTPA Soil Test Zinc, Iron, Manganese and Copper. Soil Sci. Soc. Am. J. 1978, 4, 421–428. [Google Scholar] [CrossRef]
- Karanasios, E.; Karasali, H.; Marousopoulou, A.; Akrivou, A.; Markellou, E. Monitoring of glyphosate and AMPA in soil samples from two olive cultivation areas in Greece: Aspects related to spray operators activities. Environ. Monit. Assess. 2018, 190, 361. [Google Scholar] [CrossRef] [PubMed]
- Travlos, I.S.; Cheimona, N.; Roussis, I.; Bilalis, D.J. Weed-Species Abundance and Diversity Indices in Relation to Tillage Systems and Fertilization. Front. Environ. Sci. 2018, 6, 11. [Google Scholar] [CrossRef]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Prentice, I.C. A Theory of Gradient Analysis. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1988; pp. 271–317. [Google Scholar]
- Kawanza, S.; Ndagurwa, H.G.T.; Chateya, R.J.; Muvengwi, J. Jointed cactus Opuntia aurantiaca modifies soil nutrient concentrations, grass species assemblage and biomass yield in a savanna rangeland. Acta Oecol. 2019, 101, 103477. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Cimalová, S.; Kropáč, Z.; Otýpková, Z.; Pyšek, P.; Tichý, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the Spatial Component of Ecological Variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Storkey, J.; Neve, P. What good is weed diversity? Weed Res. 2018, 58, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Fracchiolla, M.; Terzi, M.; Frabboni, L.; Caramia, D.; Lasorella, C.; De Giorgio, D.; Montemurro, P.; Cazzato, E. Influence of different soil management practices on ground-flora vegetation in an almond orchard. Renew. Agric. Food Syst. 2016, 31, 300–308. [Google Scholar] [CrossRef]
- MacLaren, C.; Bennett, J.; Dehnen-Schmutz, K. Management practices influence the competitive potential of weed communities and their value to biodiversity in South African vineyards. Weed Res. 2019, 59, 93–106. [Google Scholar] [CrossRef]
- Andert, S.; de Mol, F.; Koning, L.; Gerowitt, B. Weed response in winter wheat fields on a gradient of glyphosate use in the recent past. Agric. Ecosyst. Environ. 2022, 333, 107977. [Google Scholar] [CrossRef]
- Vilà, M.; Tessier, M.; Suehs, C.M.; Brundu, G.; Carta, L.; Galanidis, A.; Lambdon, P.; Manca, M.; Médail, F.; Moragues, E.; et al. Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J. Biogeogr. 2006, 33, 853–861. [Google Scholar] [CrossRef]
- Petsikos, C.; Dalias, P.; Troumbis, A.Y. Effects of Oxalis pes-caprae L. invasion in olive groves. Agric. Ecosyst. Environ. 2007, 120, 325–329. [Google Scholar] [CrossRef]
- Vilà, M.; Gimeno, I. Does invasion by an alien plant species affect the soil seed bank? J. Veg. Sci. 2007, 18, 423–430. [Google Scholar] [CrossRef]
- Lazzaro, L.; Ferretti, G.; Bianchi, E.; Benesperi, R. Treatment by glyphosate-based herbicide allowed recovering native species after Oxalis pes-caprae L. invasion: Indications from a Mediterranean island. Plant Biosyst. 2019, 153, 651–659. [Google Scholar] [CrossRef]
- Thorogood, C.J.; Rumsey, F.J.; Hiscock, S.J. Seed viability determination in parasitic broomrapes (Orobanche and Phelipanche) using fluorescein diacetate staining. Weed Res. 2009, 49, 461–468. [Google Scholar] [CrossRef]
- Koning, L.A.; de Mol, F.; Gerowitt, B. Effects of management by glyphosate or tillage on the weed vegetation in a field experiment. Soil Tillage Res. 2019, 186, 79–86. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. 2022. Available online: http://www.weedscience.org (accessed on 25 October 2022).
- Travlos, I.; Lysandrou, M.; Apostolidis, V. Efficacy of the herbicide GF-2581 (penoxsulam + florasulam) against broadleaf weeds in olives. Plant Soil Environ. 2014, 60, 574–579. [Google Scholar] [CrossRef]
- Juárez-Escario, A.; Conesa, J.A.; Solé-Senan, X.O. Management as a driver of functional patterns and alien species prominence in weed communities of irrigated orchards in Mediterranean areas. Agric. Ecosyst. Environ. 2017, 249, 247–255. [Google Scholar] [CrossRef]
- Iftikhar, M.; Ahmad, I.; Hameed, M.; Fatima, S.; Ahmad, F.; Ashraf, M.; Nazish, Z.; Ahmad, M.S.A.; Muneeb, A. Structural and functional responses in sun spurge (Euphorbia helioscopia L.) against post-emergence herbicides in wheat (Triticum aestivum L.). Weed Res. 2021, 61, 126–136. [Google Scholar] [CrossRef]
- Gago, P.; Cabaleiro, C.; García, J. Preliminary study of the effect of soil management systems on the adventitious flora of a vineyard in northwestern Spain. Crop. Prot. 2007, 26, 584–591. [Google Scholar] [CrossRef]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices, 4th ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Zimdahl, R.L. Fundamentals of Weed Science, 3rd ed.; Elsevier: New York, NY, USA, 2007. [Google Scholar]
- Hussain, M.; Farooq, S.; Merfield, C.; Jabran, K. Mechanical weed control. In Non-Chemical Weed Control; Jabran, K., Chauhan, B.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–155. [Google Scholar] [CrossRef]
- Huaranca, J.C.; Novaro, A.J.; Valdivia, C.E. Effects of livestock grazing on biodiversity: A meta-analysis on three trophic levels. J. Nat. Conserv. 2022, 66, 126126. [Google Scholar] [CrossRef]
- Woldu, Z.; Saleem, M.A.M. Grazing induced biodiversity in the highland ecozone of East Africa. Agric. Ecosyst. Environ. 2000, 79, 43–52. [Google Scholar] [CrossRef]
- Schuster, M.Z.; Pelissari, A.; de Moraes, A.; Harrison, S.K.; Sulc, R.M.; Lustosa, S.B.C.; Anghinoni, I.; Carvalho, P.C.F. Grazing intensities affect weed seedling emergence and the seed bank in an integrated crop–livestock system. Agric. Ecosyst. Environ. 2016, 232, 232–239. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; NOY-Meir, I.; et al. Plant trait responses to grazing? a global synthesis. Glob. Change Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Baumont, R.; Prache, S.; Meuret, M.; Morand-Fehr, P. How forage characteristics influence behaviour and intake in small ruminants: A review. Livest. Prod. Sci. 2000, 64, 15–28. [Google Scholar] [CrossRef]
- Egea, A.V.; Allegretti, L.; Lama, S.P.; Grilli, D.; Sartor, C.; Fucili, M.; Guevara, J.; Passera, C. Selective behavior of Creole goats in response to the functional heterogeneity of native forage species in the central Monte desert, Argentina. Small Rumin. Res. 2014, 120, 90–99. [Google Scholar] [CrossRef]
- Nowak, A.; Nowak, S.; Nobis, M.; Nobis, A. Crop type and altitude are the main drivers of species composition of arable weed vegetation in Tajikistan. Weed Res. 2015, 55, 525–536. [Google Scholar] [CrossRef]
- Adeux, G.; Yvoz, S.; Biju-Duval, L.; Cadet, E.; Farcy, P.; Fried, G.; Guillemin, J.-P.; Munier-Jolain, N.; Petit, S.; Cordeau, S.; et al. Cropping system diversification does not always beget weed diversity. Eur. J. Agron. 2022, 133, 126438. [Google Scholar] [CrossRef]
- Pinke, G.; Pál, R.W.; Tóth, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed vegetation of poppy (Papaver somniferum) fields in Hungary: Effects of management and environmental factors on species composition. Weed Res. 2011, 51, 621–630. [Google Scholar] [CrossRef]
- Ulber, L.; Steinmann, H.-H.; Klimek, S.; Isselstein, J. An on-farm approach to investigate the impact of diversified crop rotations on weed species richness and composition in winter wheat. Weed Res. 2009, 49, 534–543. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Brye, K.R.; Skinner, V., Jr.; Mauromoustakos, A. Relationships between soil properties and the occurrence of the most agronomically important weed species in the field margins of eastern Arkansas—Implications for weed management in field margins. Weed Res. 2017, 57, 159–171. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, Y.; An, Y.; Wang, Z.; Li, X.; Gao, H.; Wei, G.; Jiao, S. Contrasting patterns and drivers of soil micronutrient availability in paddy and maize fields of eastern China. Geoderma 2023, 431, 116342. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2009, 1–2, 100007. [Google Scholar] [CrossRef]
- Available online: https://dzumenvis.nic.in/Microbes%20and%20Metals%20Interaction/pdf/Soil%20acidification%20and%20liming.pdf (accessed on 27 December 2023).
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Delgado, A. Factors determining Zn availability and uptake by plants in soils developed under Mediterranean climate. Geoderma 2020, 376, 114509. [Google Scholar] [CrossRef]
- Cimalová, Š.; Lososová, Z. Arable weed vegetation of the northeastern part of the Czech Republic: Effects of environmental factors on species composition. Plant Ecol. 2009, 203, 45–57. [Google Scholar] [CrossRef]
- Walter, A.M.; Christensen, S.; Simmelsgaard, S.E. Spatial correlation between weed species densities and soil properties. Weed Res. 2002, 42, 26–38. [Google Scholar] [CrossRef]




| Variable | Unit | Range | Mean | ||
|---|---|---|---|---|---|
| All Sampling Sites | Peza | Meramvello | Chora | ||
| Site | |||||
| Elevation | m | 3–568 | 372.30 | 208.00 | 184.00 |
| Management variables | |||||
| Irrigation (water) | - | Yes—no | |||
| Rotary tiller | - | Yes—no | |||
| Grazing | - | Yes—no | |||
| Without control 1 | - | Yes—no | |||
| Mowing | - | Yes—no | |||
| Chemical (herbicide: glyphosate) | - | Yes—no | |||
| Soil variables | |||||
| Sand 1 | % | 13.2–75.60 | 35.28 | 34.35 | 39.02 |
| Silt 1 | % | 10.4–50 | 34.43 | 30.93 | 36.45 |
| Clay 2 | % | 10–53.60 | 30.29 | 34.72 | 24.53 |
| CEC 1 | meq/100 gr | 5.71–36.41 | 19.48 | 19.06 | 16.03 |
| pH 1 | - | 4.9–7.90 | 7.70 | 7.23 | 7.36 |
| EC 1 | mS/cm | 0.25–2.99 | 0.76 | 0.94 | 0.53 |
| C 2 | % | 0.47–2.88 | 1.66 | 2.09 | 1.22 |
| Soil organic matter (SOM) 1 | % | 0.94–5.76 | 3.32 | 4.19 | 2.44 |
| CaCO3 1 | % | 0.05–88.00 | 46.37 | 3.12 | 26.88 |
| ESP 1 | % | 0.454–12.09 | 1.27 | 2.21 | 1.95 |
| NO3-N 1 | mg/kg | 3.03–81.9 | 18.41 | 21.22 | 13.12 |
| Ntot 1 | mg/kg | 650–4600 | 2537.78 | 2130.25 | 1681.53 |
| Na 1,3 | mg/kg | 19–460.00 | 35.28 | 34.35 | 39.02 |
| K 1,3 | mg/kg | 56–1350 | 348.83 | 494.68 | 155.28 |
| Ca 1,3 | mg/kg | 507–7618 | 3624.70 | 2904.11 | 3213.93 |
| Mg | mg/kg | 39–813 | 127.26 | 227.15 | 143.54 |
| P 1,3 | mg/kg | 1.15–82.62 | 14.29 | 18.79 | 12.74 |
| Cu 1,3 | mg/kg | 0.54–115.00 | 3.27 | 17.50 | 13.97 |
| Fe 1,3 | mg/kg | 3.35–135.60 | 7.73 | 34.14 | 18.53 |
| Zn 1,3 | mg/kg | 0.25–5.96 | 0.94 | 1.78 | 0.63 |
| B 1,3 | mg/kg | 0.02–3.87 | 1.07 | 1.16 | 0.51 |
| Mn 3 | mg/kg | 0.69–41.5 | 5.20 | 2.47 | 9.41 |
| Index | Parameter | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|---|
| Summary | |||||
| Eigenvalues | 0.106 | 0.033 | 0.025 | 0.019 | |
| Species–environment correlations | 0.712 | 0.608 | 0.557 | 0.529 | |
| Cumulative percentage of variance of species–environment relation | 49.1 | 64.4 | 76.0 | 85.1 | |
| Total variance (sum of all canonical eigenvalues) | 0.215 | ||||
| Correlation matrix | |||||
| Mg | −0.1127 | −0.2104 | 0.2556 | −0.1688 | |
| Mn | 0.505 | −0.1097 | 0.1454 | 0.1238 | |
| Irrigation | −0.1158 | 0.2104 | −0.0731 | −0.1669 | |
| Rotary tiller | −0.1109 | 0.0808 | −0.1981 | 0.3145 | |
| Grazing | −0.1625 | −0.2632 | −0.1536 | −0.2386 | |
| Mowing | 0.3509 | −0.2418 | −0.0065 | 0.2003 | |
| Chemical | 0.3224 | 0.4224 | 0.0471 | −0.1398 | |
| Elevation | −0.1054 | 0.1724 | 0.2193 | 0.3248 | |
| Marginal Effects | Conditional Effects | ||||
|---|---|---|---|---|---|
| Variable | Variation Explained | Variable | Variation Explained | p | F |
| Mn | 0.06 | Mn | 0.06 | 0.001 | 7.24 |
| SOM | 0.04 | Chemical | 0.04 | 0.001 | 4.83 |
| Chemical | 0.04 | Mowing | 0.03 | 0.003 | 3.54 |
| Mowing | 0.04 | Elevation | 0.02 | 0.001 | 3.2 |
| Grazing | 0.02 | Mg | 0.02 | 0.008 | 2.43 |
| Zn | 0.02 | Rotary tiller | 0.01 | 0.023 | 2.08 |
| EC | 0.02 | Grazing | 0.02 | 0.014 | 2.12 |
| B | 0.02 | Irrigation | 0.02 | 0.023 | 2.11 |
| Control | 0.02 | pH | 0.01 | 0.109 | 1.53 |
| K | 0.02 | Silt | 0.01 | 0.042 | 1.85 |
| Mg | 0.02 | Sand | 0.01 | 0.071 | 1.61 |
| Elevation | 0.02 | SOM | 0.01 | 0.177 | 1.34 |
| Irrigation | 0.02 | P | 0.01 | 0.122 | 1.51 |
| Rotary tiller | 0.02 | B | 0.01 | 0.178 | 1.33 |
| Sand | 0.02 | Control | 0.01 | 0.293 | 1.23 |
| Fe | 0.02 | EC | 0.01 | 0.197 | 1.34 |
| N | 0.01 | K | 0.01 | 0.314 | 1.12 |
| CEC | 0.01 | Na | 0 | 0.445 | 0.99 |
| pH | 0.01 | ESP | 0.02 | 0.098 | 1.49 |
| CaCO3 | 0.01 | Ca | 0 | 0.341 | 1.06 |
| Silt | 0.01 | N | 0.01 | 0.576 | 0.86 |
| Na | 0.01 | Zn | 0 | 0.582 | 0.88 |
| Ca | 0.01 | CaCO3 | 0.01 | 0.679 | 0.79 |
| P | 0.01 | CEC | 0.01 | 0.769 | 0.67 |
| Cu | 0.01 | NO3 | 0 | 0.82 | 0.66 |
| ESP | 0.01 | Cu | 0 | 0.897 | 0.53 |
| NO3 | 0.01 | Fe | 0.01 | 0.845 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahamidis, P.; Chachalis, D.; Akrivou, A.; Karanasios, E.; Ganopoulou, M.; Argiri, A.; Mandoulaki, A.; Hatzigiannakis, E.; Arampatzis, G.; Panagopoulos, A.; et al. Weed Species’ Diversity and Composition as Shaped by the Interaction of Management, Site, and Soil Variables in Olive Groves of Southern Greece. Agronomy 2024, 14, 640. https://doi.org/10.3390/agronomy14030640
Vahamidis P, Chachalis D, Akrivou A, Karanasios E, Ganopoulou M, Argiri A, Mandoulaki A, Hatzigiannakis E, Arampatzis G, Panagopoulos A, et al. Weed Species’ Diversity and Composition as Shaped by the Interaction of Management, Site, and Soil Variables in Olive Groves of Southern Greece. Agronomy. 2024; 14(3):640. https://doi.org/10.3390/agronomy14030640
Chicago/Turabian StyleVahamidis, Petros, Demosthenis Chachalis, Antigoni Akrivou, Evangelos Karanasios, Maria Ganopoulou, Apostolia Argiri, Athanasia Mandoulaki, Evangelos Hatzigiannakis, Georgios Arampatzis, Andreas Panagopoulos, and et al. 2024. "Weed Species’ Diversity and Composition as Shaped by the Interaction of Management, Site, and Soil Variables in Olive Groves of Southern Greece" Agronomy 14, no. 3: 640. https://doi.org/10.3390/agronomy14030640







