Yield, Protein Content and Water-Related Physiologies of Spring Wheat Affected by Fertilizer System and Weather Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Research Methods
2.3. Statistical Analysis
3. Results and Discussions
3.1. Influence of Experimental Factors on Yield, Quality, and Some Physiological Indicators of Spring Wheat
3.1.1. F-Test and Statistical Probability Levels from Analysis of Variance
3.1.2. The Influence of the Year Factor
3.1.3. The Influence of Nitrogen Fertilization
3.1.4. The Influence of the Genotype Factor
3.2. Influence of Double Interactions on Yield, Quality, and Some Physiological Indicators of Spring Wheat
3.3. Influence of Triple Interactions on Yield, Quality, and Some Physiological Indicators of Spring Wheat
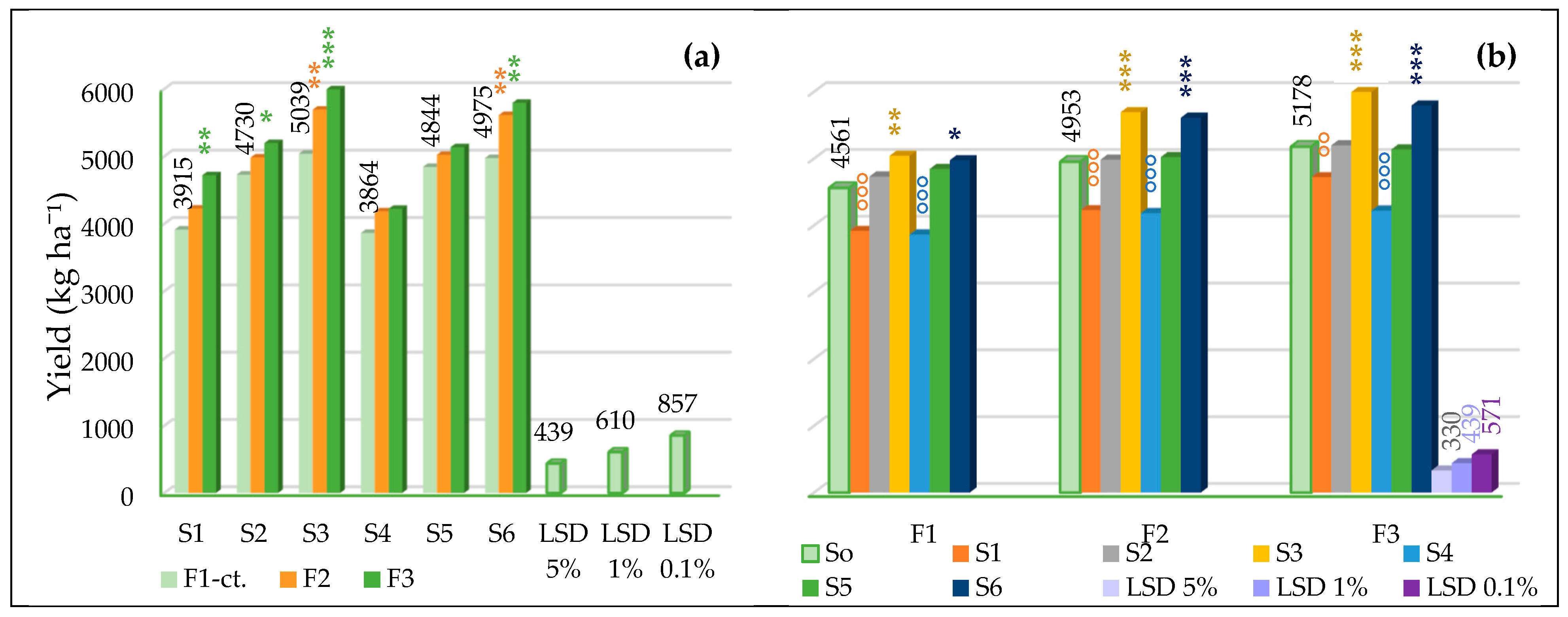
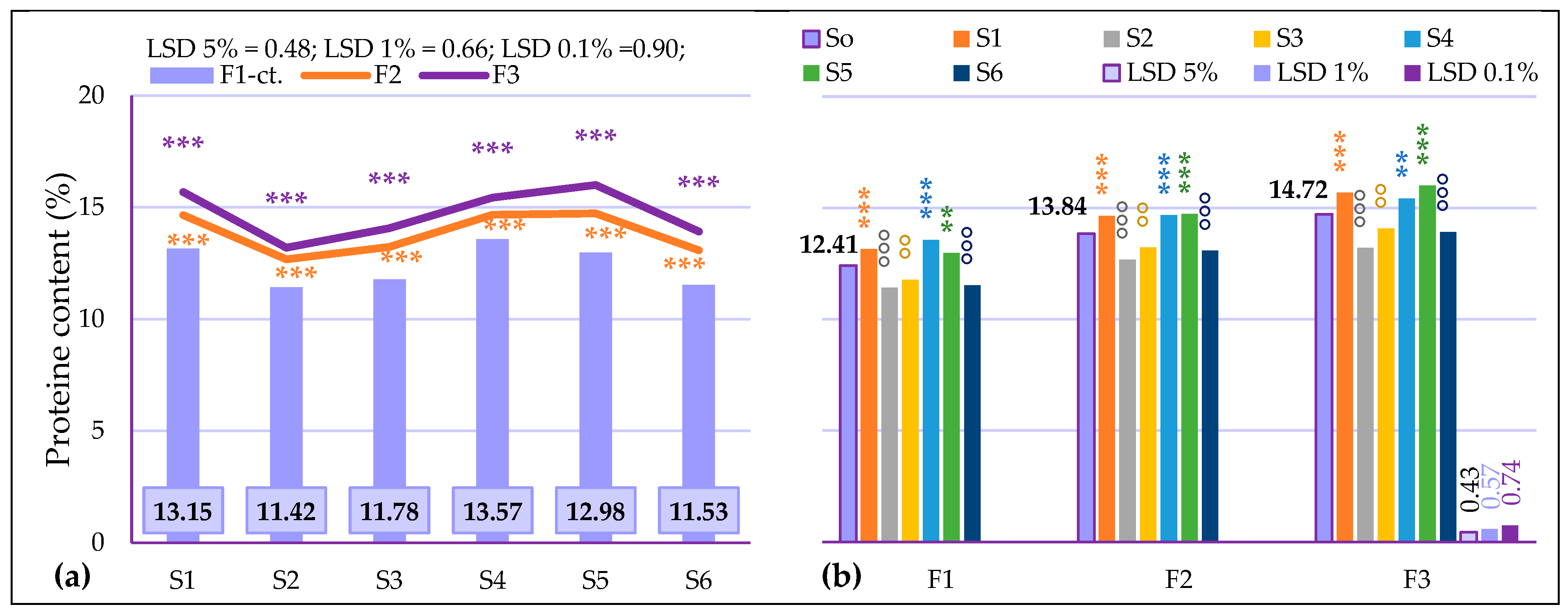
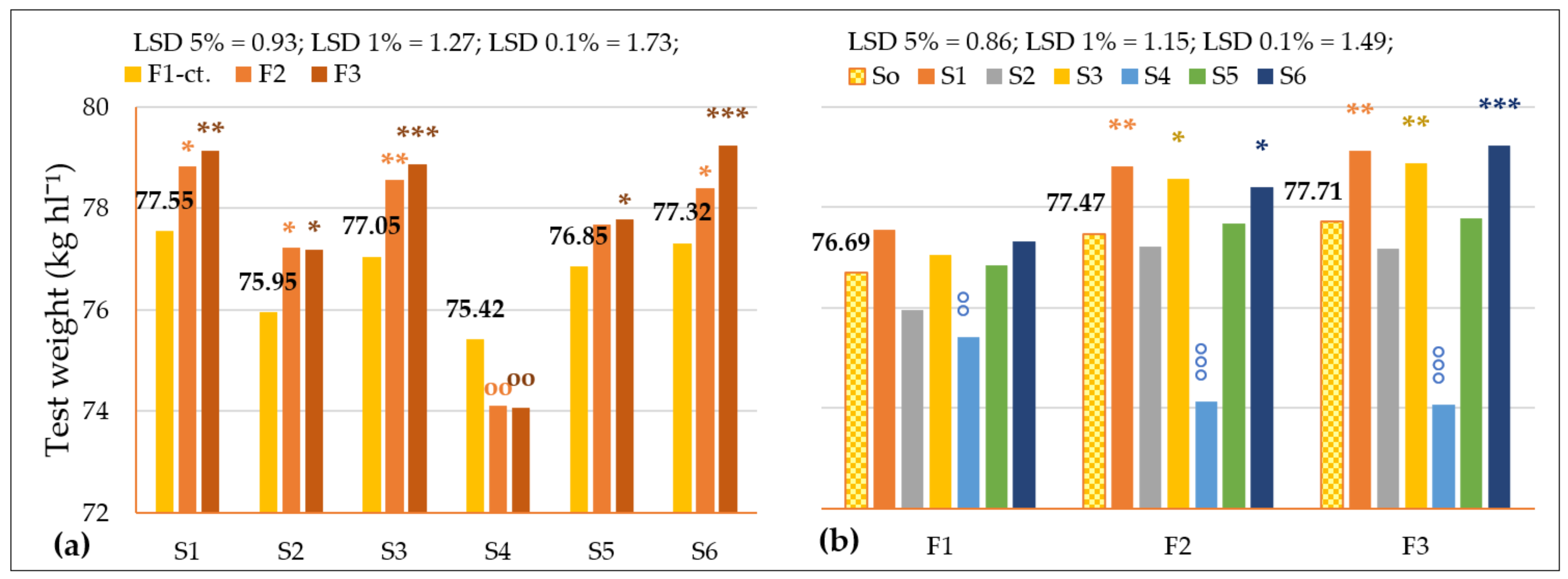
3.3.1. The Influence of the Triple Interactions on Yield and Quality of Spring Wheat
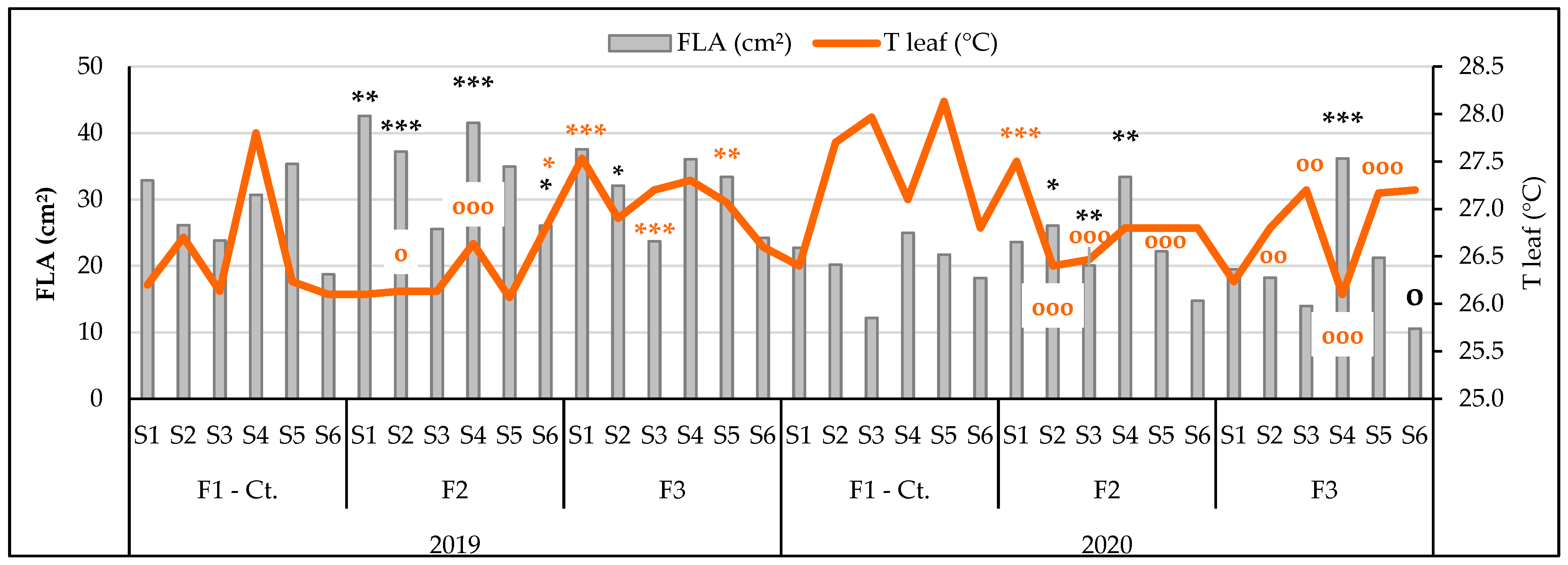
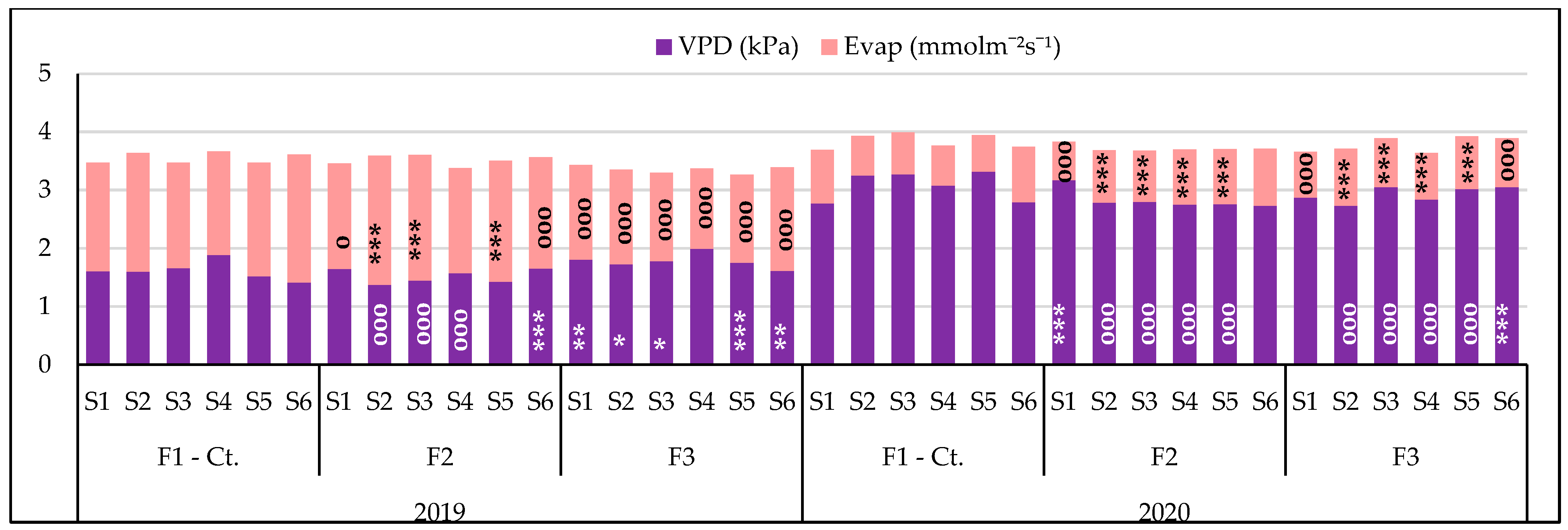
3.3.2. The Influence of the Triple Interactions on Some Physiological Indicators of Spring Wheat
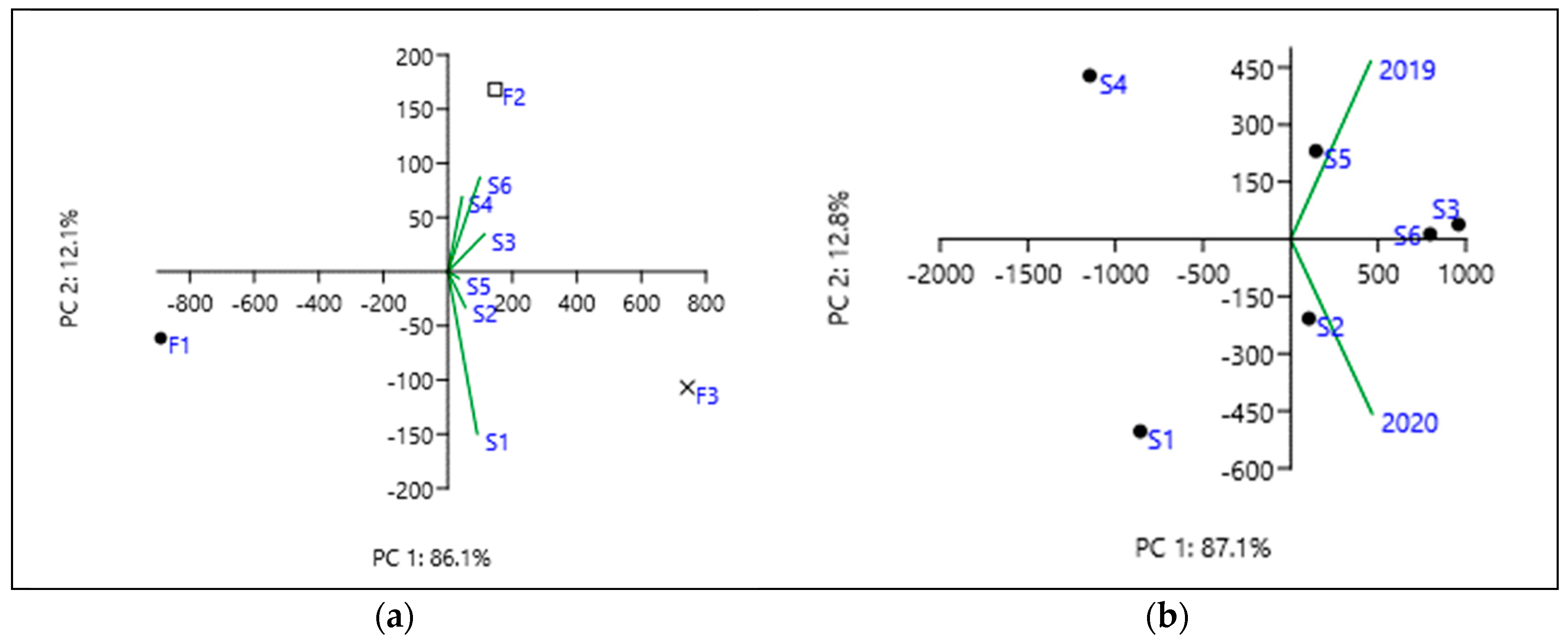
3.4. The Correlations Established between the Studied Parameters in Spring Wheat
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pandey, M.; Shrestha, J.; Subedi, S.; Shah, K.K. Role of Nutrients in Wheat: A Review. Trop. Agrobiodivers. 2020, 1, 18–23. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Khugaev, C.V.; Utkina, A.O.; Isaev, K.V.; Mohamed, E.S.; Kucher, D.E. Contribution of Eco-Friendly Agricultural Practices in Improving and Stabilizing Wheat Crop Yield: A Review. Agronomy 2023, 13, 2400. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Reynolds, M.P., Braun, H.J., Eds.; Springer: Midtown Manhattan, NY, USA, 2022; pp. 47–66. [Google Scholar]
- Alomari, D.Z.; Schierenbeck, M.; Alqudah, A.M.; Alqahtani, M.D.; Wagner, S.; Rolletschek, H.; Borisjuk, L.; Röder, M.S. Wheat Grains as a Sustainable Source of Protein for Health. Nutrients 2023, 15, 4398. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; Borrelli, G.M. Nutritional Components of Wheat Based Food: Composition, Properties, and Uses. Foods 2023, 12, 4010. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat. Foods 2021, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, Q.; Wang, C.; Feng, S.; Li, Y. Research Progress and Application Analysis of the Returning Straw Decomposition Process Based on CiteSpace. Water 2023, 15, 3426. [Google Scholar] [CrossRef]
- Cheţan, F.; Cheţan, C.; Rusu, T.; Moraru, P.I.; Ignea, M.; Şimon, A. The Influence of Unconventional Tillage Systems on Soil Water Conservation, Economic Efficiency and Yield of Winter Wheat, in Turda Area. Ser. A Agron. 2017, LX, 42–48. [Google Scholar]
- Castellini, M.; Fornaro, F.; Garofalo, P.; Giglio, L.; Rinaldi, M.; Ventrella, D.; Vitti, C.; Vonella, A.V. Effects of No-Tillage and Conventional Tillage on Physical and Hydraulic Properties of Fine Textured Soils under Winter Wheat. Water 2019, 11, 484. [Google Scholar] [CrossRef]
- Chiriţă, S.; Rusu, T.; Urdă, C.; Cheţan, F.; Racz, I. Winter Wheat Yield and Quality Depending on Chemical Fertilization, Different Treatments and Tillage Systems. AgroLife Sci. J. 2023, 12, 34–39. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Pasha, I.; Abrar, M.; Masih, S.; Hanif, M.S. Physicochemical, Functional and Rheological Properties of Wheat Varieties. J. Agric. Res. 2015, 53, 253–267. [Google Scholar]
- Hussain, N.; Mahmood, T.; Liaquat, M.; Safdar, N.; Ahmed, W.; Qayyum, A.; Abbasi, K.S.; Imran, M. Rheometry Nutrition and Gluten Microstructure Trends in Wheat Cultivars. Food Sci. Technol. 2022, 42, e60920. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, A.; Vats, S.; Tiwari, V.; Kumari, A.; Mishra, V.; Krishania, M. Vitamins in Cereals: A Critical Review of Content, Health Effects, Processing Losses, Bioaccessibility, Fortification, and Biofortification Strategies for their Improvement. Front. Nutr. 2021, 8, 586815. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Hameed, A.; Tahir, M.F. Wheat Quality: A Review on Chemical Composition, Nutritional Attributes, Grain Anatomy, Types, Classification, and Function of Seed Storage Proteins in Bread Making Quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Hussain, M.; Jabran, K.; Farooq, M.; Farooq, S.; Gašparovič, K.; Barboricova, M.; Aljuaid, B.S.; El-Shehawi, A.M.; Zuan, A.T.K. The Impact of Different Crop Rotations by Weed Management Strategies’ Interactions on Weed Infestation and Productivity of Wheat (Triticum aestivum L.). Agronomy 2021, 11, 2088. [Google Scholar] [CrossRef]
- Auzins, A.; Leimane, I.; Krievina, A.; Morozova, I.; Miglavs, A.; Lakovskis, P. Evaluation of Environmental and Economic Performance of Crop Production in Relation to Crop Rotation, Catch Crops, and Tillage. Agriculture 2023, 13, 1539. [Google Scholar] [CrossRef]
- Esaulko, A.; Sitnikov, V.; Pismennaya, E.; Vlasova, O.; Golosnoi, E.; Ozheredova, A.; Ivolga, A.; Erokhin, V. Productivity of Winter Wheat Cultivated by Direct Seeding: Measuring the Effect of Hydrothermal Coefficient in the Arid Zone of Central Fore-Caucasus. Agriculture 2023, 13, 55. [Google Scholar] [CrossRef]
- Chai, Y.; Zhao, Z.; Lu, S.; Chen, L.; Hu, Y. Field Evaluation of Wheat Varieties Using Canopy Temperature Depression in Three Different Climatic Growing Seasons. Plants 2022, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.L.; Liebig, M.A.; Krupinsky, J.M.; Merrill, S.D. Crop Sequence Influences on Sustainable Spring Wheat Production in the Northern Great Plains. Sustainability 2010, 2, 3695–3709. [Google Scholar] [CrossRef]
- Nhamo, L.; Matchaya, G.; Mabhaudhi, T.; Nhlengethwa, S.; Nhemachena, C.; Mpandeli, S. Cereal Production Trends under Climate Change: Impacts and Adaptation Strategies in Southern Africa. Agriculture 2019, 9, 30. [Google Scholar] [CrossRef]
- Hussain, J.; Khaliq, T.; Rahman, M.H.U.; Ullah, A.; Ahmed, I.; Srivastava, A.K.; Gaiser, T.; Ahmad, A. Effect of Temperature on Sowing Dates of Wheat under Arid and Semi-Arid Climatic Regions and Impact Quantification of Climate Change through Mechanistic Modeling with Evidence from Field. Atmosphere 2021, 12, 927. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Salmerón Cortasa, M.; Loges, R. Winter Wheat Roots Grow Twice as Deep as Spring Wheat Roots, is this Important for N Uptake and N Leaching Losses? Plant Soil 2009, 322, 101–114. [Google Scholar] [CrossRef]
- Joshi, P.; Dhillon, G.S.; Gao, Y.; Kaur, A.; Wheeler, J.; Chen, J. An Optimal Model to Improve Genomic Prediction for Protein Content and Test Weight in a Diverse Spring Wheat Panel. Agriculture 2024, 14, 347. [Google Scholar] [CrossRef]
- Manley, M.; Engelbrecht, M.L.; Williams, P.C.; Kidd, M. Assessment of Variance in the Measurement of Hectolitre Mass of 701. Wheat, using Equipment from Different Grain Producing and Exporting Countries. Biosyst. Eng. 2009, 103, 176–186. [Google Scholar] [CrossRef]
- Protic, R.; Miric, M.; Protic, N.; Jovanovic, Ž.; Jovin, P. The Test Weight of Several Winter Wheat Genotypes under Various Sowing Dates and Nitrogen Fertilizer Rates. Rom. Agric. Res. 2007, 24, 43–46. [Google Scholar]
- Marinciu, C.M.; Șerban, G.; Mandea, V.; Săulescu, N.N. Cultivar and Crop Management Effects on Test Weight in Winter Wheat (Triticum aestivum). Rom. Agric. Res. 2021, 38, 46. Available online: https://new.incda-fundulea.ro/images/rar/nr38/rar3814.pdf (accessed on 10 March 2024). [CrossRef]
- Muntean, L.S.; Cernea, S.; Morar, G.; Duda, M.M.; Vârban, D.I.; Muntean, S.; Moldovan, C. Phytotechnics; Risoprint: Cluj-Napoca, Romania, 2014; pp. 74–145. [Google Scholar]
- Wyzińska, M.; Grabiński, J. The Influence of Autumn Sowing date on the Productivity of Spring Wheat (Triticum aestivum L.). In Proceedings of the Research for Rural Development. Agricultural Sciences (Crop Sciences, Animal Sciences); 2018; Volume 2, pp. 35–41. Available online: https://www.researchgate.net/publication/331711853_The_influence_of_autumn_sowing_date_on_the_productivity_of_spring_wheat_Triticum_aestivum_L (accessed on 17 March 2024).
- Hlisnikovský, L.; Menšík, L.; Kunzová, E. Development and the Effect of Weather and Mineral Fertilization on Grain Yield and Stability of Winter Wheat following Alfalfa—Analysis of Long-Term Field Trial. Plants 2023, 12, 1392. [Google Scholar] [CrossRef]
- Afuye, G.A.; Kalumba, A.M.; Orimoloye, I.R. Characterisation of Vegetation Response to Climate Change: A Review. Sustainability 2021, 13, 7265. [Google Scholar] [CrossRef]
- Moayedi, S.; Elias, E.M.; Manthey, F.A. Effect of Weather on Grain Quality Traits of Durum Wheat Grown in the Northern Plains of USA. Am. J. Plant Sci. 2021, 12, 1894–1911. [Google Scholar] [CrossRef]
- Clauw, H.; Van de Put, H.; Sghaier, A.; Kerkaert, T.; Debonne, E.; Eeckhout, M.; Steppe, K. The Impact of a Six-Hour Light–Dark Cycle on Wheat Ear Emergence, Grain Yield, and Flour Quality in Future Plant-Growing Systems. Foods 2024, 13, 750. [Google Scholar] [CrossRef]
- Swify, S.; Mažeika, R.; Baltrusaitis, J.; Drapanauskaitė, D.; Barčauskaitė, K. Review: Modified Urea Fertilizers and Their Effects on Improving Nitrogen Use Efficiency (NUE). Sustainability 2024, 16, 188. [Google Scholar] [CrossRef]
- Roumia, H.; Kókai, Z.; Mihály-Langó, B.; Csobod, É.C.; Benedek, C. Ancient Wheats—A Nutritional and Sensory Analysis Review. Foods 2023, 12, 2411. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kim, J.-Y. Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization. Plants 2021, 10, 2282. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Sanmartin, C.; Taglieri, I.; Macaluso, M.; Venturi, F.; Napoli, M.; Mancini, M.; Fabbri, C.; Zinnai, A. Effect of Fertilization Regime of Common Wheat (Triticum aestivum) on Flour Quality and Shelf-Life of PDO Tuscan Bread. Foods 2023, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yan, X. Responses of Wheat Protein Content and Protein Yield to Future Climate Change in China during 2041–2060. Sustainability 2023, 15, 14204. [Google Scholar] [CrossRef]
- Chen, X.; Donghong, M.; Tauqeer, A.Y.; Yin-Gang, H. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 2012, 137, 195–201. [Google Scholar] [CrossRef]
- Sobolewska, M.; Wenda-Piesik, A.; Jaroszewska, A.; Stankowski, S. Effect of Habitat and Foliar Fertilization with K, Zn and Mn on Winter Wheat Grain and Baking Qualities. Agronomy 2020, 10, 276. [Google Scholar] [CrossRef]
- Mureșan, D.; Varadi, A.; Racz, I.; Kadar, R.; Ceclan, A.; Duda, M.M. Effect of Genotype and Sowing Date on Yield and Yield Components of Facultative Wheat in Transylvania Plain. AgroLife Sci. J. 2020, 9, 237–247. [Google Scholar]
- Dobre, S.P.; Lazăr, C. Surface Determinations of Flag Leaf for a Set of Mutant DH Wheat Lines. In Genetics and Plant Breeding; INCDA: Fundulea, Romania, 2014; Volume LXXXII, pp. 7–16. Available online: https://www.incda-fundulea.ro/anale/82/82.1.pdf (accessed on 17 March 2024).
- Berdhal, J.D.; Rasmusson, D.C.; Moss, D.N. Effect of Leaf Area on Photosynthetic Rates, Light Penetration and Grain Yield in Barley. Crop Sci. 1972, 20, 117–180. [Google Scholar]
- Ibrahim, H.; Elenein, R.A.A. The Relative Contribution of Different Wheat Leaves and Awns to the Grain Yield and its Protein Content. Z. Acker Pflanzenbau 1977, 144, 1–7. [Google Scholar]
- Liu, Y.; Li, M.J.; Li, J.; Li, X.; Yang, X.; Tong, Y.; Zhang, A.; Li, B.; Lin, J.; Kuang, T.; et al. Dynamic Changes in Flag Leaf Angle Contribute to High Photosynthetic Capacity. Chin. Sci. Bull. 2009, 54, 3045–3052. [Google Scholar] [CrossRef]
- Available online: https://istis.ro/catalog-oficial/ (accessed on 15 March 2024).
- Grecu, C.; Haş, I.; Nagy, C. ARDS Turda, 50th Anniversary 1957–2007. In Research and Development Results; SC Ela Design SRL: Bucharest, Romania, 2007; pp. 9–12. [Google Scholar]
- SRTS. The Romanian System of Soil Taxonomy; Estfalia: Bucharest, Romania, 2012. [Google Scholar]
- Chețan, F.; Chețan, C. Researches Regarding the Influence of the Unconventional Soil Tillage Systems upon Weeding and Soybean Yield, in Pedoclimatic Conditions in the Transylvanian Plain. In Agrarian Economy and Rural Development—Realities and Perspectives for Romania; Volume of International Symposium; ICEADR: Bucharest, Romania, 2020; Volume 11, pp. 91–99. Available online: https://symposium.iceadr.ro/wp-content/uploads/2023/03/Volum-simpozion-ICEADR-2020-ENG-compressed.pdf (accessed on 10 March 2024).
- Bardas, M.; Rusu, T.; Popa, A.; Russu, F.; Simon, A.; Chețan, F.; Racz, I.; Popescu, S.; Topan, C. Effect of Foliar Fertilization on the Physiological Parameters, Yield and Quality Indices of the Winter Wheat. Agronomy 2024, 14, 73. [Google Scholar] [CrossRef]
- Available online: https://i0.wp.com/www.mississippi-crops.com/wp-content/uploads/2015/02/Wheat_FeekesScale_graphic.png?ssl=1 (accessed on 15 March 2024).
- Available online: https://www.terra-preta.ro/produse/pp-systems (accessed on 15 March 2024).
- Chanda, S.; Singh, Y. Estimation of Leaf Area in Wheat using Linear Measurements. Plant Breed. Seed Sci. 2002, 46, 75–79. [Google Scholar]
- Turda Meteorological Station, Longitude: 23′47-Latitude 46′35′-Altitude 427 m.
- PolyFact. ANOVA Test PC Program for Variant Analyses; USAMV: Cluj-Napoca, Romania, 2020. [Google Scholar]
- Kadar, R.; Muntean, L.; Racz, I.; Ona, A.D.; Ceclan, A.; Hirișcău, D. The Effect of Genotype, Climatic Conditions and Nitrogen Fertilization on Yield and Grain Protein Content of Spring Wheat (Triticum aestivum L.). Not. Bot. Horti Agrobot. 2019, 47, 515–521. [Google Scholar] [CrossRef]
- Szmigiel, A.; Kołodziejczyk, M.; Oleksy, A.; Kulig, B. Efficiency of Nitrogen Fertilization in Spring Wheat. Int. J. Plant Prod. 2016, 10, 447–456. [Google Scholar]
- Sasani, S.; Amiri, R.; Sharifi, H.R.; Lotfi, A. Impact of Sowing date on Bread Wheat Kernel Quantitative and Qualitative Traits under Middle East Climate Conditions. Zemdirb. Agric. 2020, 107, 279–286. [Google Scholar] [CrossRef]
- Gauer, L.E.; Grant, C.A.; Gehl, D.T.; Bailey, L.D. Effects of Nitrogen Fertilization on Grain Protein Content, Nitrogen Uptake, and Nitrogen use Efficiency of Six Spring Wheat (Triticum aestivum L.) Cultivars, in Relation to Estimated Moisture Supply. Can. J. Plant Sci. 1992, 72, 235–241. [Google Scholar] [CrossRef]
- Orloff, S. Effect of Nitrogen Fertilization Practices on Spring Wheat Protein Content. Final Report. 2011. Available online: http://cawheat.org/uploads/resources/542/wheatcommissionfinalrptnfert12.pdf (accessed on 15 March 2024).
- Jahan, A.H.S.; Hossain, A.; Alam, N.; Ali, A.; Saif, H.B.; Kizilgeci, F.; Omer, K.; Barutcular, C.; Sabagh, A.E. Yield and Grain Protein of Wheat (Triticum Aestivum L.) Is Influenced by The Application of Different Levels of Nitrogen. Fresenius Environ. Bull. 2020, 29, 5704–5714. [Google Scholar]
- Protic, R.; Jovin, P.; Protic, N.; Jankovics, S.; Jovanovic, Z. Mass of 1000 Grains in Several Winter Wheat Genotypes, at Different Dates of Sowing and Rates of Nitrogen Fertilizer. Rom. Agric. Res. 2007, 24, 39–43. [Google Scholar]
- Wu, W.; Zhou, L.; Chen, J.; Qiu, Z.; He, Y. Gain TKW: A Measurement System of Thousand Kernel Weight Based on the Android Platform. Agronomy 2018, 8, 178. [Google Scholar] [CrossRef]
- Dobreva, S.S.; Muhova, A.; Bonchev, B. Nitrogen and Phosphorus Fertilizers Affecting the Quality and Quantity of the Durum Wheat. Sci. Pap. Ser. A Agron. 2022, LXV, 533–539. [Google Scholar]
- Walsh, O.S.; Walsh, W.L. Nitrogen Fertilizer Rate and Time Effect on Dryland No-till Hard Red Spring Wheat Production. Agrosyst. Geosci. Environ. 2020, 3, e20093. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Kehel, Z.; Marrou, H.; Sinclair, T.R. Seasonal and Climatic Variation of Weighted VPD for Transpiration Estimation. Eur. J. Agron. 2020, 113, 125966. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant Responses to Rising Vapor Pressure Deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Cutress, D. The Importance of Vapour Pressure Deficits (VPD) in Agricultural Plant Growth, Technical Articles, Key Sector: Forestry, Horticulture, Organic; Theme: Business, Land. 2021. Available online: https://businesswales.gov.wales/farmingconnect/news-and-events/technical-articles/importance-vapour-pressure-deficits-vpd-agricultural-plant-growth (accessed on 15 March 2024).
- Pettigrew, W.T.; Hesketh, J.D.; Peters, D.B.; Wolley, J.T. A Vapor Pressure Deficit Effect on Crop Canopy Photosynthesis. Photosynth. Res. 1990, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Noor, H.; Yan, Z.; Sun, P.; Zhang, L.; Ding, P.; Li, L.; Ren, A.; Sun, M.; Gao, Z. Effects of Nitrogen on Photosynthetic Productivity and Yield Quality of Wheat (Triticum aestivum L.). Agronomy 2023, 13, 1448. [Google Scholar] [CrossRef]
- Kibler, C.L.; Trugman, A.T.; Dar, A.R.; Still, J.C.; Russell, L.; Scott, K.K.; Caylor, J.C.S.; Singer, M.B. Evapotranspiration Regulates Leaf Temperature and Respiration in Dryland Vegetation. Agric. For. Meteorol. 2023, 339, 109560. [Google Scholar] [CrossRef]
- El Nadi, A.H. The Significance of Leaf Area in Evapotranspiration. Ann. Bot. 1974, 38, 607–611. [Google Scholar] [CrossRef]
- Litke, L.; Zinta, G.; Ruža, A. Nitrogen Fertilizer Influence on Winter Wheat Yield and Yield components depending on soil tillage and forecrop. Res. Rural Dev. 2017, 2, 54–61. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Jiang, G.; Yin, H.; Che, Z. Effects of Nitrogen Application Strategy on Nitrogen Enzyme Activities and Protein Content in Spring Wheat Grain. Agriculture 2022, 12, 1891. [Google Scholar] [CrossRef]
- Subedi, K.D.; Ma, B.L.; Xue, A.G. Planting Date and Nitrogen Effects on Grain Yield and Protein Content of Spring Wheat. Crop Sci. 2007, 47, 36–44. [Google Scholar] [CrossRef]
- Erdemci, I. Investigation of genotype x environment interaction in chickpea genotypes using AMMI and GGE biplot analysis. Turk. J. Field Crop 2018, 23, 20–26. [Google Scholar] [CrossRef]
- Wodebo, K.Y.; Tolemariam, T.; Demeke, S.; Garedew, W.; Tesfaye, T.; Zeleke, M.; Gemiyu, D.; Bedeke, W.; Wamatu, J.; Sharma, M. AMMI and GGE Biplot Analyses for Mega-Environment Identification and Selection of Some High-Yielding Oat (Avena sativa L.) Genotypes for Multiple Environments. Plants 2023, 12, 3064. [Google Scholar] [CrossRef] [PubMed]
- Ravier, C.; Meynard, J.M.; Cohan, J.P.; Gate, P.; Jeuffroy, M.H. Early Nitrogen Deficiencies Favor High Yield, Grain Protein Content and N Use Efficiency in Wheat. Eur. J. Agron. 2017, 89, 16–24. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L.; Fan, J.J.; Sun, M.; Yi, Y.; Guo, W.S.; Voldeng, H.D. Management of Nitrogen Fertilization to Balance Reducing Lodging Risk and Increasing Yield and Protein Content in Spring Wheat. Field Crops Res. 2019, 241, 107584. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Dvojković, K.; Ivić, M.; Plavšin, I.; Novoselović, D. Gluten Protein Compositional Changes in Response to Nitrogen Application Rate. Agronomy 2021, 11, 325. [Google Scholar] [CrossRef]
- Clarke, J.M.; Campbell, C.A.; Cutforth, H.W.; DePauw, R.M.; Wilkleman, G.E. Nitrogen and Phosphorus Uptake, Translocation, and Utilization Efficiency of Wheat in Relation to Environment and Cultivar Yield and Protein Levels. Can. J. Plant Sci. 1990, 70, 965–977. [Google Scholar] [CrossRef]
- Szmigiel, A.; Oleksy, A.; Kołodziejczyk, M. Effect of Nitrogen Fertilization on Quality and Quantity in Spring Wheat. Electron. J. Pol. Agric. Univ. 2014, 17, 1–10. [Google Scholar]
- Warechowska, M.; Stepien, A.; Wojtkowiak, K.; Nawrocka, A. The Impact of Nitrogen Fertilization Strategies on Selected Qualitative Parameters of Spring Wheat Grain and Flour. Pol. J. Nat. Sci. 2019, 34, 199–212. [Google Scholar]
- Blandino, M.; Marinaccio, F.; Reyneri, A. Effect of late-season nitrogen fertilization on grain yield and on flour rheological quality and stability in common wheat, under different production situations. Ital. J. Agron. 2016, 11, 107–113. [Google Scholar] [CrossRef]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of adding picoxystrobin, azoxystrobin and nitrogen to a triazole programme on disease control, flag leaf senescence, yield and grain quality of winter wheat. Crop Prot. 2003, 22, 975–987. [Google Scholar] [CrossRef]
- Kosmolak, F.G.; Crowle, W.L. An Effect of Nitrogen Fertilization on the Agronomic Traits and Dough Mixing Strength of Five Canadian Hard Red Spring Wheat Cultivars. Can. J. Plant Sci. 1980, 60, 1071–1076. Available online: https://cdnsciencepub.com/doi/10.4141/cjps80-157 (accessed on 17 March 2024). [CrossRef]
- Ministry of Agriculture and Rural Development (MARD). Order 228/July 5th, 2017 Regarding the Approval of the Grading Handbook for Seeds for Consumption. 2017. Available online: https://www.madr.ro/comunicare/3919-a-fost-aprobat-manualul-de-gradare-pentru-semintele-de-consum.html (accessed on 15 March 2024).
- Lin, H.; Chen, Y.J.; Zhang, H.L.; Fu, P.L.; Fan, Z.X. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. Available online: https://besjournals.onlinelibrary.wiley.com/doi/10.1111/1365-2435.12923 (accessed on 17 March 2024). [CrossRef]
- Yang, F.; Zhang, Q.; Wang, R.; Zhou, J. Evapotranspiration Measurement and Crop Coefficient Estimation over a Spring Wheat Farmland Ecosystem in the Loess Plateau. PLoS ONE 2014, 9, e100031. [Google Scholar] [CrossRef] [PubMed]
- Vinod, N.; Slot, M.; McGregor, I.R.; Ordway, E.M.; Smith, M.N.; Taylor, T.C.; Sack, L.; Buckley, T.N.; Anderson-Teixeira, K.J. Thermal sensitivity across forest vertical profiles: Patterns, mechanisms, and ecological implications. New Phytol. 2022, 237, 22–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, L.; Wang, S.; Chi, Y.; Chen, J. Leaf Temperature and Vapour Pressure Deficit (VPD) Driving Stomatal Conductance and Biochemical Processes of Leaf Photosynthetic Rate in a Subtropical Evergreen Coniferous Plantation. Sustainability 2018, 10, 4063. [Google Scholar] [CrossRef]
- The Ultimate Vapor Pressure Deficit (VPD) Guide. Available online: https://pulsegrow.com/blogs/learn/vpd (accessed on 15 March 2024).
- Gâdea, Ș. Plant Physiology; Academic Press: Cambridge, MA, USA, 2013; p. 97. [Google Scholar]
- Bramley, H.; Ranawana, S.R.W.M.C.J.K.; Palta, J.A.; Stefanova, K.; Siddique, K.H.M. Transpirational Leaf Cooling Effect Did Not Contribute Equally to Biomass Retention in Wheat Genotypes under High Temperature. Plants 2022, 11, 2174. [Google Scholar] [CrossRef]



| Month | 2019 | 2020 | 60 Years Average | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall, [mm] | Temperatures, [°C] | Rainfall, [mm] | Temperatures, [°C] | Rainfall, [mm] | Temperatures, [°C] | |||||
| m.a. | dev. | m.a. | dev. | m.a. | dev. | m.a. | dev. | |||
| March | 12.3 | −11.3 | 7.3 | 2.6 | 34 | 10.4 | 6.1 | 1.4 | 23.6 | 4.7 |
| April | 62.6 | 16.7 | 11.3 | 1.4 | 17.8 | −28.1 | 10.3 | 0.4 | 45.9 | 9.9 |
| May | 152.4 | 83.7 | 13.6 | −1.4 | 44.4 | −24.3 | 13.7 | −1.3 | 68.7 | 15.0 |
| June | 68.8 | −16.0 | 21.8 | 3.9 | 166.6 | 81.8 | 19.1 | 1.2 | 84.8 | 17.9 |
| July | 35.0 | −42.1 | 20.4 | 0.7 | 86.8 | 9.7 | 20.2 | 0.5 | 77.1 | 19.7 |
| August | 63.8 | 7.2 | 22.1 | 2.8 | 58 | 1.5 | 21.5 | 2.2 | 56.5 | 19.3 |
| Source of Variance | Year (Y) | Fertilization (F) | Genotype (S) | Y × F | Y × S | F × S | Y × F × S |
|---|---|---|---|---|---|---|---|
| GY, kg ha−1 Probability levels, [%] | 19.843 5 | 10.133 1 | 79.996 1 | 5.881 5 | 11.766 1 | 1.758 NS | 1.123 NS |
| P, % Probability levels, [%] | 1299.228 0.1 | 181.415 0.1 | 128.080 0.1 | 38.265 1 | 31.346 1 | 2.336 5 | 1.093 NS |
| TKW, g Probability levels, [%] | 66.058 5 | 5.675 5 | 7.549 1 | 4.272 NS | 1.622 NS | 4.215 1 | 3.189 1 |
| TW, kg hl−1 Probability levels, [%] | 120.499 0.1 | 11.870 1 | 72.181 0.1 | 22.119 1 | 102.219 0.1 | 4.818 1 | 4.571 1 |
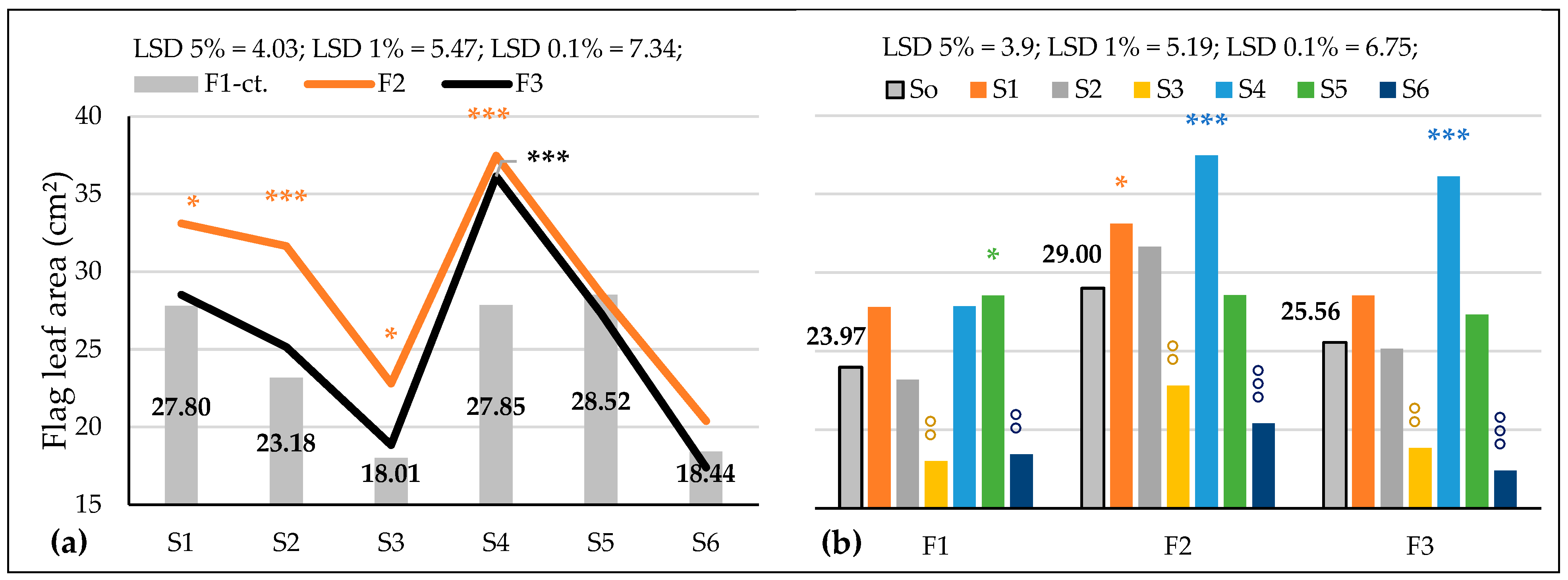
| FLA, cm2 Probability levels, [%] | 163.463 1 | 19.402 1 | 53.671 0.1 | 2.724 NS | 5.777 1 | 2.603 5 | 2.493 5 |
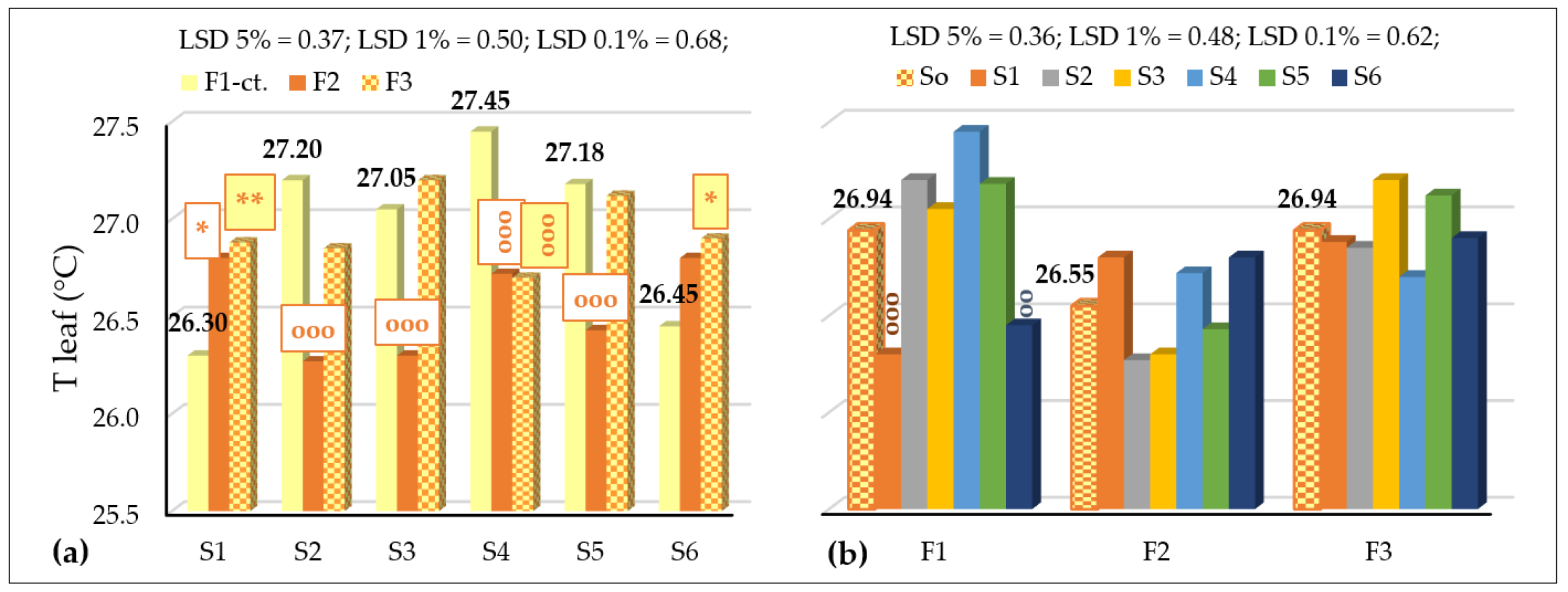
| T leaf, °C Probability levels, [%] | 21.761 5 | 17.086 1 | 2.452 5 | 29.184 1 | 12.977 1 | 8.330 1 | 7.824 1 |
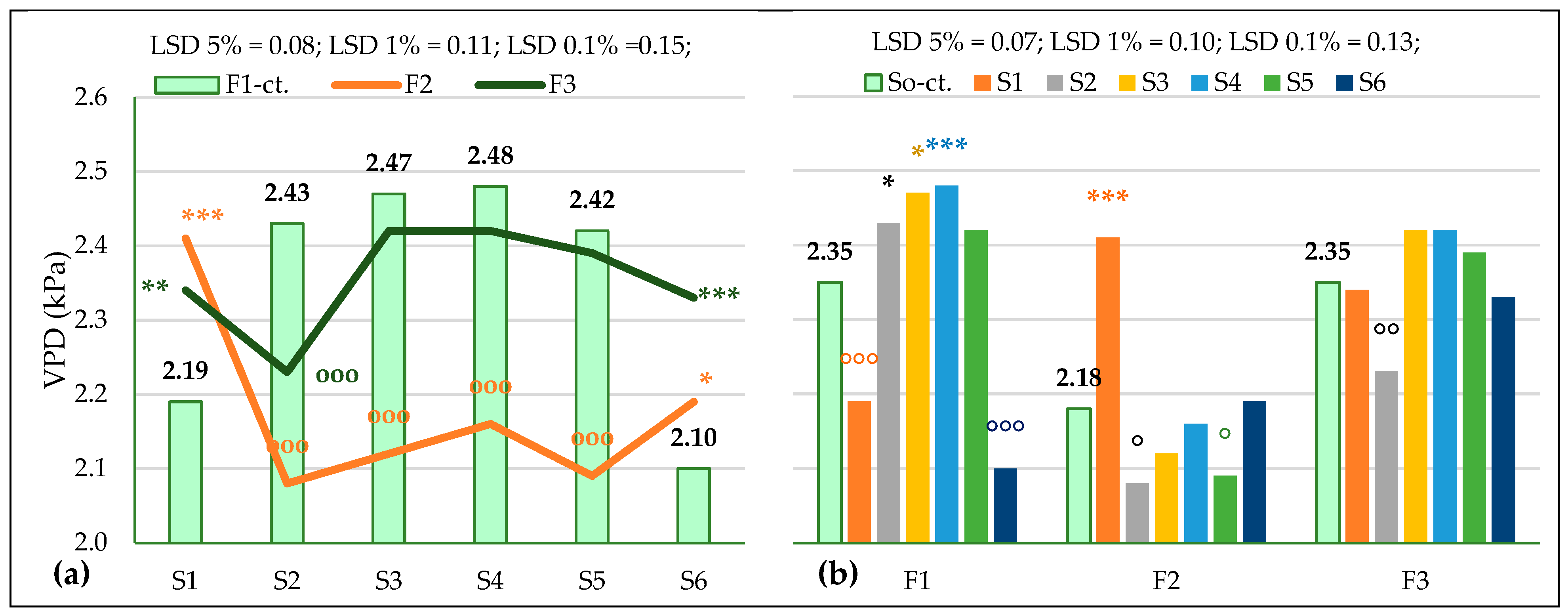
| VPD, kPa Probability levels, [%] | 18,667.82 0.1 | 61.951 0.1 | 13.006 1 | 38.970 1 | 20.645 1 | 25.755 1 | 14.722 1 |
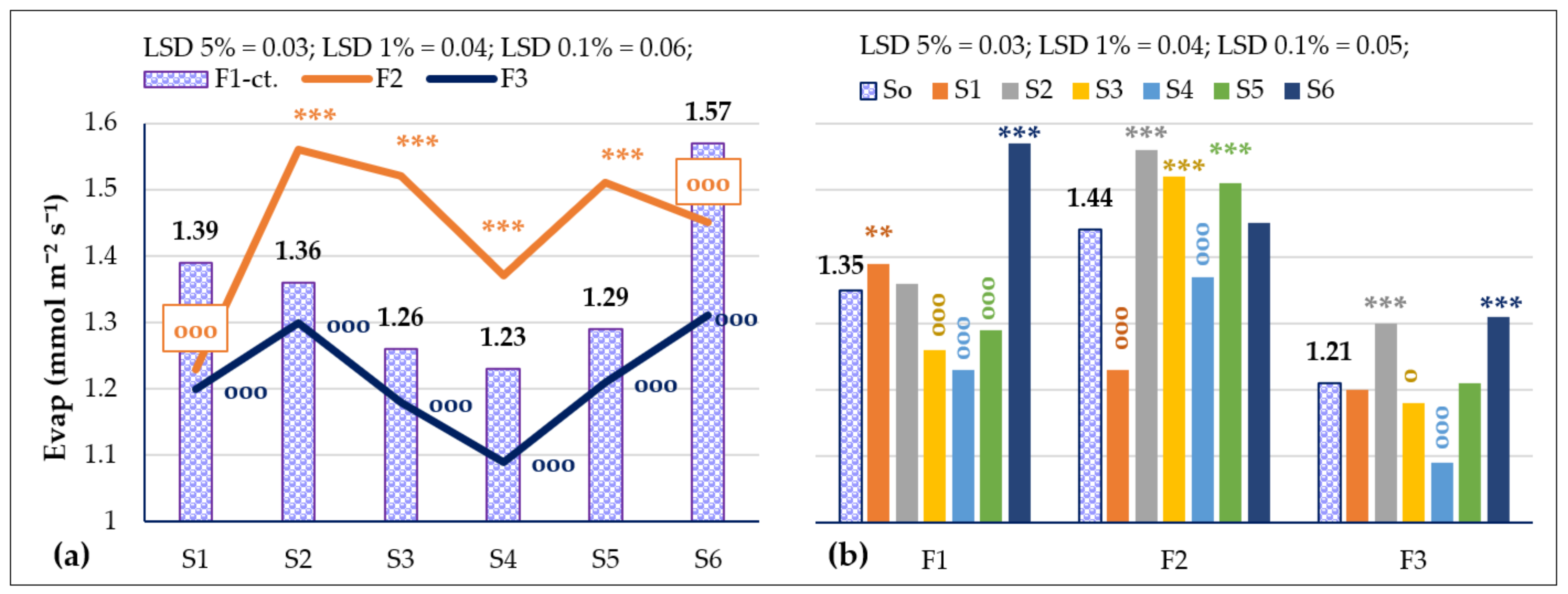
| Evap, mmol m−2 s−1 Probability levels, [%] | 5262.036 0.1 | 642.407 0.1 | 168.062 0.1 | 792.707 0.1 | 51.747 0.1 | 80.095 0.1 | 64.164 0.1 |
| Source of Variation | GY, [kg ha−1] | P, [%] | TKW, [g] | TW, [kg hl−1] | FLA, [cm2] | T leaf, [°C] | VPD, [kPa] | Evap, [mmol m−2 s−1] | |
|---|---|---|---|---|---|---|---|---|---|
| Year | Y0–ct. | 4897.39 | 13.65 | 33.83 | 77.29 | 26.17 | 26.81 | 2.29 | 1.33 |
| 2019 | 4795.96 | 12.67 °° | 31.33 | 76.67 ° | 31.26 * | 26.65 | 1.64 °°° | 1.83 *** | |
| 2020 | 4998.82 | 14.64 ** | 36.33 | 77.91 * | 21.09 ° | 26.98 | 2.95 *** | 0.83 °°° | |
| LSD | 5% | 195.82 | 0.24 | 2.65 | 0.48 | 3.42 | 0.30 | 0.04 | 0.06 |
| 1% | 452.20 | 0.54 | 6.11 | 1.12 | 7.89 | 0.70 | 0.09 | 0.14 | |
| 0.1% | 1439.02 | 1.73 | 19.44 | 3.55 | 25.12 | 2.23 | 0.30 | 0.43 | |
| Fertilization | F1–ct. | 4561.33 | 12.41 | 32.89 | 76.69 | 23.97 | 26.94 | 2.35 | 1.35 |
| F2 | 4953.08 * | 13.84 *** | 33.98 | 77.47 ** | 29.00 *** | 26.55 °°° | 2.18 °°° | 1.44 *** | |
| F3 | 5177.75 ** | 14.72 *** | 34.63 * | 77.71 ** | 25.56 | 26.94 | 2.35 | 1.21 °°° | |
| LSD | 5% | 320.15 | 0.28 | 1.21 | 0.51 | 1.91 | 0.18 | 0.04 | 0.01 |
| 1% | 465.67 | 0.41 | 1.75 | 0.74 | 2.77 | 0.26 | 0.06 | 0.02 | |
| 0.1% | 698.50 | 0.62 | 2.63 | 1.10 | 4.16 | 0.39 | 0.09 | 0.03 | |
| Genotype | S0–ct. | 4897.39 | 13.65 | 33.83 | 77.29 | 26.17 | 26.81 | 2.29 | 1.33 |
| S1 | 4287.11 °°° | 14.49 *** | 32.19 | 78.50 *** | 29.81 ** | 26.66 | 2.31 | 1.28 °°° | |
| S2 | 4970.17 | 12.43 °°° | 35.21 | 76.78 ° | 26.66 | 26.77 | 2.24 ° | 1.40 *** | |
| S3 | 5575.56 *** | 13.03 °°° | 32.31 | 78.16 *** | 19.89 °°° | 26.85 | 2.33 | 1.32 | |
| S4 | 4089.89 °°° | 14.56 *** | 34.00 | 74.53 °°° | 33.81 *** | 26.96 | 2.35 ** | 1.23 °°° | |
| S5 | 5001.22 | 14.57 *** | 36.93 ** | 77.44 | 28.13 | 26.91 | 2.30 | 1.33 | |
| S6 | 5460.39 *** | 12.84 °°° | 32.37 | 78.32 *** | 18.75 °°° | 26.72 | 2.21 °°° | 1.44 *** | |
| LSD | 5% | 190.66 | 0.25 | 1.99 | 0.50 | 2.25 | 0.21 | 0.04 | 0.02 |
| 1% | 253.58 | 0.33 | 2.65 | 0.66 | 3.00 | 0.27 | 0.06 | 0.02 | |
| 0.1% | 329.84 | 0.43 | 3.44 | 0.86 | 3.90 | 0.36 | 0.07 | 0.03 | |
| GY | P | TKW | TW | FLA | T leaf | VPD | Evap | |
|---|---|---|---|---|---|---|---|---|
| GY | 1 | |||||||
| P | −0.133 | 1 | ||||||
| TKW | 0.046 | 0.160 | 1 | |||||
| TW | 0.617 | 0.048 | −0.125 | 1 | ||||
| FLA | −0.702 | 0.538 | −0.127 | −0.548 | 1 | |||
| T leaf | −0.004 | 0.057 | 0.443 | −0.122 | −0.182 | 1 | ||
| VPD | −0.173 | 0.138 | 0.167 | −0.120 | −0.054 | 0.860 | 1 | |
| Evap | 0.210 | −0.521 | −0.166 | 0.149 | −0.198 | −0.679 | −0.858 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chețan, F.; Hirișcău, D.; Rusu, T.; Bărdaș, M.; Chețan, C.; Șimon, A.; Moraru, P.I. Yield, Protein Content and Water-Related Physiologies of Spring Wheat Affected by Fertilizer System and Weather Conditions. Agronomy 2024, 14, 921. https://doi.org/10.3390/agronomy14050921
Chețan F, Hirișcău D, Rusu T, Bărdaș M, Chețan C, Șimon A, Moraru PI. Yield, Protein Content and Water-Related Physiologies of Spring Wheat Affected by Fertilizer System and Weather Conditions. Agronomy. 2024; 14(5):921. https://doi.org/10.3390/agronomy14050921
Chicago/Turabian StyleChețan, Felicia, Diana Hirișcău, Teodor Rusu, Marius Bărdaș, Cornel Chețan, Alina Șimon, and Paula Ioana Moraru. 2024. "Yield, Protein Content and Water-Related Physiologies of Spring Wheat Affected by Fertilizer System and Weather Conditions" Agronomy 14, no. 5: 921. https://doi.org/10.3390/agronomy14050921






