Assessing Microbial Contributions to N2O Impacts Following Biochar Additions
Abstract
:1. Introduction
2. Results
2.1. Soil and Biochar Characterization
| Property | Prairie Soil | Agricultural Soil | Forest Soil | Sterilized Sand | Biochar |
|---|---|---|---|---|---|
| Organic Matter (%) | 4.9 | 4.4 | 3.3 | 0.1 | 71.63 |
| Total Nitrogen (%) | 0.2 | <0.1 | <0.1 | <0.1 | 0.88 |
| Cation Exchange Capacity (%) | 23.5 | 15.1 | 4 | 16.1 | - |
| pH (1:5 distilled H2O) | 7.2 | 6.5 | 4.7 | 9.5 | 6.2 |
| Potassium (ppm) | 180 | 145 | 63 | 16 | <0.10 |
| Calcium (ppm) | 4250 | 2433 | 358 | 3840 | 0.15 |
| Magnesium (ppm) | 1100 | 527 | 53 | 102 | 0.07 |
| Iron (ppm) | 75 | 190 | 252 | 10 | 4353 |
| Nitrate (NO3, ppm) | 3.4 | 15.4 | 0.9 | 0.2 | <0.1 |
| Nitrite (NO2, ppm) | 0.3 | <0.1 | <0.1 | <0.1 | <0.1 |
| Ammonium (NH4, ppm) | 9.1 | 2.8 | 1.1 | <0.1 | <0.1 |
2.2. Impact of Biochar on Soil CO2 Production

2.3. Impact of Biochar on Soil N2O Production
2.4. Impacts of Biochar Addition on Soil Microbial Biomass
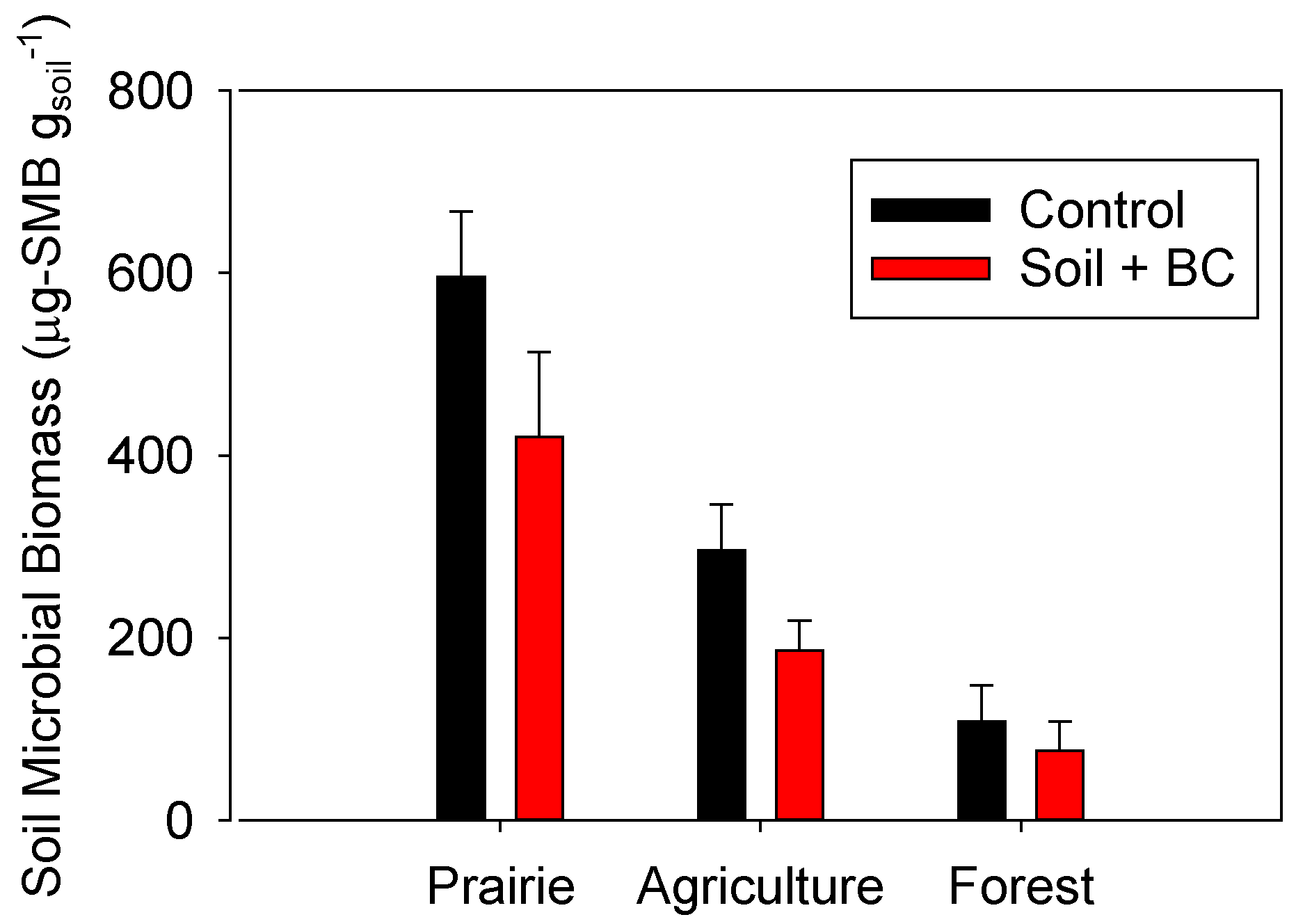
2.5. Microbial Group Functional Assessment of N2O and CO2 Production
| Treatments | CO2 | N2O | ||
|---|---|---|---|---|
| Control | Biochar | Control | Biochar | |
| μg-C g−1 h−1 | μg-C g−1 h−1 | ng-N g−1 h−1 | ng-N g−1 h−1 | |
| Glucose Addition | 26.9 (3.2) a | 18.90 (4.20) a | 32.5 (2.2) a | 22.70 (4.10) a |
| Streptomycin + Glucose | 19.9 (2.3) b | 16.58 (5.10) a | 22.6 (3.4) b | 18.85 (2.00) a |
| Cyclohexamide + Glucose | 8.4 (3.7) c | 9.50 (4.20) b | 16.9 (4.5) c | 14.55 (3.10) b |
| Both inhibitors + Glucose | 9.4 (3.4) c | 10.00 (3.50) b | 12.8 (3.6) c | 16.52 (4.20) b |
| Calculations | ||||
| Net Microbial Inhibition (%) | 64.84 | 47.09 | 60.82 | 27.22 |
| Bacterial Contribution (%) | 26.07 | 12.28 * | 30.70 | 16.96 * |
| Fungal (%) | 68.57 | 49.74 * | 48.22 | 35.90 |
| IAR | 1.46 | 1.32 | 1.30 | 1.94 |
| Fungal: Bacterial | 2.6 | 4.1 * | 1.6 | 2.1 * |
| Treatments | Control Biochar | Control Biochar | ||
|---|---|---|---|---|
| CO2 | CO2 | N2O | N2O | |
| μg-C-CO2 g−1 h−1 | μg-C-CO2 g−1 h−1 | ng-N-N2O g−1 h−1 | ng-N-N2O g−1 h−1 | |
| Glucose Addition | 13.3 (1.2) a | 8.4 (1.5) a | 27.7 (2.0) a | 10.1 (2.0) a |
| Streptomycin + Glucose | 7.8 (1.3) b | 6.9 (1.1) a | 18.5 (1.8) b | 9.5 (0.6) a |
| Cyclohexamide + Glucose | 5.4 (0.6) c | 3.2 (0.7) b | 14.8 (2.2) b | 9.0 (0.8) a |
| Both inhibitors + Glucose | 4.3 (1.4) c | 3.0 (1.1) b | 10.4 (3.4) c | 8.5 (0.9) a |
| Calculations | ||||
| Net Microbial Inhibition (%) | 68.09 | 64.35 | 62.45 | 15.84 * |
| Bacterial Contribution (%) | 41.25 | 18.06 * | 33.39 | 5.94 * |
| Fungal (%) | 59.20 | 62.32 | 46.68 | 10.89 * |
| IAR | 1.48 | 1.25 | 1.28 | 1.06 |
| Fungal: Bacterial | 1.4 | 3.5 * | 1.4 | 1.8 * |
| Treatments | Control | Soil + Biochar | Control | Soil + Biochar |
|---|---|---|---|---|
| CO2 | CO2 | N2O | N2O | |
| µg-C-CO2 g−1 h−1 | µg-C-CO2 g−1 h−1 | ng-N-N2O g−1 h−1 | ng-N-N2O g−1 h−1 | |
| Glucose Addition | 4.9 (1.0) a | 3.44 (0.80) a | 3.6 (1.0) a | 2.70 (0.50) a |
| Streptomycin + Glucose | 1.9 (0.9) b | 3.30 (0.90) a | 1.9 (0.9) a | 2.50 (0.90) a |
| Cyclohexamide + Glucose | 3.6 (0.6) a | 3.20 (1.00) a | 2.8 (0.8) a | 2.60 (0.40) a |
| Both inhibitors + Glucose | 2.1 (0.5) b | 2.50 (0.90) a | 2.0 (0.8) a | 2.50 (0.56) a |
| Calculations | ||||
| Net Microbial Inhibition (%) | 57.73 | 27.33 * | 45.07 | 7.41 * |
| Bacterial Contribution (%) | 60.82 | 4.07 * | 46.48 | 7.41 * |
| Fungal (%) | 26.80 | 6.98 * | 21.97 | 3.70 * |
| IAR | 1.52 | 0.40 | 1.52 | 1.50 |
| Fungal: Bacterial | 0.4 | 1.7 * | 0.5 | 0.5 |
3. Discussion
4. Experimental Section
4.1. Soils and Biochar
4.2. Incubations


4.3. Measurements and Analysis

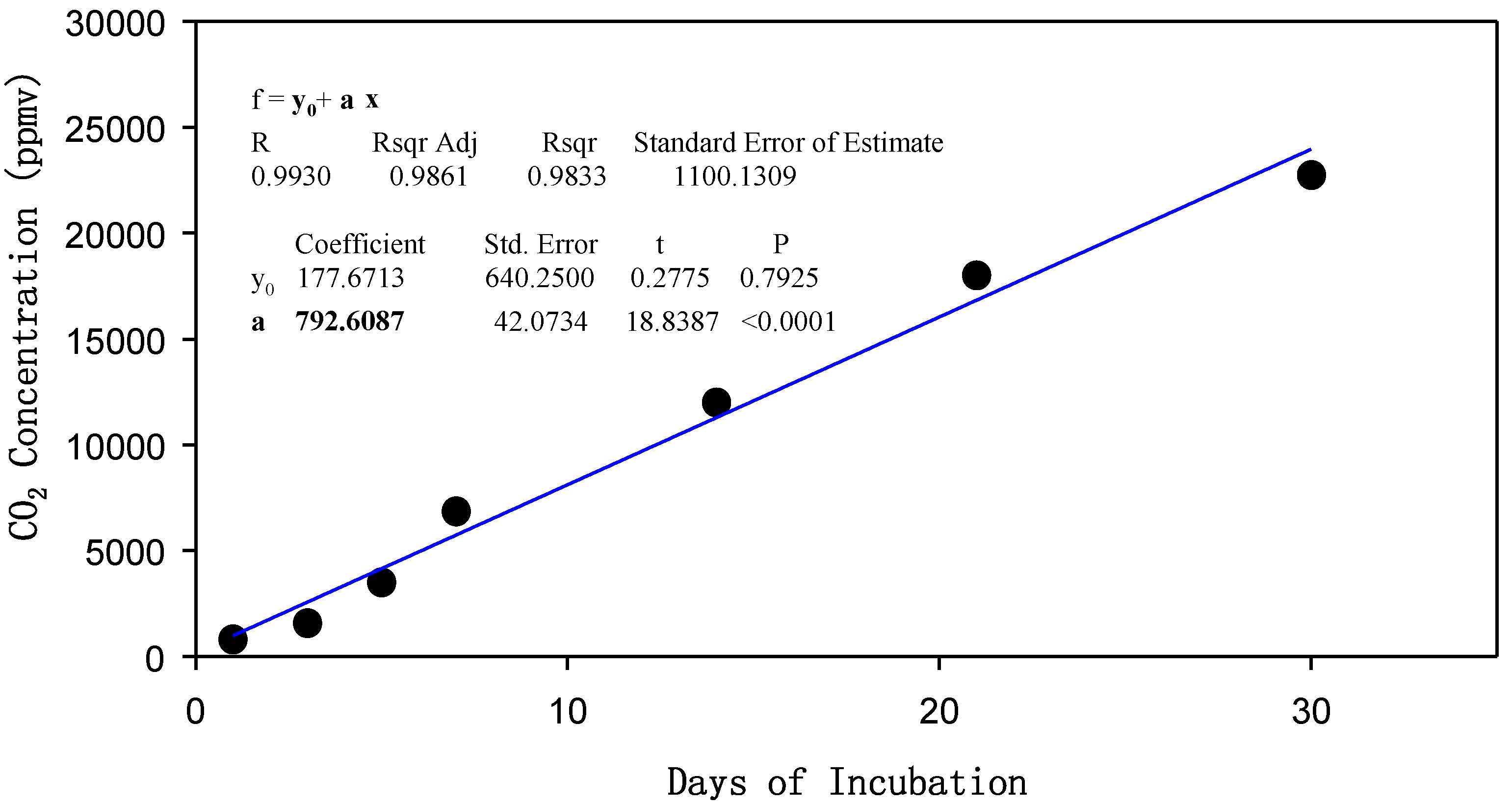
4.4. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [PubMed]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agriculutral use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Garcia, M.; Förster, B.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Singh, B.P.; Kimber, S.W.L.; Murphy, D.V.; Macdonald, L.M.; Rust, J.; Morris, S. An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agric. Ecosyst. Environ. 2014, 191, 53–62. [Google Scholar] [CrossRef]
- Martin, S.M.; Kookana, R.S.; Van Zwieten, L.; Krull, E. Marked changes in herbicide sorption–desorption upon ageing of biochars in soil. J. Hazard. Mater. 2012, 231–232, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A systematic review of biochar research, with a focus on its stability in situ and its promise as a climate mitigation strategy. PLoS One 2013, 8, e75932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar]
- Cayuela, M.L.; Oenema, O.; Kuikman, P.J.; Bakker, R.R.; Van Groenigen, J.W. Bioenergy by-products as soil amendments? Implications for carbon sequestration and greenhouse gas emissions. GCB Bioenergy 2010, 2, 201–213. [Google Scholar]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Spokas, K.; Reicosky, D. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 2009, 3, 179–193. [Google Scholar]
- Spokas, K.A. Impact of biochar field aging on laboratory greenhouse gas production potentials. GCB Bioenergy 2013, 5, 165–176. [Google Scholar] [CrossRef]
- Mills, R.T.E.; Dewhirst, N.; Sowerby, A.; Emmett, B.A.; Jones, D.L. Interactive effects of depth and temperature on CH4 and N2O flux in a shallow podzol. Soil Biol. Biochem. 2013, 62, 1–4. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar]
- Angst, T.E.; Patterson, C.J.; Reay, D.S.; Anderson, P.; Peshkur, T.A.; Sohi, S.P. Biochar diminishes nitrous oxide and nitrate leaching from diverse nutrient sources. J. Environ. Qual. 2013, 42, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. B 2012, 367, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Cornelissen, G.; Rutherford, D.; Arp, H.P.H.; Doersch, P.; Kelly, C.N.; Rostad, C.E. Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ. Sci. Technol. 2013, 47, 7704–7712. [Google Scholar] [CrossRef] [PubMed]
- Hanono, F.; Lerner, E. Adsorption of NO and N2O on charcoal between 195 and 300 K. J. Catal. 1978, 51, 398–405. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Mechanism of ammonia retention on graphite oxides: Role of surface chemistry and structure. J. Phys. Chem. C 2007, 111, 15596–15604. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil—The role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a Southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Meller Harel, Y.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar]
- Nelson, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Plant Biol. 2012, 63, 107–130. [Google Scholar] [CrossRef]
- Clough, T.J.; Bertram, J.E.; Ray, J.L.; Condron, L.M.; O’Callaghan, M.; Sherlock, R.R.; Wells, N.S. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Sci. Soc. Am. J. 2010, 74, 852–860. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; duSaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 2010, 333, 443–452. [Google Scholar] [CrossRef]
- Spokas, K.; Novak, J.; Venterea, R. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.; Sherlock, R.; Condron, L. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar]
- Keeley, J.E.; Morton, B.A.; Pedrosa, A.; Trotter, P. Role of allelopathy, heat and charred wood in the germination of chaparral herbs and suffrutescents. J. Ecol. 1985, 73, 445–458. [Google Scholar] [CrossRef]
- Inderjit; Callaway, R.M. Experimental designs for the study of allelopathy. Plant Soil 2003, 256, 1–11. [Google Scholar]
- Putnam, A.R.; Duke, W.B. Allelopathy in agroecosystems. Annu. Rev. Phytopathol. 1978, 16, 431–451. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J 2014, 8, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar]
- Feng, Y.; Xu, Y.; Yu, Y.; Xie, Z.; Lin, X. Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol. Biochem. 2012, 46, 80–88. [Google Scholar] [CrossRef]
- Castaldi, S.; Riondino, M.; Baronti, S.; Esposito, F.R.; Marzaioli, R.; Rutigliano, F.A.; Vaccari, F.P.; Miglietta, F. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 2011, 85, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H., Jr. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Ducey, T.F.; Ippolito, J.A.; Cantrell, K.B.; Novak, J.M.; Lentz, R.D. Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl. Soil Ecol. 2013, 65, 65–72. [Google Scholar] [CrossRef]
- Bustin, S. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial sourceas well as the magnitude of N2O emission from soil. Biol. Fertility Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Lu, X.; Yan, Y.; Fan, J.; Wang, X. Gross nitrification and denitrification in alpine grassland ecosystems on the Tibetan Plateau. Arct. Antarct. Alp. Res. 2012, 44, 188–196. [Google Scholar] [CrossRef]
- Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol. Ecol. 2010, 74, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Dancer, W.; Peterson, L.; Chesters, G. Ammonification and nitrification of N as influenced by soil pH and previous N treatments. Soil Sci. Soc. Am. J. 1973, 37, 67–69. [Google Scholar] [CrossRef]
- Durán, U.; Val del Río, A.; Campos, J.; Mosquera-Corral, A.; Méndez, R. Enhanced ammonia removal at room temperature by pH controlled partial nitrification and subsequent anaerobic ammonium oxidation. Environ. Technol. 2014, 35, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Sutka, R.L.; Ostrom, N.; Ostrom, P.; Breznak, J.; Gandhi, H.; Pitt, A.; Li, F. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl. Environ. Microbiol. 2006, 72, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Liiri, M.; Ilmarinen, K.; Setälä, H. The significance of Cognettia sphagnetorum (Enchytraeidae) on nitrogen availability and plant growth in wood ash-treated humus soil. Plant Soil 2002, 246, 31–39. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Baggs, E.M.; Johnson, D. Nitrous oxide production by the ectomycorrhizal fungi Paxillus involutus and Tylospora fibrillosa. FEMS Microbiol. Lett. 2011, 316, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Crenshaw, C.; Lauber, C.; Sinsabaugh, R.; Stavely, L. Fungal control of nitrous oxide production in semiarid grassland. Biogeochemistry 2008, 87, 17–27. [Google Scholar] [CrossRef]
- Martens, D.A.; Loeffelmann, K.L. Soil amino acid composition quantified by acid hydrolysis and anion chromatography-pulsed amperometry. J. Agric. Food Chem. 2003, 51, 6521–6529. [Google Scholar] [CrossRef]
- McLain, J.E.; Martens, D.A. N2O production by heterotrophic N transformations in a semiarid soil. Appl. Soil Ecol. 2006, 32, 253–263. [Google Scholar] [CrossRef]
- Laughlin, R.J.; Stevens, R.J. Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Sci. Soc. Am. J. 2002, 66, 1540–1548. [Google Scholar] [CrossRef]
- Chalk, P.M.; Smith, C.J. Chemodenitrification. In Gaseous Loss of Nitrogen from Plant-Soil Systems; Freney, J.R., Simpson, J.R., Eds.; Springer Press: Amsterdam, Netherlands, 1983; Volume 9, pp. 65–89. [Google Scholar]
- Hansen, H.C.B.; Koch, C.B.; Nancke-Krogh, H.; Borggaard, O.K.; Sørensen, J. Abiotic nitrate reduction to ammonium: Key role of green rust. Environ. Sci. Technol. 1996, 30, 2053–2056. [Google Scholar] [CrossRef]
- Moraghan, J.T.; Buresh, R.J. Chemical reduction of nitrite and nitrous oxide by ferrous iron. Soil Sci. Soc. Am. J. 1977, 41, 47–50. [Google Scholar] [CrossRef]
- Chao, T.-T.; Kroontje, W. Inorganic nitrogen transformations through the oxidation and reduction of iron. Soil Sci. Soc. Am. J. 1966, 30, 193–196. [Google Scholar] [CrossRef]
- Van Cleemput, O.; Baert, L. Nitrite stability influenced by iron compounds. Soil Biol. Biochem. 1983, 15, 137–140. [Google Scholar]
- Sorensen, J. Nitrate reduction in marine sediment: Pathways and interactions with iron and sulfur cycling. Geomicrobiol. J. 1987, 5, 401–421. [Google Scholar]
- Samarkin, V.A.; Madigan, M.T.; Bowles, M.W.; Casciotti, K.L.; Priscu, J.C.; McKay, C.P.; Joye, S.B. Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nat. Geosci. 2010, 3, 341–344. [Google Scholar] [CrossRef]
- Zhu, X.; Silva, L.C.R.; Doane, T.A.; Horwath, W.R. Iron: The forgotten driver of nitrous oxide production in agricultural soil. PLoS ONE 2013, 8, e60146. [Google Scholar] [CrossRef] [PubMed]
- Postma, D. Kinetics of nitrate reduction by detrital Fe(II)-silicates. Geochim. Cosmochim. Acta 1990, 54, 903–908. [Google Scholar] [CrossRef]
- Noda, K.; Chambrion, P.; Kyotani, T.; Tomita, A. A study of the N2 formation mechanism in carbon–N2O reaction by using isotope gases. Energy Fuel. 1999, 13, 941–946. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sust. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Atkinson, C.; Fitzgerald, J.; Hipps, N. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Bailey, V.; Smith, J.; Bolton, H. Novel antibiotics as inhibitors for the selective respiratory inhibition method of measuring fungal: Bacterial ratios in soil. Biol. Fertility Soils 2003, 38, 154–160. [Google Scholar] [CrossRef]
- Semenov, M.V.; Stolnikova, E.V.; Ananyeva, N.D.; Ivashchenko, K.V. Structure of the microbial community in soil catena of the right bank of the Oka River. Biol. Bull. 2013, 40, 266–274. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Castaldi, S.; Stolnikova, E.V.; Kudeyarov, V.N.; Valentini, R. Fungi-to-bacteria ratio in soils of European Russia. Arch. Agron. Soil Sci. 2014, in press. [Google Scholar]
- Cooper, D.C.; Picardal, F.F.; Coby, A.J. Interactions between microbial iron reduction and metal geochemistry: Effect of redox cycling on transition metal speciation in iron bearing sediments. Environ. Sci. Technol. 2006, 40, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Coby, A.J.; Picardal, F.W. Inhibition of NO3− and NO2− reduction by microbial Fe (III) reduction: Evidence of a reaction between NO2− and cell surface-bound Fe2+. Appl. Environ. Microbiol. 2005, 71, 5267–5274. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Uchimiya, M.; Sposito, G. Iron (III) bioreduction in soil in the presence of added humic substances. Soil Sci. Soc. Am. J. 2009, 73, 65–71. [Google Scholar] [CrossRef]
- Sang, C.; Kim, B.H.; Lund, C.R. Effect of NO upon N2O decomposition over Fe/ZSM-5 with low iron loading. J. Phys. Chem. B 2005, 109, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- March, J. Advanced Organic Chemistry, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Petit, C.; Bandosz, T.J. Role of aluminum oxycations in retention of ammonia on modified activated carbons. J. Phys. Chem. C. 2007, 111, 16445–16452. [Google Scholar] [CrossRef]
- Huang, C.-C.; Li, H.-S.; Chen, C.-H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Jastrow, J. Changes in soil aggregation associated with tallgrass prairie restoration. Am. J. Bot. 1987, 74, 1656–1664. [Google Scholar] [CrossRef]
- Chang, A.C.; Lund, L.J.; Page, A.L.; Warneke, J.E. Physical properties of fly ash-amended soils. J. Environ. Qual. 1977, 6, 267–270. [Google Scholar] [CrossRef]
- Huang, Z.T.; Petrovic, A.M. Physical properties of sand as affected by clinoptilolite zeolite particle size and quantity. J. Turfgrass Manag. 1994, 1, 1–15. [Google Scholar] [CrossRef]
- Groffman, P.M.; Tiedje, J.M. Denitrification hysteresis during wetting and drying cycles in soil. Soil Sci. Soc. Am. J. 1988, 52, 1626–1629. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci. Soc. Am. J. 2003, 67, 798–805. [Google Scholar] [CrossRef]
- Tortoso, A.C.; Hutchinson, G. Contributions of autotrophic and heterotrophic nitrifiers to soil NO and N2O emissions. Appl. Environ. Microbiol. 1990, 56, 1799–1805. [Google Scholar] [PubMed]
- Soil quality—Determination of soil microbial biomass—Part 1: Substrate-induced respiration method, ISO Standard 14240.1997. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=21530 (accessed on 30 October 2014).
- Klemedtsson, L.; Svensson, B.H.; Rosswall, T. Relationships between soil moisture content and nitrous oxide production during nitrification and denitrification. Biol. Fertility Soils 1988, 6, 106–111. [Google Scholar]
- Klemedtsson, L.; Svensson, B.H.; Rosswall, T. A method of selective inhibition to distinguish between nitrification and denitrification as sources of nitrous oxide in soil. Biol. Fertility Soils 1988, 6, 112–119. [Google Scholar]
- Marusenko, Y.; Huber, D.P.; Hall, S.J. Fungi mediate nitrous oxide production but not ammonia oxidation in aridland soils of the southwestern US. Soil Biol. Biochem. 2013, 63, 24–36. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- A fan is used to ensure that ambient laboratory air is mixed and properly sampled, particularly avoiding the impact of laboratory personnel exhaling and influencing the air sample, 2000
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Spokas, K.A.; Venterea, R.T.; Zhang, R.; Baker, J.M.; Feyereisen, G.W. Assessing Microbial Contributions to N2O Impacts Following Biochar Additions. Agronomy 2014, 4, 478-496. https://doi.org/10.3390/agronomy4040478
Lin X, Spokas KA, Venterea RT, Zhang R, Baker JM, Feyereisen GW. Assessing Microbial Contributions to N2O Impacts Following Biochar Additions. Agronomy. 2014; 4(4):478-496. https://doi.org/10.3390/agronomy4040478
Chicago/Turabian StyleLin, Xiurong, Kurt A. Spokas, Rodney T. Venterea, Renduo Zhang, John M. Baker, and Gary W. Feyereisen. 2014. "Assessing Microbial Contributions to N2O Impacts Following Biochar Additions" Agronomy 4, no. 4: 478-496. https://doi.org/10.3390/agronomy4040478
APA StyleLin, X., Spokas, K. A., Venterea, R. T., Zhang, R., Baker, J. M., & Feyereisen, G. W. (2014). Assessing Microbial Contributions to N2O Impacts Following Biochar Additions. Agronomy, 4(4), 478-496. https://doi.org/10.3390/agronomy4040478





