Responses of Fourteen Vietnamese Rice (Oryza sativa L.) Cultivars to High Temperatures during Grain Filling Period under Field Conditions
Abstract
:1. Introduction
2. Results
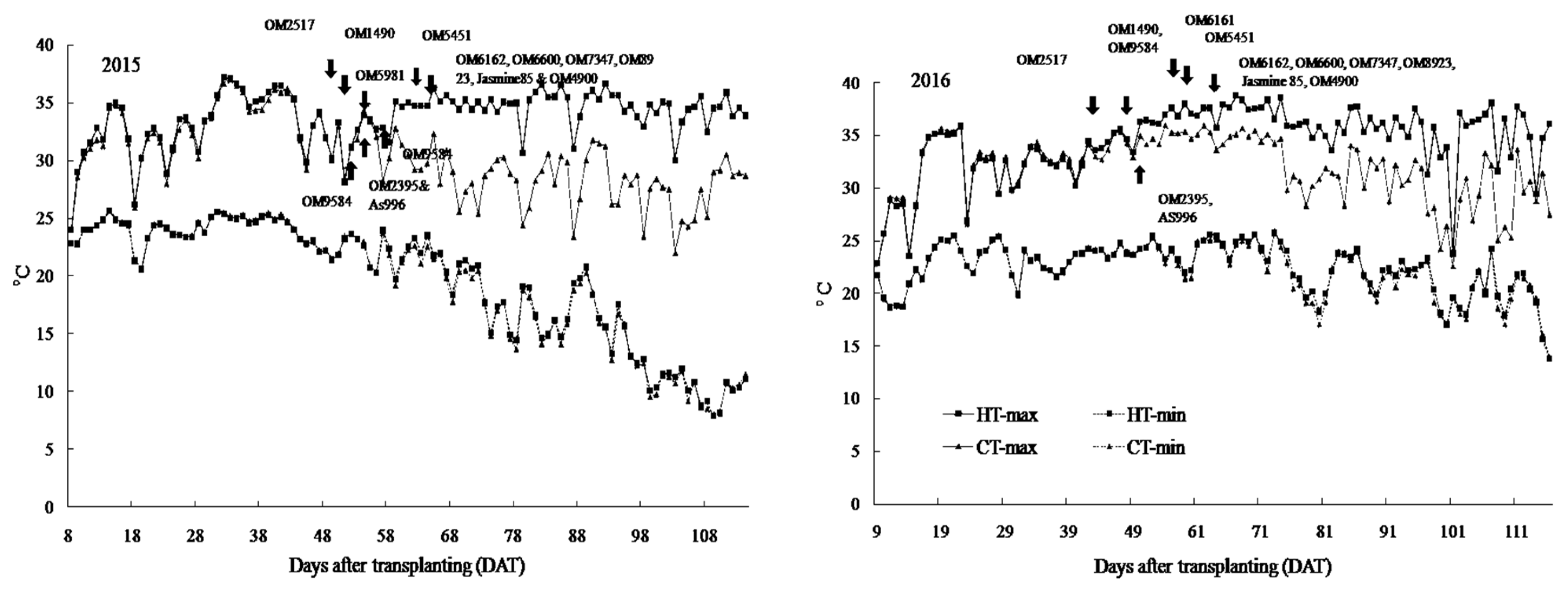
2.1. Air Temperature
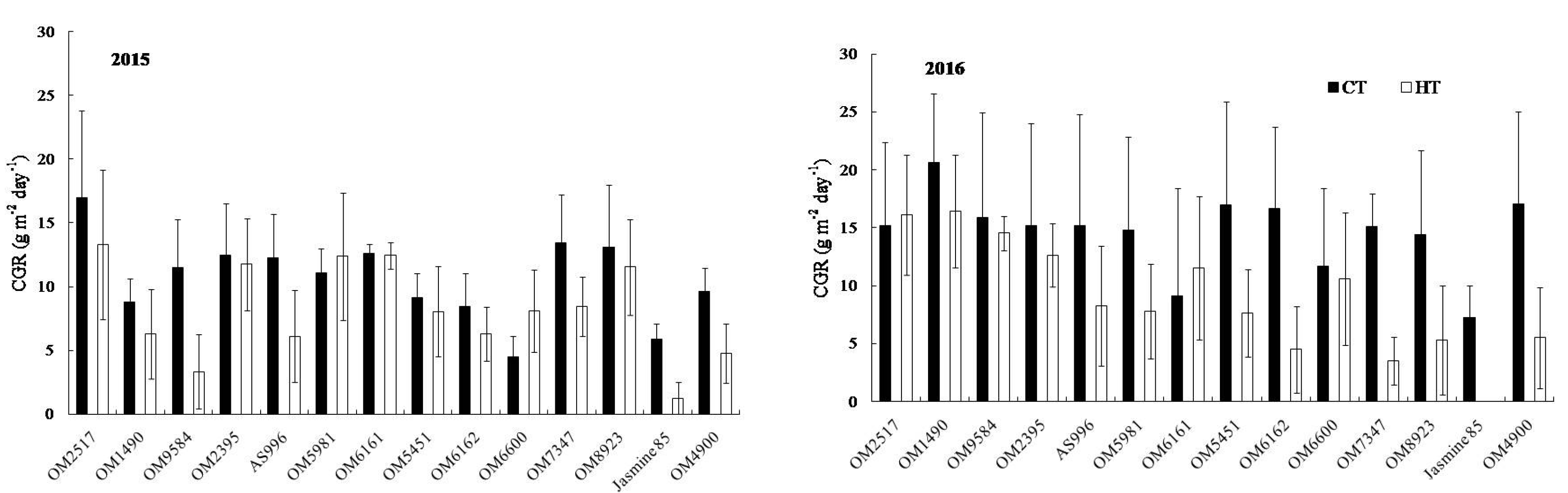
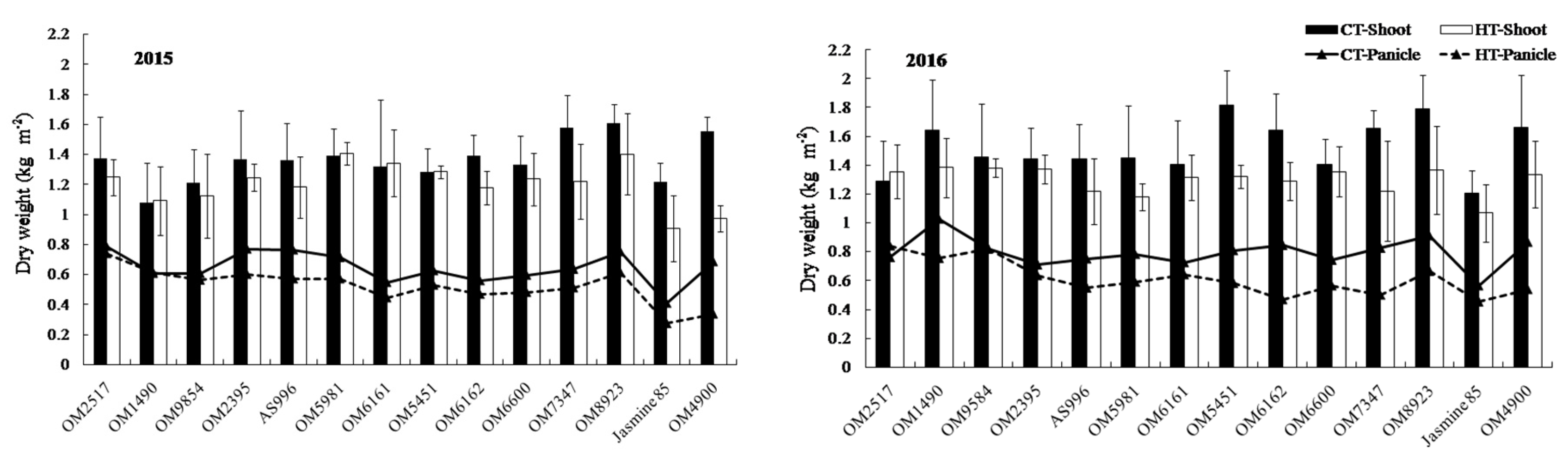
2.2. Dry Weight and Crop Growth Rate
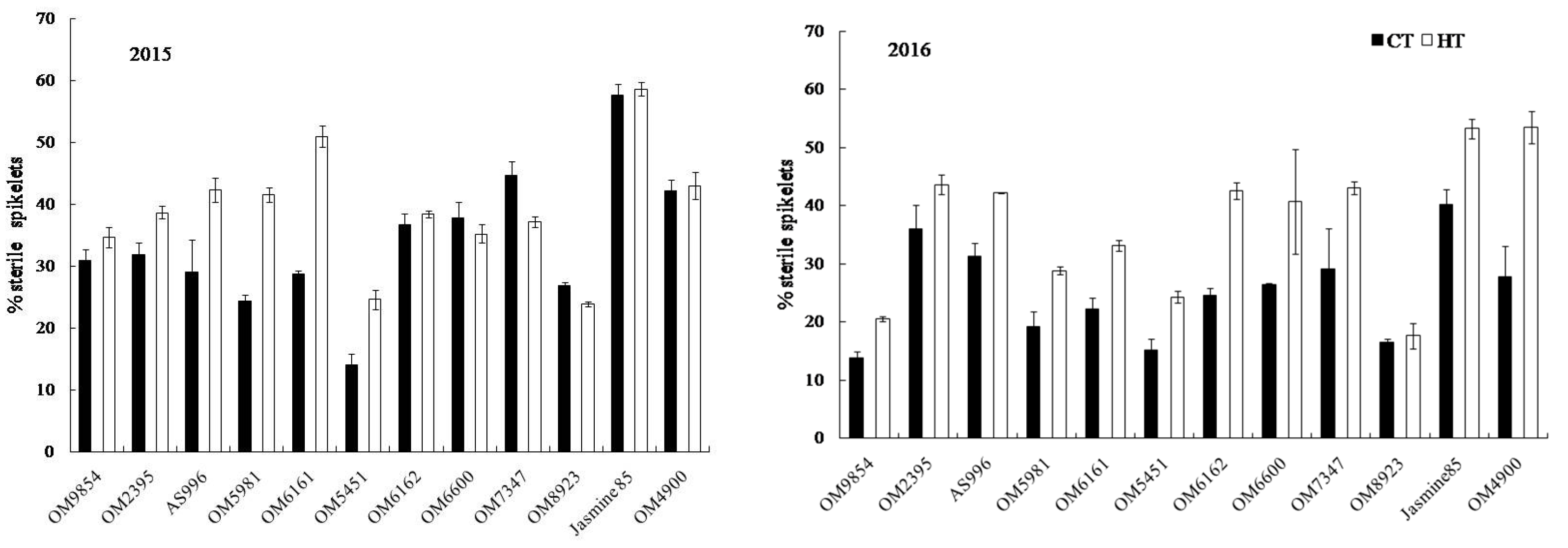
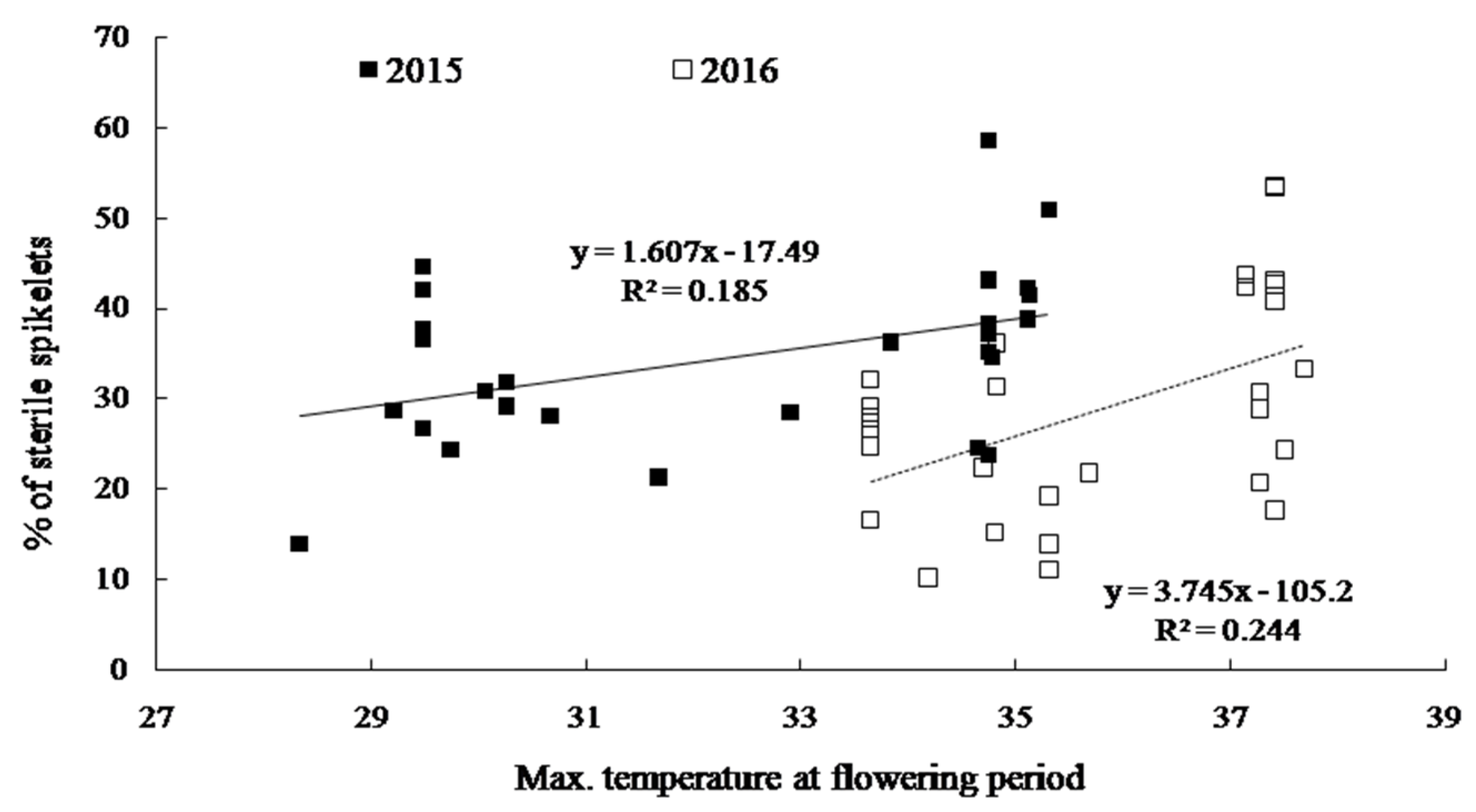
2.3. Yield and Yield Components
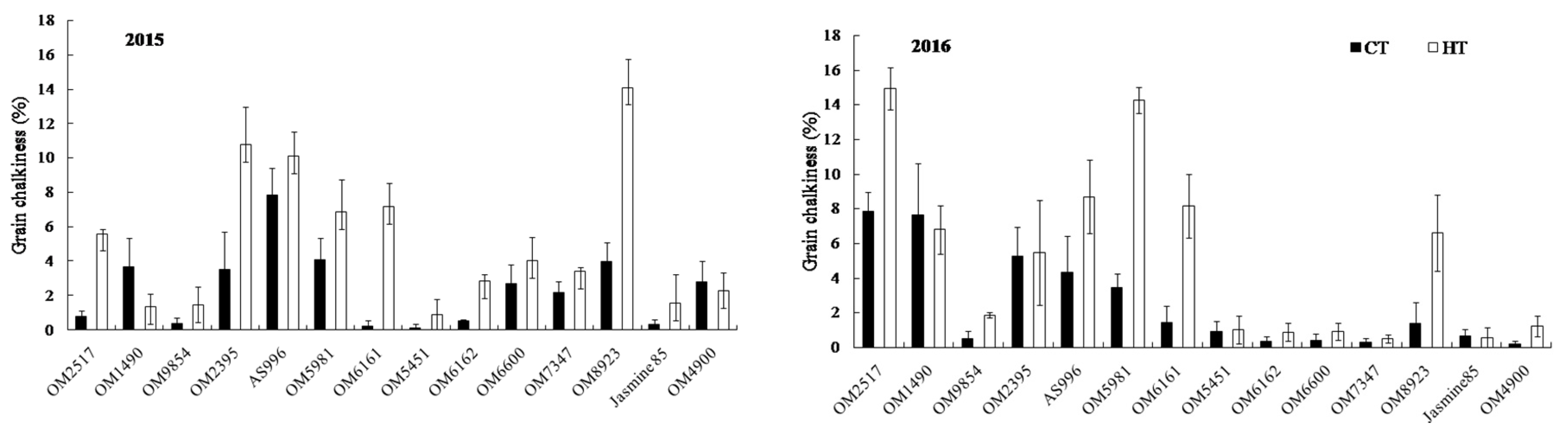
2.4. Grain Appearance Quality
3. Discussion
4. Materials and Methods
4.1. Rice Cultivation
4.2. Temperature Treatment
4.3. Measurement of Air Temperature
4.4. Growth and Dry Weight
- W1: The dry weight at the corresponding date t1.
- W2: The dry weight at the corresponding date t2.
4.5. Yield and Yield Components
4.6. Grain Appearance Quality
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). RICE: Food Outlook Global Market Analysis; FAO: Rome, Italy, 2008. [Google Scholar]
- Cooper, N.T.W.; Siebenmorgen, T.J.; Counce, P.A. Effects of Nighttime Temperature during Kernel Development on Rice Physicochemical Properties. Cereal Chem. J. 2008, 85, 276–282. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2001: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; ISBN 978-0-521-80767-8. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; ISBN 978-0-521-88009-1. [Google Scholar]
- Thang, N.; Trung, L.D.; Dat, V.H.; Phuong, N.T. Poverty, Poverty Reduction and Poverty Dynamics in Vietnam; Background Paper for the Chronic Poverty Report; Chronic Poverty Research Center: Manchester, UK, 2006. [Google Scholar]
- Yin, X.; Kropff, M.J.; Goudriaan, J. Differential Effects of Day and Night Temperature on Development to Flowering in Rice. Ann. Bot. 1996, 77, 203–213. [Google Scholar] [CrossRef]
- Oh-e, I.; Saitoh, K.; Kuroda, T. Effects of High Temperature on Growth, Yield and Dry-Matter Production of Rice Grown in the Paddy Field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H.; Boote, K.J. Response of rice to carbon dioxide and temperature. Agric. For. Meteorol. 1992, 60, 153–166. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Hoshikawa, K. Science of the Rice Plant: Physiology; Food and Agriculture Policy Research Center: Tokyo, Japan, 1993. [Google Scholar]
- Xie, X.J.; Shen, S.H.H.; Li, Y.X.; Zhao, X.Y.; Li, B.B.; Xu, D.F. Effect of photosynthetic characteristic and dry matter accumulation of rice under high temperature at heading stage. Afr. J. Agric. Res. 2011, 6, 1931–1940. [Google Scholar]
- Karimi, M.M.; Siddique, K.H.M. Crop growth and relative growth rates of old and modern wheat cultivars. Aust. J. Agric. Res. 1991, 42, 13–20. [Google Scholar] [CrossRef]
- Takai, T.; Matsuura, S.; Nishio, T.; Ohsumi, A.; Shiraiwa, T.; Horie, T. Rice yield potential is closely related to crop growth rate during late reproductive period. Field Crops Res. 2006, 96, 328–335. [Google Scholar] [CrossRef]
- Ministry of Education, Culture, Sports, Science and Technology (MEXT); Japan Meteorological Agency (JMA) and Ministry of the Environment (MOE). Climate Change and Its Impact in Japan: Synthetic Report on Observations, Projections and Impact Assessments of Climate Change; MEXT; JMA; MOE: Tokyo, Japan, 2009.
- Nguyen, D.-N.; Lee, K.-J.; Kim, D.-I.; Anh, N.T.; Lee, B.-W. Modeling and validation of high-temperature induced spikelet sterility in rice. Field Crops Res. 2014, 156, 293–302. [Google Scholar] [CrossRef]
- Das, S.; Krishnan, P.; Nayak, M.; Ramakrishnan, B. High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot. 2014, 101, 36–46. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. Comparison between Anthers of two Rice (Oryza saliva L.) Cultivars with Tolerance to High Temperatures at Flowering or Susceptibility. Plant Prod. Sci. 2001, 4, 36–40. [Google Scholar] [CrossRef]
- Yoshida, S.; Hara, T. Effects of air temperature and light on grain filling of an indica and a japonica rice (Oryza sativa L.) under controlled environmental conditions. Soil Sci. Plant Nutr. 1977, 23, 93–107. [Google Scholar] [CrossRef]
- Kim, H.R.; Young, Y.H. The Effects of the Elevated CO2 Concentration and Increased Temperature on Growth, Yield and Physiological Responses of Rice (Oryza sativa L. cv. Junam). Adv. Biores. 2010, 1, 46–50. [Google Scholar]
- Wang, F.; Chen, S.; Cheng, F.; Liu, Y.; Zhang, G. The Differences in Grain Weight and Quality Within a Rice (Oryza sativa L.) Panicle as Affected by Panicle Type and Source-sink Relation. J. Agron. Crop Sci. 2007, 193, 63–73. [Google Scholar] [CrossRef]
- Murakami, M.K.M. Manual of handmade field-portable forced ventilation thermometer. Bull. Terr. Environ. Res. Cent. Univ. Tsukuba 2010, 11, 29–33. [Google Scholar]
- Zakaria, S.; Matsuda, T.; Tajima, S.; Nitta, Y. Effect of High Temperature at Ripening Stage on the Reserve Accumulation in Seed in Some Rice Cultivars. Plant Prod. Sci. 2002, 5, 160–168. [Google Scholar] [CrossRef]
- MONRE (Ministry of Natural Resourse and Environment of Vietnam). Climate Change, Sea Level Rise Scenarios for Vietnam; MONRE: Ha Noi, Vietnam, 2009.
- Asian Development Bank (ADB). Viet Nam: Environment and Climate Change Assessment; ADB: Manila, Philippine, 2013; ISBN 978-92-9254-132-3. [Google Scholar]
| Cultivar | Treatment | No. of Panicles m−2 | Spikelets Panicle−1 | % of Ripened Grain | 1000 Grains Weight (g) | Grain Yield (g m−2) |
|---|---|---|---|---|---|---|
| Mean 2015 | CT | 275 | 124 | 67.5 | 25.1 | 568 |
| HT | 250 | 119 | 61.8 | 24.7 | 463 | |
| Mean 2016 | CT | 209 | 155 | 73.9 | 24.9 | 636 |
| HT | 207 | 148 | 61.3 | 24.7 | 505 | |
| Cultivar | ** | ** | ** | ** | ** | |
| Treatment | * | ** | ** | NS | ** |
| Cultivar | Treatment | No. of Panicles m−2 | Spikelets Panicle−1 | % of Ripened Grain | 1000 Grains Weight (g) | Grain Yield (g m−2) |
|---|---|---|---|---|---|---|
| OM2517 | CT | 288 | 108 | 75.9 | 25.2 | 596.1 |
| HT | 285 | 110 | 68.2 | 25.3 | 539.1 | |
| OM1490 | CT | 341 | 111 | 69.4 | 23.2 | 609.6 |
| HT | 306 | 127 | 60.5 | 22.4 | 534.0 | |
| OM9854 | CT | 307 | 113 | 65.0 | 23.9 | 535.1 |
| HT | 271 | 113 | 59.1 | 23.9 | 434.2 | |
| OM2395 | CT | 300 | 120 | 64.0 | 27.1 | 628.1 |
| HT | 240 | 121 | 56.5 | 27.4 | 450.7 | |
| AS996 | CT | 245 | 129 | 66.4 | 26.8 | 561.9 |
| HT | 278 | 124 | 54.0 | 26.8 | 499.9 | |
| OM5981 | CT | 327 | 105 | 72.7 | 25.6 | 637.9 |
| HT | 294 | 107 | 56.1 | 25.2 | 443.7 | |
| OM6161 | CT | 299 | 108 | 70.5 | 25.2 | 571.3 |
| HT | 257 | 103 | 47.6 | 25.5 | 318.9 | |
| OM5451 | CT | 286 | 101 | 80.8 | 24.7 | 574.3 |
| HT | 271 | 98 | 71.3 | 24.7 | 465.6 | |
| OM6162 | CT | 209 | 147 | 60.2 | 24.5 | 452.9 |
| HT | 228 | 135 | 59.4 | 17.4 | 317.2 | |
| OM6600 | CT | 219 | 152 | 58.5 | 24.5 | 478.4 |
| HT | 207 | 144 | 62.3 | 25.0 | 463.0 | |
| OM7347 | CT | 216 | 138 | 61.3 | 25.5 | 465 |
| HT | 233 | 147 | 51.5 | 25.2 | 442.7 | |
| OM8923 | CT | 318 | 118 | 70.3 | 25.5 | 670.0 |
| HT | 269 | 105 | 72.0 | 25.1 | 511.5 | |
| Jasmine85 | CT | 237 | 128 | 39.4 | 25.7 | 307.0 |
| HT | 192 | 112 | 38.2 | 26.2 | 215.5 | |
| OM4900 | CT | 250 | 146 | 54.3 | 25.1 | 497.1 |
| HT | 191 | 131 | 53.2 | 25.3 | 337.0 | |
| Cultivar | ** | ** | ** | ** | ** | |
| Treatment | ** | NS | * | NS | ** |
| Cultivar | Treatment | No. of Panicles m−2 | Spikelets Panicle−1 | % of Ripened Grain | 1000 Grains Weight (g) | Grain Yield (g m−2) |
|---|---|---|---|---|---|---|
| OM2517 | CT | 228 | 119 | 87.1 | 26.6 | 692 |
| HT | 220 | 120 | 75.0 | 26.2 | 572 | |
| OM1490 | CT | 211 | 139 | 87.3 | 24.1 | 677 |
| HT | 236 | 142 | 66.1 | 23.9 | 583 | |
| OM9854 | CT | 209 | 129 | 81.7 | 24.7 | 598 |
| HT | 220 | 137 | 70.5 | 23.9 | 559 | |
| OM2395 | CT | 193 | 140 | 59.9 | 26.6 | 474 |
| HT | 207 | 141 | 52.9 | 27.3 | 464 | |
| AS996 | CT | 227 | 133 | 65.4 | 25.7 | 558 |
| HT | 226 | 127 | 54.6 | 26.9 | 463 | |
| OM5981 | CT | 219 | 135 | 76.2 | 24.7 | 611 |
| HT | 237 | 134 | 65.0 | 25.6 | 579 | |
| OM6161 | CT | 260 | 141 | 76.5 | 25.2 | 778 |
| HT | 216 | 136 | 64.4 | 25.1 | 521 | |
| OM5451 | CT | 233 | 127 | 81.4 | 24.7 | 652 |
| HT | 223 | 137 | 71.8 | 23.8 | 574 | |
| OM6162 | CT | 174 | 210 | 72.4 | 23.7 | 691 |
| HT | 162 | 182 | 55.4 | 23.5 | 422 | |
| OM6600 | CT | 192 | 202 | 70.5 | 23.8 | 715 |
| HT | 199 | 188 | 57.3 | 22.7 | 529 | |
| OM7347 | CT | 163 | 202 | 68.8 | 24.7 | 613 |
| HT | 164 | 182 | 54.9 | 24.4 | 440 | |
| OM8923 | CT | 251 | 138 | 81.6 | 25.2 | 783 |
| HT | 259 | 131 | 79.2 | 24.4 | 723 | |
| Jasmine85 | CT | 151 | 168 | 55.8 | 24.5 | 380 |
| HT | 140 | 149 | 44.6 | 24.4 | 249 | |
| OM4900 | CT | 191 | 185 | 70.4 | 24.6 | 673 |
| HT | 199 | 168 | 45.3 | 24.4 | 404 | |
| Cultivar | ** | ** | ** | ** | ** | |
| Treatment | NS | * | ** | NS | ** |
| Cultivar | Pedigree | Year of Release in Vietnam | Year | Heading Date |
|---|---|---|---|---|
| OM2517 | OM1235/OMCS94 | 2004 | 2015 | 21-August |
| 2016 | 5-August | |||
| OM1490 | OM606/IR44592-62 | 1999 | 2015 | 24-August |
| 2016 | 10-August | |||
| OM9854 | OM6976/OM5451 | _ | 2015 | 27-August |
| 2016 | 10-August | |||
| OM2395 | IR63356/TN1 | 2004 | 2015 | 28-August |
| 2016 | 11-August | |||
| AS996 | IR64/Oryza rufipugon | 2002 | 2015 | 28-August |
| 2016 | 11-August | |||
| OM5981 | IR28/AS996 | 2010 | 2015 | 29-August |
| 2016 | 11-August | |||
| OM6161 | C51/Jasmine85 | 2009 | 2015 | 1-September |
| 2016 | 14-August | |||
| OM5451 | Jasmine85/OM2940 | 2010 | 2015 | 7-September |
| 2016 | 22-August | |||
| OM6162 | C50/Jasmine85 | 2009 | 2015 | 9-September |
| 2016 | 23-August | |||
| OM6600 | C43/Jamine85//C43 | 2011 | 2015 | 9-September |
| 2016 | 25-August | |||
| OM7347 | Khaodawkmali/BL/BL | 2010 | 2015 | 9-September |
| 2016 | 25-August | |||
| OM8923 | OM3536/AS996 | 2011 | 2015 | 9-September |
| 2016 | 25-August | |||
| Jasmine85 | Peta/Taichung Native 1//Khaodawkmali 105 | 1993 | 2015 | 9-September |
| 2016 | 25-August | |||
| OM4900 | C53/Jasmine85//Japonica | 2008 | 2015 | 9-September |
| 2016 | 25-August |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuy, T.L.; Saitoh, K. Responses of Fourteen Vietnamese Rice (Oryza sativa L.) Cultivars to High Temperatures during Grain Filling Period under Field Conditions. Agronomy 2017, 7, 57. https://doi.org/10.3390/agronomy7030057
Thuy TL, Saitoh K. Responses of Fourteen Vietnamese Rice (Oryza sativa L.) Cultivars to High Temperatures during Grain Filling Period under Field Conditions. Agronomy. 2017; 7(3):57. https://doi.org/10.3390/agronomy7030057
Chicago/Turabian StyleThuy, Tran Loc, and Kuniyiki Saitoh. 2017. "Responses of Fourteen Vietnamese Rice (Oryza sativa L.) Cultivars to High Temperatures during Grain Filling Period under Field Conditions" Agronomy 7, no. 3: 57. https://doi.org/10.3390/agronomy7030057
APA StyleThuy, T. L., & Saitoh, K. (2017). Responses of Fourteen Vietnamese Rice (Oryza sativa L.) Cultivars to High Temperatures during Grain Filling Period under Field Conditions. Agronomy, 7(3), 57. https://doi.org/10.3390/agronomy7030057






