Geographic and Research Center Origins of Rice Resistance to Asian Planthoppers and Leafhoppers: Implications for Rice Breeding and Gene Deployment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbivores
2.2. Plant Materials
2.3. Genotyping
2.3.1. Genotyping Assay
2.3.2. DNA Extraction
2.3.3. Genotyping Using Infinium 6K Array
2.4. Phenotyping for Planthopper Resistance
2.4.1. SSSTs
2.4.2. MSSTs
2.4.3. Evaluation of Resistance
2.5. Phenotyping for Leafhopper Resistance
2.6. Responses by Nilaparvata lugens to Resistant Varieties
2.6.1. Nymph Survival and Weight Gain
2.6.2. Oviposition
2.6.3. Biomass Build-Up
2.6.4. Honeydew Production
2.7. Data Analyses
3. Results
3.1. Phylogenetics of the Rice Collection
3.2. Virulence among Nilaparvata lugens Colonies
3.3. Virulence among Sogatella furcifera Colonies
3.4. Response of Nilaparvata lugens to Resistant Rice
3.5. Resistance against Nephotettix virescens
4. Discussion
4.1. Aspects of Virulence Adaptation in Nilaparvata lugens
4.2. Virulence Adaptation among Sogatella furcifera in East Asia
4.3. Responses by Planthoppers to Resistant Rice Varieties and Interpretation of Seedbox Tests
4.4. Origins of Resistance and Local Adaptation
4.5. Research Center Origins of Resistance against Nephotettix virescens
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Painter, R.H. Insect Resistance in Crop Plants; MacMillan: New York, NY, USA, 1951. [Google Scholar]
- Smith, C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches; Springer Science and Business Media: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Savary, S.; Horgan, F.; Willocquet, L.; Heong, K.L. A review of principals for sustainable pest management in rice. Crop Prot. 2012, 32, 54–63. [Google Scholar] [CrossRef]
- Pelletier, Y.; Horgan, F.G.; Pompon, J. Potato resistance to insects. Am. J. Plant Sci. 2011, 5, 37–51. [Google Scholar]
- Horgan, F.G. Integrated pest management for sustainable rice cultivation: A holistic approach. In Achieving Sustainable Cultivation of Rice—Cultivation, Pest and Disease Management; Sasaki, T., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 2, pp. 309–342. [Google Scholar]
- Heinrichs, E.A. Perspectives and directions for the continued development of insect-resistant rice varieties. Agric. Ecosyst. Environ. 1986, 18, 9–36. [Google Scholar] [CrossRef]
- Fujita, D.; Kohli, A.; Horgan, F.G. Rice resistance to planthoppers and leafhoppers. Crit. Rev. Plant Sci. 2013, 32, 162–191. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, C.; He, Y. Recent progress on the genetics and molecular breeding of brown planthopper resistance in rice. Rice 2016, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Khush, G.S.; Virk, P.S. IR Varieties and Their Impact; International Rice Research Institute: Los Baños, Philippines, 2005. [Google Scholar]
- Horgan, F.G.; Ramal, A.F.; Bentur, J.S.; Kumar, R.; Bhanu, K.V.; Sarao, P.S.; Iswanto, E.H.; Van Chien, H.; Phyu, M.H.; Bernal, C.C. Virulence of brown planthopper (Nilaparvata lugens) populations from South and South East Asia against resistant rice varieties. Crop Prot. 2015, 78, 222–231. [Google Scholar] [CrossRef]
- Jairin, J.; Phengrat, K.; Teangdeerith, S.; Vanavichit, A.; Toojinda, T. Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol. Breed. 2007, 19, 35–44. [Google Scholar] [CrossRef]
- Jairin, J.; Sansen, K.; Wongboon, W.; Kothcharerk, J. Detection of a brown planthopper resistance gene bph4 at the same chromosome position of Bph3 using different genetic backgrounds of rice. Breed. Sci. 2010, 60, 71–75. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Wu, C.; Yang, C.; Hua, H.; Gao, G.; Xiao, J.; He, Y. Pyramiding and evaluation of the brown planthopper resistance genes Bph14 and Bph15 in hybrid rice. Mol. Breed. 2012, 29, 61–69. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, M.; Gao, G.; Zhang, Q.; Xiao, J.; He, Y. Pyramiding and evaluation of three dominant brown planthopper resistance genes in the elite indica rice 9311 and its hybrids. Pest Manag. Sci. 2013, 69, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Hechanova, S.L.; Verdeprado, H.; Prahalada, G.D.; Kim, S.-R. Development of 25 near-isogenic lines (NILs) with ten BPH resistance genes in rice (Oryza sativa L.): Production, resistance spectrum, and molecular analysis. Theor. Appl. Genet. 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, T.S.; Almazan, M.L.P.; Bernal, C.C.; Fujita, D.; Ramal, A.F.; Yasui, H.; Subbarayalu, M.K.; Horgan, F.G. Current utility of the BPH25 and BPH26 genes and possibilities for further resistance against plant- and leafhoppers from the donor cultivar ADR52. Appl. Entomol. Zool. 2015, 50, 533–543. [Google Scholar] [CrossRef]
- Horgan, F.G.; Peñalver Cruz, A.; Bernal, C.C.; Ramal, A.F.; Almazan, M.L.P.; Wilby, A. Antiherbivore resistance facilitates tolerance to the brown planthopper, Nilaparvata lugens (Stål) in rice under high nitrogen conditions. Field Crop. Res. 2017. accepted. [Google Scholar]
- Claridge, M.; Den Hollander, J. Virulence to rice cultivars and selection for virulence in populations of the brown planthopper Nilaparvata lugens. Entomol. Exp. Appl. 1982, 32, 213–221. [Google Scholar] [CrossRef]
- Claridge, M.; Den Hollander, J.; Furet, I. Adaptations of brown planthopper (Nilaparvata lugens) populations to rice varieties in Sri Lanka. Entomol. Exp. Appl. 1982, 32, 222–226. [Google Scholar] [CrossRef]
- Ferrater, J.B.; Horgan, F.G. Does Nilaparvata lugens gain tolerance to rice resistance genes through conspecifics at shared feeding sites? Entomol. Exp. Appl. 2016, 160, 77–82. [Google Scholar] [CrossRef]
- Ferrater, J.B.; Naredo, A.I.; Almazan, M.L.P.; de Jong, P.W.; Dicke, M.; Horgan, F.G. Varied responses by yeast-like symbionts during virulence adaptation in a monophagous phloem-feeding insect. Arthropod-Plant Interact. 2015, 9, 215–224. [Google Scholar] [CrossRef]
- Alam, S.N.; Cohen, M.B. Durability of brown planthopper, Nilaparvata lugens, resistance in rice variety IR64 in greenhouse selection studies. Entomol. Exp. Appl. 1998, 89, 71–78. [Google Scholar] [CrossRef]
- Cohen, M.B.; Alam, S.N.; Medina, E.B.; Bernal, C.C. Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: Mechanism and role in successful N. lugens management in Central Luzon, Philippines. Entomol. Exp. Appl. 1997, 85, 221–229. [Google Scholar] [CrossRef]
- Horgan, F.G. Mechanisms of resistance: A major gap in understanding planthopper-rice interactions. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 281–302. [Google Scholar]
- Widawsky, D.; Rozelle, S.; Jin, S.; Huang, J. Pesticide productivity, host-plant resistance and productivity in China. Agric. Econ. 1998, 19, 203–217. [Google Scholar] [CrossRef]
- Horgan, F.G.; Crisol, E. Hybrid rice and insect herbivores in Asia. Entomol. Exp. Appl. 2013, 148, 1–19. [Google Scholar] [CrossRef]
- Ali, M.; Alghamdi, S.; Begum, M.; Anwar Uddin, A.; Alam, M.; Huang, D. Screening of rice genotypes for resistance to the brown planthopper, Nilaparvata lugens Stål. Cereal Res. Commun. 2012, 40, 502–508. [Google Scholar] [CrossRef]
- Bhanu, K.V.; Lakshmi, V.J.; Katti, G.; Reddy, A.V. Antibiosis and tolerance mechanisms of resistance in rice varieties carrying brown planthopper resistance genes. Asian J. Biol. Life Sci. 2014, 3, 108–113. [Google Scholar]
- Horgan, F.G.; Srinivasan, T.S.; Naik, B.S.; Ramal, A.F.; Bernal, C.C.; Almazan, M.L.P. Effects of nitrogen on egg-laying inhibition and ovicidal response in planthopper-resistant rice varieties. Crop Prot. 2016, 89, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.J. High-Throughput SNP genotyping to accelerate crop improvement. Plant Breed. Biotechnol. 2014, 2, 195–212. [Google Scholar] [CrossRef]
- Velusamy, R.; Heinrichs, E.A.; Medrano, F.G. Greenhouse techniques to identify field resistance to the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae), in rice cultivars. Crop Prot. 1986, 5, 328–333. [Google Scholar] [CrossRef]
- Khan, Z.R.; Saxena, R.C. Behavioral and physiological responses of Sogatella furcifera (Homoptera: Delphacidae) to selected resistant and susceptible rice cultivars. J. Econ. Entomol. 1985, 78, 1280–1286. [Google Scholar] [CrossRef]
- Khan, Z.R.; Saxena, R.C. Varietal resistance in rice against Sogatella furcifera (Horváth). Crop Prot. 1986, 5, 15–25. [Google Scholar] [CrossRef]
- Hirae, M.; Fukuta, Y.; Tamura, K.; Oya, S. Artificial selection of biotypes of green rice leafhopper, Nephotettix cincticeps Uhler (Homoptera: Cidadellidae), and virulence to resistant rice varieties. Appl. Entomol. Zool. 2007, 42, 97–107. [Google Scholar] [CrossRef]
- Vu, Q.; Quintana, R.; Fujita, D.; Bernal, C.C.; Yasui, H.; Medina, C.D.; Horgan, F.G. Responses and adaptation by Nephotettix virescens to monogenic and pyramided rice lines with Grh-resistance genes. Entomol. Exp. Appl. 2014, 150, 179–190. [Google Scholar] [CrossRef]
- Verma, S.K.; Pathak, P.K.; Singh, B.N.; Lal, M.N. Indian biotypes of the brown planthopper. Int. Rice Res. Newsl. 1979, 4, 7. [Google Scholar]
- Pathak, P.K.; Verma, S.K. Distinct geographic populations of brown planthopper in India. Int. Rice Res. Newsl. 1980, 5, 12. [Google Scholar]
- Kabir, M.A.; Alam, M.S. Varietal screening for resistance to brown planthopper and its biotype in Bangladesh. Int. Rice Res. Newsl. 1981, 6, 8–9. [Google Scholar]
- Huynh, N.V. New biotype of brown planthopper in the Mekong Delta of Vietnam. Int. Rice Res. Newsl. 1977, 2, 10. [Google Scholar]
- Thuat, N.C.; Thans, D.V. Population dynamics of the brown planthopper (BPH) in the Mekong Delta. Int. Rice Res. Newsl. 1984, 9, 14–15. [Google Scholar]
- Phuong, L.T.; Chau, L.M. Resistance of varieties derived from Oryza sativa/Oryza officinalis to brown planthopper in the Mekong Delta, Vietnam. Int. Rice Res. Newsl. 1997, 22, 26–27. [Google Scholar]
- Peñalver Cruz, A.; Arida, A.; Heong, K.L.; Horgan, F.G. Aspects of brown planthopper adaptation to resistant rice varieties with the Bph3 gene. Entomol. Exp. Appl. 2011, 141, 245–257. [Google Scholar] [CrossRef]
- Myint, K.K.M.; Yasui, H.; Takagi, M.; Matsumura, M. Virulence of long-term laboratory populations of the brown planthopper, Nilaparvata lugens (Stål), and whitebacked planthopper, Sogatella furcifera (Horváth) (Homoptera: Delphacidae), on rice differential varieties. Appl. Entomol. Zool. 2009, 44, 149–153. [Google Scholar] [CrossRef]
- Heinrichs, E.A.; Rapusas, H.R. Responses to selection for virulence of Nephotettix virescens (Homoptera: Cicadellidae) on resistant rice varieties. Environ. Entomol. 1990, 19, 167–175. [Google Scholar] [CrossRef]
- Sharma, N.P.; Torii, A.; Takumi, S.; Mori, N.; Nakamura, C. Marker-assisted pyramiding of brown planthopper (Nilaparvata lugens Stål) resistance genes Bph1 and bph2 on rice chromosome 12. Hereditas 2004, 140, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Z.; Cao, J.; Collins, H.L.; Bates, S.L.; Roush, R.T.; Earle, E.D.; Shelton, A.M. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhang, L.; Ma, Y.; Liu, B.; Zhao, Y.; Yu, H.; Zhou, X.; Qin, R.; Zhu, L.; He, G. Genome-wide mapping of virulence in brown planthopper identifies loci that break down host plant resistance. PLoS ONE 2014, 9, e98911. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yamamoto, K.; Suetsugu, Y.; Kuwazaki, S.; Hattori, M.; Jairin, J.; Sanada-Morimura, S.; Matsumura, M. Genetic mapping of the rice resistance-breaking gene of the brown planthopper Nilaparvata lugens. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140726. [Google Scholar] [CrossRef] [PubMed]
- Romena, A.M.; Rapusas, H.R.; Heinrichs, E.A. Evaluation of rice accessions for resistance to the whitebacked planthopper Sogatella furcifera (Horvath) (Homoptera: Delphacidae). Crop Prot. 1986, 5, 334–340. [Google Scholar] [CrossRef]
- Heinrichs, E.A.; Rapusas, H.R. Levels of resistance to the whitebacked planthopper, Sogatella furcifera (Homoptera: Delphacidae), in rice varieties with different resistance genes. Environ. Entomol. 1983, 12, 1793–1797. [Google Scholar] [CrossRef]
- Alam, S.N.; Cohen, M.B. Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor. Appl. Genet. 1998, 97, 1370–1379. [Google Scholar] [CrossRef]
- Horgan, F.G.; Naik, B.S.; Iswanto, K.H.; Almazan, M.L.P.; Ramal, A.F.; Bernal, C.C. Responses by the brown planthopper, Nilaparvata lugens, to conspecific density on resistant and susceptible rice varieties. Entomol. Exp. Appl. 2016, 158, 284–294. [Google Scholar] [CrossRef]
- Mishra, N.C.; Misra, B.C. Preference of white-backed planthopper, Sogatella furcifera to different rice varieties. Entomol. Exp. Appl. 1991, 59, 87–92. [Google Scholar] [CrossRef]
- Budiyanto, E.; Kamal, I.; Ito, K.; Matsui, M.; Okada, M. Differences of susceptibility in rice varieties to the whitebacked planthopper, Sogatella furcifera Horváth (Homoptera: Delphacidae). Appl. Entomol. Zool. 1986, 21, 629–631. [Google Scholar] [CrossRef]
- Velusamy, R. Resistance of wild rices, Oryza spp., to the whitebacked planthopper Sogatella furcifera (Horváth) (Homoptera: Delphacidae). Crop Prot. 1989, 8, 265–270. [Google Scholar] [CrossRef]
- Mishra, N.C.; Misra, B.C. Mortality of white-backed planthopper (Sogatella furcifera) on resistant varieties of rice (Oryza sativa). Indian J. Agric. Sci. 1992, 62, 235–237. [Google Scholar]
- Nalini, R.; Gunathilagaraj, K. Measure of tolerance level in rice (Oryza sativa) accessions resistant to white-backed planthopper (Sogatella furcifera). Indian J. Agric. Sci. 1994, 64, 583–587. [Google Scholar]
- Kimmins, F.M. Electrical penetration graphs from Nilaparvata lugens on resistant and susceptible rice varieties. Entomol. Exp. Appl. 1989, 50, 69–79. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, L.; He, G. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 2013, 6, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Liu, G.; Qiang, Q. Prevalence of whitebacked planthoppers in Chinese hybrid rice and whitebacked planthopper resistance in Chinese japonica rice. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 257–280. [Google Scholar]
- Brar, D.S.; Virk, P.S.; Jena, K.K.; Khush, G.S. Breeding for resistance to planthoppers in rice. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 401–428. [Google Scholar]
- Dahal, G.; Hibino, H.; Aguiero, V.M. Population characteristics and tungro transmission by Nephotettix virescens (Hemiptera: Cicadellidae) on selected resistant rice cultivars. Bull. Entomol. Res. 1997, 87, 387–395. [Google Scholar] [CrossRef]
- Heong, K.L.; Manza, A.; Catindig, J.; Villareal, S.; Jacobsen, T. Changes in pesticide use and arthropod biodiversity in the IRRI research farm. Outlooks Pest Manag. 2007, 18, 229–233. [Google Scholar] [CrossRef]
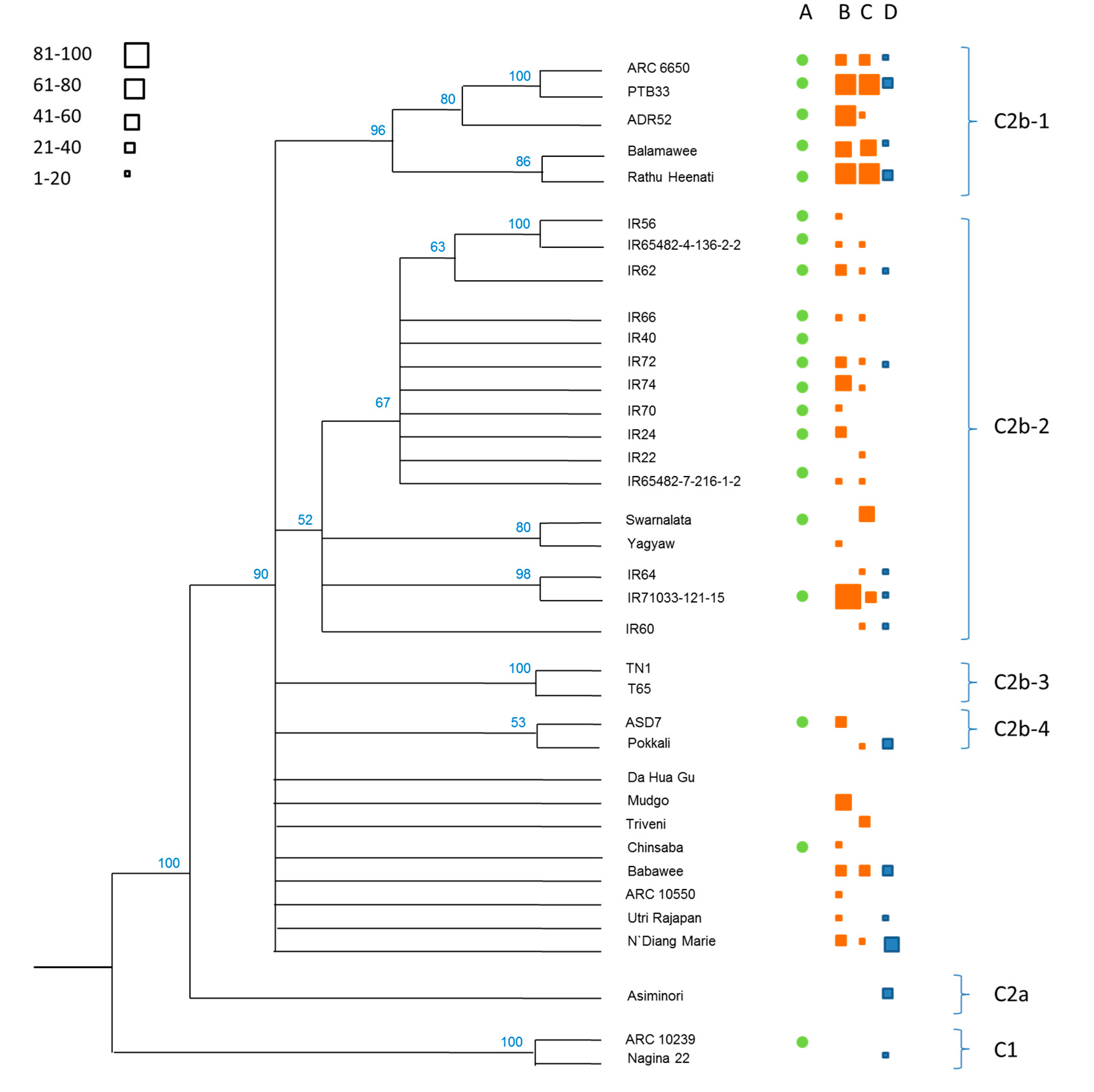

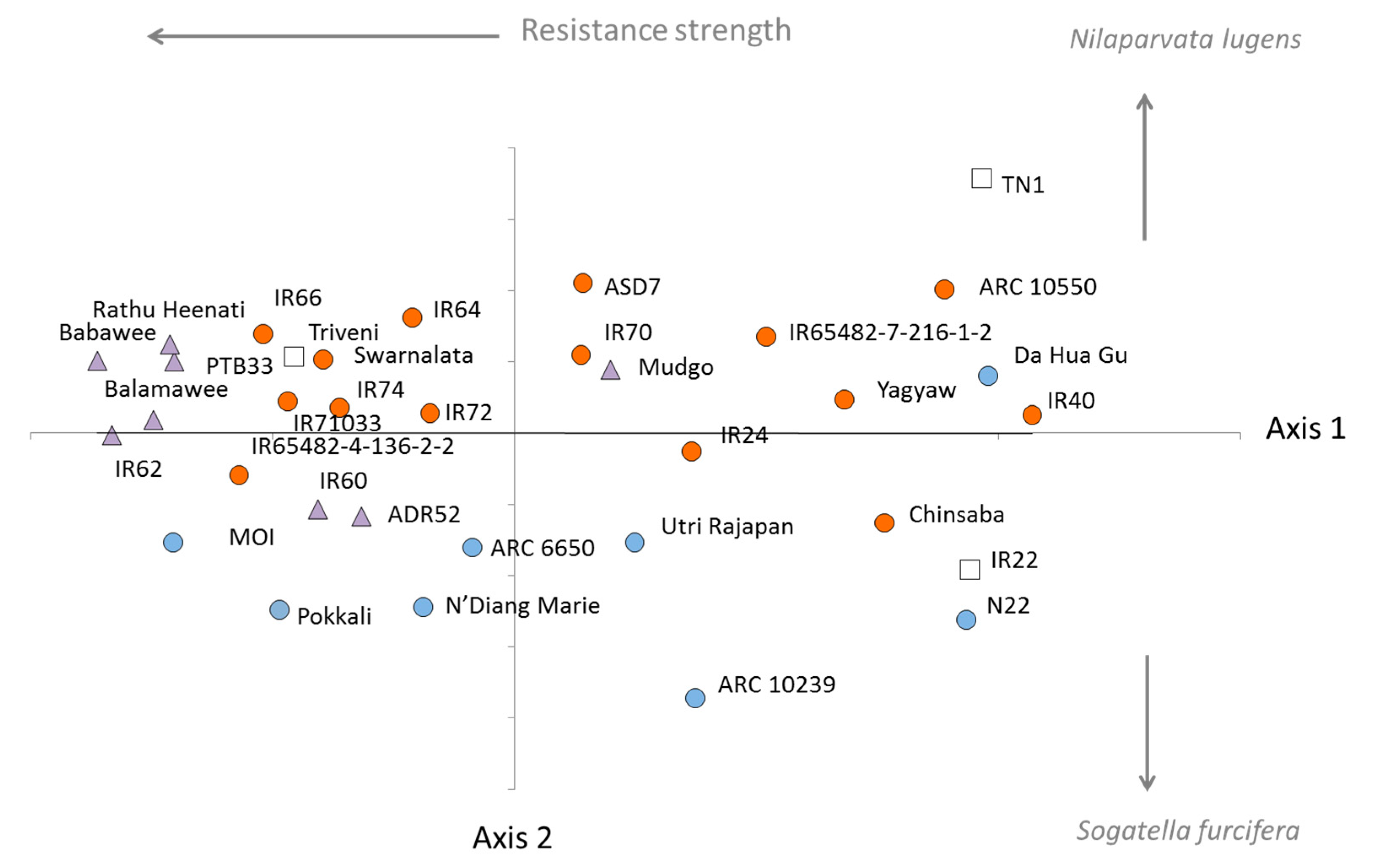
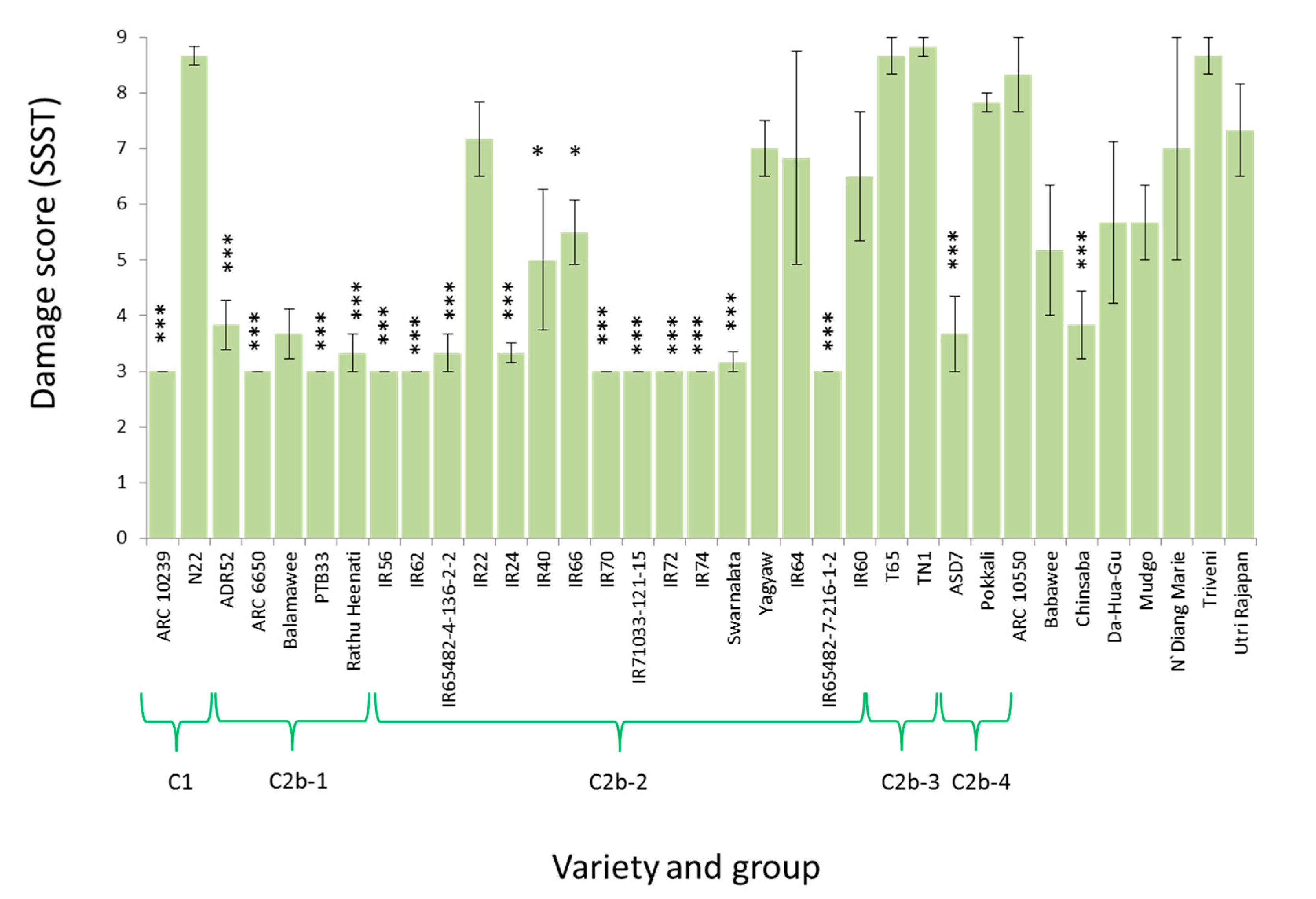
| Clade | Variety (Accession) | CAES (Taiwan) | IRRI (Philippines) | ||
|---|---|---|---|---|---|
| SSST 1 | MSST 1 | SSST 1 | MSST 1 | ||
| C1 | ARC 10239 | 3.00 (0.00) *** | 3.67 (0.67) ** | 6.33 (1.00) | 3.00 (0.00) |
| Nagina 22 | 7.00 (1.15) | 5.67 (0.67) | 2.33 (1.00) *** | 1.00 (1.00) | |
| C2b-1 | ARC 6650 | 5.00 (2.00) | 3.67 (0.67) ** | 5.00 (0.00) | 3.00 (1.73) |
| PTB33 | 4.33 (1.33) | 3.00 (0.00) *** | 4.00 (1.00) | 0.00 (0.00) *** | |
| ADR52 | 5.00 (1.15) | 4.33 (0.67) | 3.00 (2.00) | 1.00 (1.00) | |
| Balamawee | 5.00 (1.15) | 3.00 (0.00) *** | 3.00 (0.00) | 1.00 (0.76) | |
| Rathu Heenati | 5.00 (1.15) | 3.00 (0.00) *** | 3.67 (0.00) | 0.00 (0.00) *** | |
| C2b-2 | IR56 | - | - | 5.67 (1.00) | 3.00 (0.00) |
| IR65482-4-136-2-2 | 4.33 (0.67) | 4.33 (0.67) | 3.00 (2.00) | 2.00 (1.00) | |
| IR62 | 4.33 (0.67) | 3.00 (0.00) *** | 4.33 (0.00) | 2.00 (1.00) | |
| IR66 | 5.00 (0.00) | 4.33 (0.67) | 4.33 (1.00) | 3.67 (0.67) | |
| IR40 | 8.33 (0.67) | 7.00 (0.00) | 4.33 (1.00) | 3.67 (0.67) | |
| IR72 | 5.67 (0.67) | 3.67 (0.67) ** | 5.00 (0.00) | 3.00 (1.00) | |
| IR74 | 4.33 (0.67) | 4.33 (0.67) | 4.33 (1.00) | 3.00 (0.00) | |
| IR70 | 5.67 (0.67) | 6.33 (0.67) | 4.33 (1.00) | 3.67 (0.67) | |
| IR22 | 5.00 (0.00) | 5.00 (0.00) | 5.67 (1.00) | 4.33 (1.33) | |
| IR24 | 5.00 (1.15) | 4.33 (0.67) | 5.00 (1.00) | 3.00 (0.00) | |
| IR65482-7-216-1-2 | 4.33 (0.67) | 5.67 (0.67) | 4.33 (1.00) | 3.00 (0.00) | |
| Swarnalata | 5.00 (1.15) | 4.33 (0.67) | 4.33 (0.00) | 3.00 (0.00) | |
| Yagyaw | 7.00 (0.00) | 6.33 (0.67) | 5.00 (1.00) | 3.00 (0.00) | |
| IR64 | 3.67 (0.67) | 3.67 (0.67) ** | 5.00 (1.00) | 2.00 (1.00) | |
| IR71033-121-15 | 4.33 (1.33) | 3.67 (0.67) ** | 4.33 (1.00) | 2.00 (1.00) | |
| IR60 | 4.33 (1.33) | 3.67 (0.67) ** | 4.33 (0.00) | 3.00 (0.00) | |
| C2b-4 | ASD7 | 7.00 (0.00) | 5.00 (0.00) | 5.67 (1.00) | 1.00 (1.00) |
| Pokkali | 4.33 (1.33) | 3.00 (0.00) *** | 2.33 (1.00) *** | 3.00 (0.00) | |
| C2b | Da Hua Gu | 7.67 (0.67) | 7.00 (1.15) | 5.00 (4.00) | 3.00 (0.00) |
| Mudgo | 5.67 (0.67) | 5.67 (0.67) | 5.67 (3.00) | 1.00 (1.00) | |
| Triveni | 5.00 (1.15) | 4.33 (0.67) | 4.33 (1.00) | 1.00 (1.00) | |
| Chinsaba | 5.67 (0.67) | 5.67 (0.67) | 3.67 (1.00) | 1.00 (1.00) | |
| Babawee | 5.67 (0.67) | 3.67 (0.67) ** | 3.00 (2.00) | 0.00 (0.00) *** | |
| ARC 10550 | 7.67 (0.67) | 7.67 (0.67) | 5.00 (2.00) | 4.33 (2.96) | |
| Utri Rajapan | 4.33 (0.67) | 4.33 (0.67) | 5.33 (0.00) | 0.00 (0.00) *** | |
| N‘Diang Marie | 5.00 (0.00) | 3.00 (0.00) *** | 2.33 (1.00) *** | 0.00 (0.00) *** | |
| C2a | Asiminori | - | - | 5.67 (1.00) | 0.00 (0.00) *** |
| - | ARC 11367 | 7.00 (1.15) | 8.33 (0.67) | 7.00 (0.00) | - |
| - | Jia Nong 66 | - | - | - | 2.00 (1.00) |
| - | MO1 | 3.00 (0.00) *** | 3.00 (0.00) *** | 3.00 (2.00) | - |
| C2b-3 | T65 | - | - | 5.00 (1.00) | 3.00 (0.00) |
| TN1 | 8.33 (0.67) | 7.67 (0.67) | 7.17 (1.00) | 5.33 (1.20) | |
| F-variety | 3.059 *** | 7.483 *** | 1.921 ** | 3.549 *** | |
| DF | 34.00 | 34.00 | 37.00 | 36.00 | |
| DF (error) | 70.00 | 70.00 | 76.00 | 74.00 | |
| Clade | Varieties | Nymph Biomass (mg) 1,3 | Number of Eggs per Plant 1,3 | Planthopper Biomass (mg) after 30 Days 1,3 | Honeydew Excretion (mm2) 2,3 |
|---|---|---|---|---|---|
| C2b-1 | PTB33 | 1.08 (0.17) *** | 31.30 (7.16) | 10.61 (2.79) * | 10.67 (0.92) *** |
| Balamawee | 0.69 (0.17) *** | 21.80 (3.26) * | 17.97 (8.85) | 13.67 (2.59) *** | |
| Rathu Heenati | 0.86 (0.16) *** | 24.60 (4.04) * | 19.80 (2.10) | 13.40 (2.87) *** | |
| C2b-2 | IR65482-4-136-2-2 | 1.67 (0.17) | 40.80 (10.50) | 14.49 (5.48) * | - |
| IR62 | 1.71 (0.18) | 31.30 (6.65) | 78.79 (35.39) | 35.33 (3.24) *** | |
| IR66 | 1.78 (0.13) | 33.40 (4.32) | 59.44 (19.44) | 41.83 (16.18) *** | |
| IR40 | 1.73 (0.16) | 72.97 (9.99) | 160.00 (40.00) | 36.00 (12.95) *** | |
| IR74 | 2.25 (0.10) | 72.60 (10.80) | - | 57.67 (11.04) *** | |
| IR24 | 1.78 (0.16) | 86.40 (7.57) | 107.46 (27.76) | 60.00 (5.52) *** | |
| IR22 | 1.83 (0.25) | 82.60 (10.23) | 147.98 (20.23) | 28.00 (4.34) *** | |
| IR65482-7-216-1-2 | 2.46 (0.10) | 68.00 (16.79) | 147.98 (20.23) | 33.80 (7.93) *** | |
| Swarnalata | 2.24 (0.15) | 39.70 (9.17) | 111.34 (24.53) | 28.67 (8.01) *** | |
| Yagyaw | 2.05 (0.12) | 53.70 (13.88) | 98.08 (19.15) | 21.50 (4.30) *** | |
| IR64 | 2.52 (0.20) | 76.20 (18.32) | 77.73 (11.65) | 38.80 (11.34) *** | |
| IR60 | - | - | - | 28.60 (8.04) *** | |
| C2b-4 | ASD7 | 2.15 (0.24) | 63.70 (12.10) | 52.51 (11.56) | - |
| Pokkali | - | - | - | 61.00 (5.13) *** | |
| C2b | Mudgo | 1.91 (0.30) | 96.40 (16.85) | 102.90 (22.58) | 38.25 (5.02) *** |
| Triveni | 1.52 (0.12) | 82.20 (26.64) | 93.10 (13.08) | 123.25 (10.73) | |
| Chinsaba | 2.01 (0.19) | 53.00 (17.83) | 21.54 (4.79) | 17.20 (4.26) *** | |
| Babawee | 1.59 (0.21) * | 37.90 (8.95) | 17.14 (2.20) | 39.67 (13.65) *** | |
| Utri Rajapan | 1.95 (0.17) | 56.80 (12.57) | 128.67 (23.46) | 72.33 (18.51) *** | |
| C2b-3 | TN1 | 2.40 (0.52) | 81.80 (29.11) | 107.57 (38.62) | 133.00 (19.40) |
| F-values | 6.273 *** | 3.007 *** | 4.638 *** | 8.266 *** | |
| Df | 20 | 20 | 19 | 21 | |
| Df (error) | 174 | 174 | 80 | 93 |
| Parameter | Egg Laying | Nymph (Biomass) | Population (Biomass) | SSST | MSST |
|---|---|---|---|---|---|
| Egg laying | - | 0.579 1 | 0.688 2 | 0.662 1 | 0.431 1 |
| Nymph (biomass) | 0.006 | - | 0.555 2 | 0.482 1 | 0.389 1 |
| Population (biomass) | 0.001 | 0.011 | - | 0.503 2 | 0.587 2 |
| SSST | 0.001 | 0.027 | 0.024 | - | 0.419 3 |
| MSST | 0.051 | 0.082 | 0.007 | 0.014 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horgan, F.G.; Srinivasan, T.S.; Bentur, J.S.; Kumar, R.; Bhanu, K.V.; Sarao, P.S.; Chien, H.V.; Almazan, M.L.P.; Bernal, C.C.; Ramal, A.F.; et al. Geographic and Research Center Origins of Rice Resistance to Asian Planthoppers and Leafhoppers: Implications for Rice Breeding and Gene Deployment. Agronomy 2017, 7, 62. https://doi.org/10.3390/agronomy7040062
Horgan FG, Srinivasan TS, Bentur JS, Kumar R, Bhanu KV, Sarao PS, Chien HV, Almazan MLP, Bernal CC, Ramal AF, et al. Geographic and Research Center Origins of Rice Resistance to Asian Planthoppers and Leafhoppers: Implications for Rice Breeding and Gene Deployment. Agronomy. 2017; 7(4):62. https://doi.org/10.3390/agronomy7040062
Chicago/Turabian StyleHorgan, Finbarr G., Thanga Suja Srinivasan, Jagadish S. Bentur, Ram Kumar, K. Vasanta Bhanu, Preetinder Singh Sarao, Ho Van Chien, Maria Liberty P. Almazan, Carmencita C. Bernal, Angelee Fame Ramal, and et al. 2017. "Geographic and Research Center Origins of Rice Resistance to Asian Planthoppers and Leafhoppers: Implications for Rice Breeding and Gene Deployment" Agronomy 7, no. 4: 62. https://doi.org/10.3390/agronomy7040062






