Polyaspartic Acid Improves Maize (Zea mays L.) Seedling Nitrogen Assimilation Mainly by Enhancing Nitrate Reductase Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Measurement of Biomass and Nitrogen Accumulation
2.3. Measurement of Nitrate Reductase (NR, EC 1.6.6.1) Activity
2.4. Measurement of Glutamine Synthetase (GS, EC 6.3.1.2) Activity
2.5. Measurement of Alanine Aminotransferase (AlaAT EC 2.6.1.2) and Aspartate Aminotransferase (AspAT EC 2.6.1.1) Activity
2.6. Real-Time Quantitative PCR (qPCR)
2.7. Nitrate Reductease Protein Extraction and Quantification by Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis of Data
3. Results
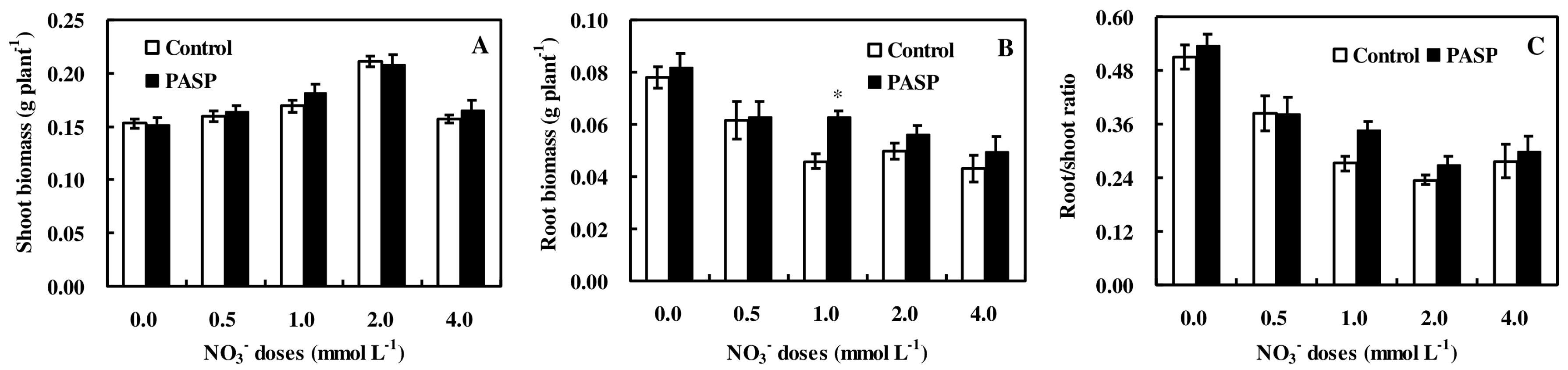
3.1. Changes in Plant Biomass Accumulation in Maize Seedlings
3.2. Changes in Nitrogen Accumulation in Maize Seedlings
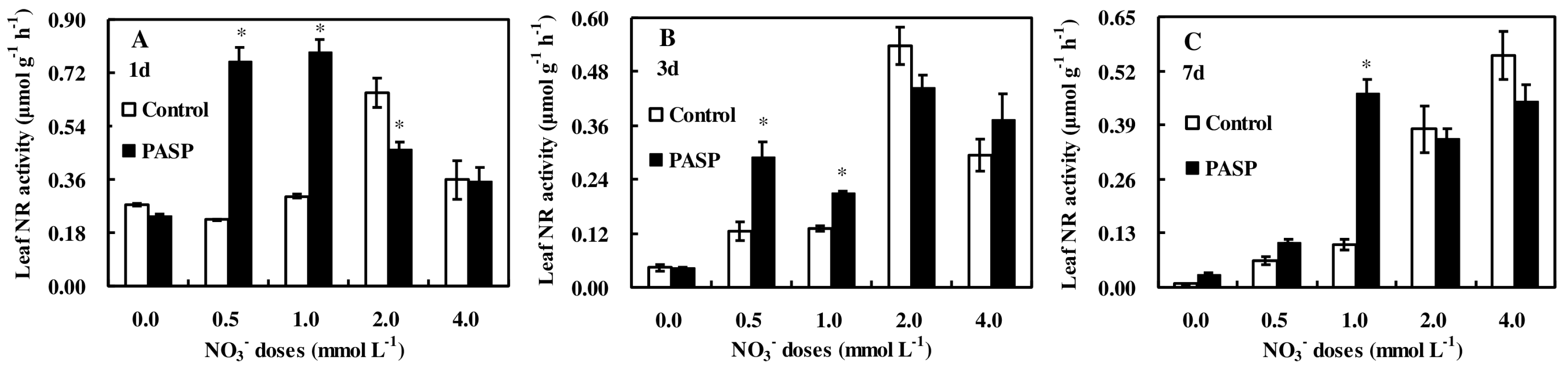
3.3. Changes in Enzyme Activities Correlated to Nitrogen Metabolism in Leaves and Roots of Maize Seedlings
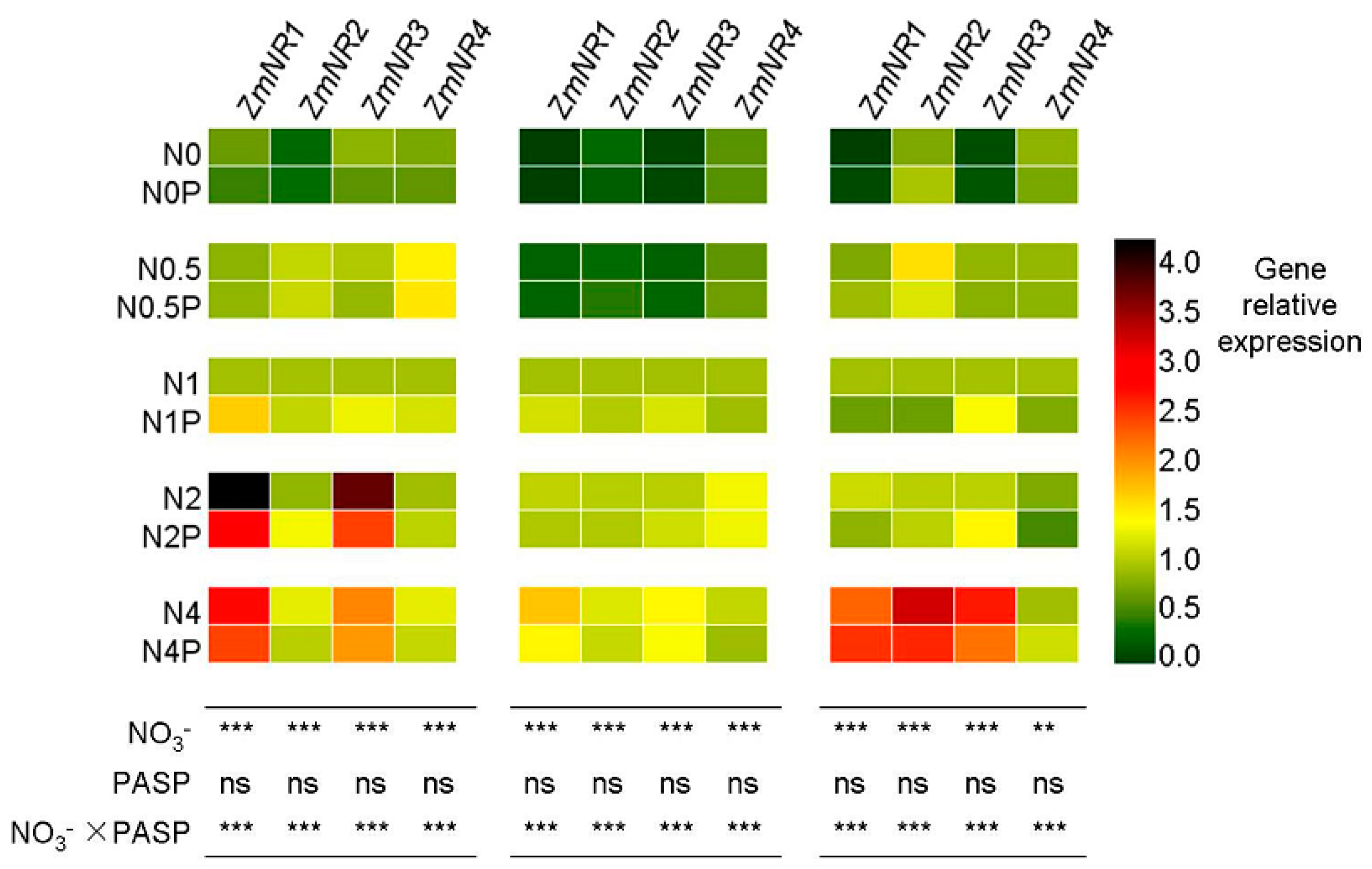
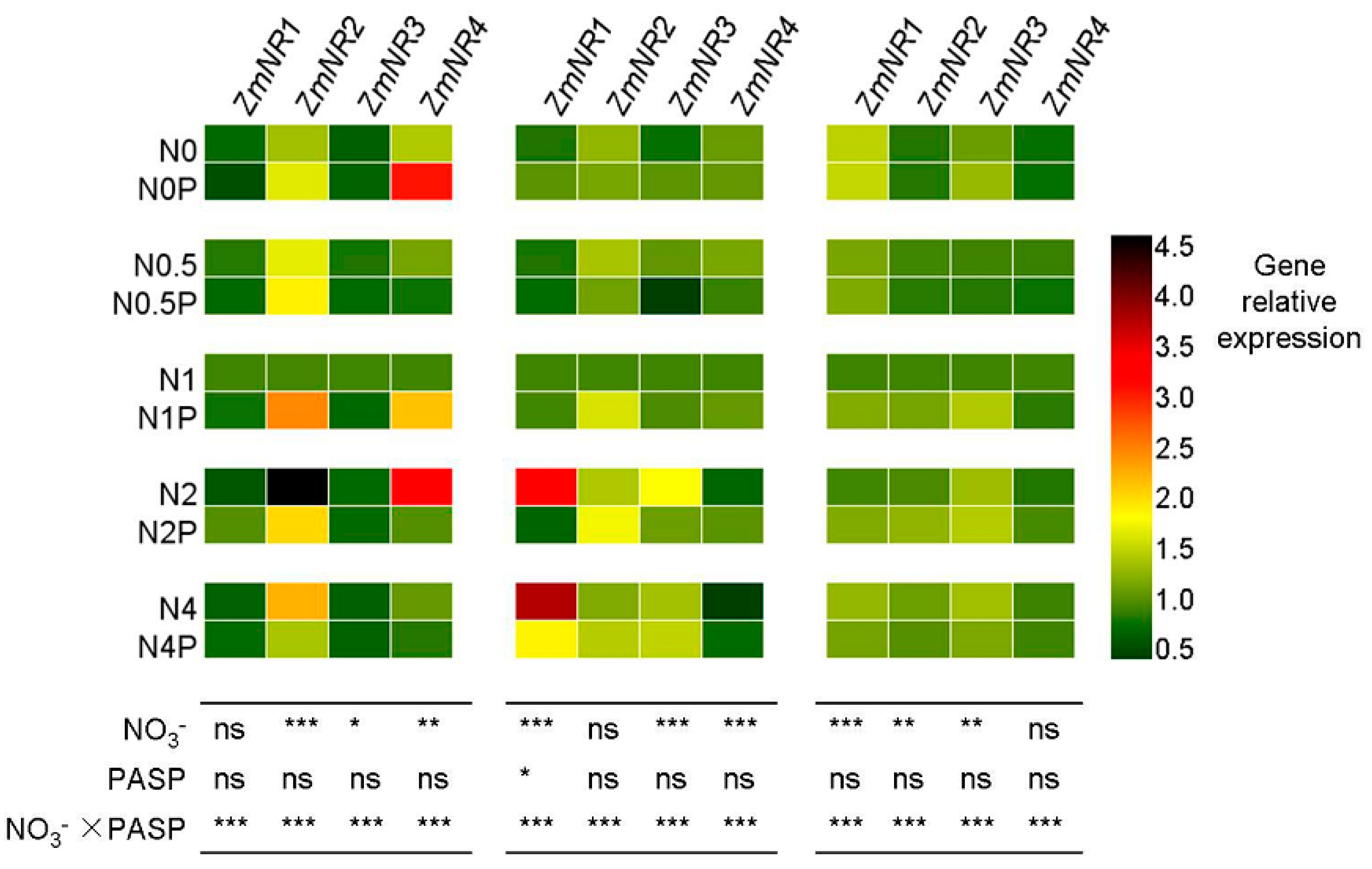
3.4. Changes in NR Gene Expression Levels in Maize Seedlings
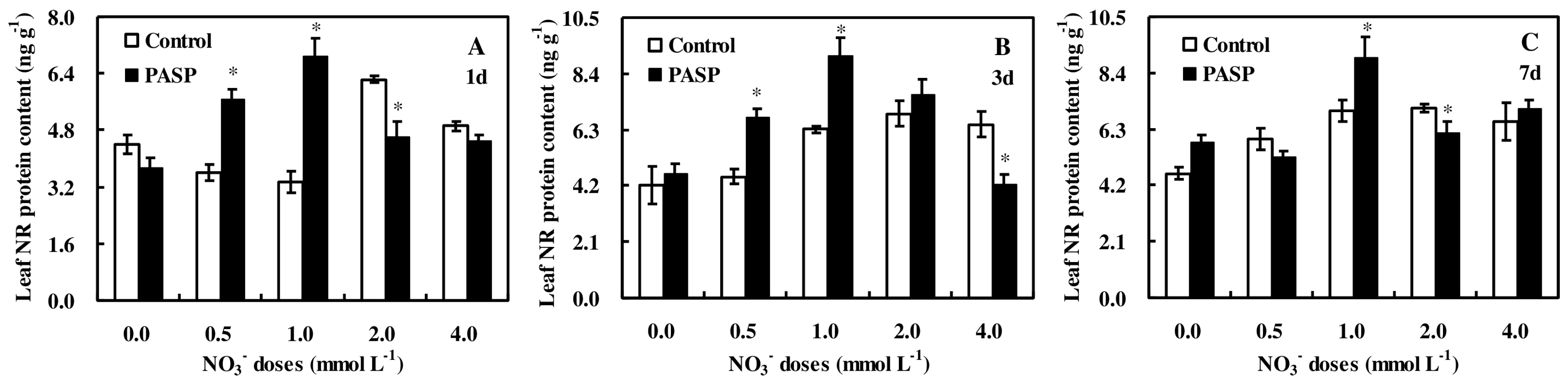
3.5. Changes in Nitrate Reductase Protein Accumulation in Maize Seedlings
4. Discussion
4.1. PASP Promoted Seedling Growth and Nitrogen Accumulation in Maize under Low Nitrogen Conditions
4.2. The Improvement of PASP on Nitrogen Accumulation in Maize Was Primarily Attributed to Changes in NR Activities
4.3. The Regulation of PASP on NR Activity Was Mainly Due to the Increasing Accumulation of Protein Rather than Gene Transcription
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Q.; Miao, Y.X.; Feng, G.H.; Gao, X.W.; Liu, B.; Liu, Y.Q.; Li, F.; Khosla, R.; Mulla, D.J.; Zhang, F.S. Improving nitrogen use efficiency with minimal environmental risks using an active canopy sensor in a wheat-maize cropping system. Field Crops Res. 2017, 214, 365–372. [Google Scholar] [CrossRef]
- Norse, D.; Ju, X. Environmental costs of China’s food security. Agric. Ecosyst. Environ. 2015, 209, 5–14. [Google Scholar] [CrossRef]
- Zhang, W.F.; Dou, Z.X.; He, P.; Ju, X.T.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.L.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Nat. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhang, Y.; Han, W.X.; Tang, A.H.; Shen, J.L.; Cui, Z.L.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, P.; Santiveri, F.; Boixadera, J.; Lloveras, J. Nitrogen fertilization of irrigated maize under Mediterranean conditions. Eur. J. Agron. 2009, 30, 163–171. [Google Scholar] [CrossRef]
- Good, A.G.; Beatty, P.H. Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Gallais, A. Nitrogen use efficiency—Physiological, molecular and genetic investigations towards crop improvement. In Advances in Maize; Prioul, J.L., Thévenot, C., Molnar, T., Eds.; Society for Experimental Biology: London, UK, 2011; Volume 3, pp. 285–310. [Google Scholar]
- Deng, F.; Wang, L.; Ren, W.J.; Mei, X.F.; Li, S.X. Optimized nitrogen managements and polyaspartic acid urea improved dry matter production and yield of indica hybrid rice. Soil Tillage Res. 2015, 145, 1–9. [Google Scholar] [CrossRef]
- Hu, H.Y.; Ning, T.Y.; Li, Z.J.; Han, H.F.; Zhang, Z.Z.; Qin, S.J.; Zheng, Y.H. Coupling effects of urea types and subsoiling on nitrogen—Water use and yield of different varieties of maize in northern China. Field Crops Res. 2013, 142, 85–94. [Google Scholar] [CrossRef]
- Deng, F.; Wang, L.; Ren, W.J.; Mei, X.F.; Li, S.X. Enhancing nitrogen utilization and soil nitrogen balance in paddy fields by optimizing nitrogen management and using polyaspartic acid urea. Field Crops Res. 2014, 169, 30–38. [Google Scholar] [CrossRef]
- Du, Z.J.; Yang, H.; Wang, Y.Z.; Luo, H.Y.; Xu, L.; Wang, K.L.; Wang, B. Effects on yield and phosphorus nutrition absorbing for rice using homologous polypeptides of polyaspartic acids. Mod. Agric. Sci. Technol. 2012, 18, 12–13, 15, (In Chinese with English abstract). [Google Scholar]
- Xu, Y.; Zhao, L.L.; Wang, L.N.; Xu, S.Y.; Cui, Y.C. Synthesis of polyaspartic acid–melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide. Desalination 2012, 286, 285–289. [Google Scholar] [CrossRef]
- Tomida, M.; Nakato, T.; Matsunami, S.; Kakuchi, T. Convenient synthesis of high molecular weight poly (succinimide) by acid-catalysed polycondensation of L-aspartic acid. Polymer 1997, 38, 4733–4736. [Google Scholar] [CrossRef]
- Vega-Chacón, J.; Amaya Arbeláez, M.I.; Jorge, J.H.; Marques, R.F.C.; Jafelicci, M., Jr. pH-responsive poly(aspartic acid) hydrogel-coated magnetite nanoparticles for biomedical applications. Mater. Sci. Eng. C 2017, 77, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Polyaspartic Acid—A Versatile Green Chemical. Chem. Sci. Rev. Lett. 2012, 1, 162–167. [Google Scholar]
- Chiriac, A.P.; Nita, L.E.; Neamtu, I. Poly(ethylene glycol) functionalized by polycondensing procedure with poly(succinimide). Polymer 2010, 55, 641–645. [Google Scholar]
- Jiang, W.; Zhou, D.B.; Zhang, H.S.; Zhang, Y.S. The effect of polyaspartic acid on maize growth at seedling stage under different fertilizer applied condition. J. Maize Sci. 2007, 15, 121–124, (In Chinese with English Abstract). [Google Scholar]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Plett, D.; Baumann, U.; Schreiber, A.W.; Holtham, L.; Kalashyan, E.; Toubia, J.; Nau, J.; Beatty, M.; Rafalski, A.; Dhugga, K.S.; et al. Maize maintains growth in response to decreased nitrate supply through a highly dynamic and developmental stage-speciic transcriptional response. Plant Biotechnol. J. 2016, 14, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Lea, U.S.; Leydecker, M.T.; Quillere, I.; Meyer, C.; Lillo, C. Post-translational regulation of nitrate reductase strongly affects the levels of free amino acids and nitrate, whereas transcriptional regulation has only minor inluence. Plant Physiol. 2006, 140, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Sasaki, Y.; Ida, S.; Morikawa, H. Nitrite reductase gene enrichment improves assimilation of NO2 in Arabidopsis. Plant Physiol. 2001, 126, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Moller, A.L.B.; Dionisio, G.; Kichey, T.; Jahn, T.P.; Dubois, F.; Baudo, M.; Lopes, M.S.; Terce-Laforgue, T.; Foyer, C.H.; et al. Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 67, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [PubMed]
- Swarbreck, S.M.; Defoin-Platel, M.; Hindle, M.; Saqi, M.; Habash, D.Z. New perspectives on glutamine synthetase in grasses. J. Exp. Bot. 2011, 62, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, T.; Kusano, M. Evidence supporting distinct functions of three cytosolic glutamine synthetases and two NADH-glutamate synthases in rice. J. Exp. Bot. 2014, 65, 5519–5525. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, F.; Cañas, R.A.; Pascual, M.B.; Avila, C.; Cánovas, F.M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 2014, 65, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, F.; El-Azaz, J.; Ávila, C.; Cánovas, F.M. Deciphering the role of aspartate and prephenate aminotransferase activities in plastid nitrogen metabolism. Plant Physiol. 2014, 164, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.H.; Shrawat, A.K.; Carroll, R.T.; Zhu, T.; Good, A.G. Transcriptome analysis of nitrogen-efficient rice over-expressing alanine aminotransferase. Plant Biotechnol. J. 2009, 7, 562–576. [Google Scholar] [CrossRef] [PubMed]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.X.; Sun, Z.G.; Wang, L.H.; Zhou, Q.; Huang, X.H. Analysis of the combined effects of lanthanum and acid rain, and their mechanisms, on nitrate reductase transcription in plants. Ecotoxicol. Environ. Saf. 2017, 138, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Chu, C.C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 4, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cañas, R.A.; Quilleré, I.; Lea, P.J.; Hirel, B. Analysis of amino acid metabolism in the ear of maize mutants deficient in two cytosolic glutamine synthetase isoenzymes highlights the importance of asparagine for nitrogen translocation within sink organs. Plant Biotechnol. J. 2010, 8, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.H. Study on the Application of Environmental Friendly Polymer PASP in Agriculture. Master’s Thesis, East China University of Science and Technology, Shanghai, China, 2005. [Google Scholar]
- Majláth, I.; Darko, E.; Palla, B.; Nagy, Z.; Janda, T.; Szalai, G. Reduced light and moderate water deficiency sustain nitrogen assimilation and sucrose degradation at low temperature in durum wheat. J Plant Physiol. 2016, 191, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Pereira, S.; Canovas, F.; Salema, R. Glutamine synthetase of potato (Solanum tuberosum L. cv. Desiree) plants: Cell- and organ-specific expression and differential developmental regulation reveal specific roles in nitrogen assimilation and mobilization. J. Exp. Bot. 2015, 56, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Blaesing, O.E.; Hannemann, J.; Carillo, P.; Hohne, M.; Hendriks, J.H.M.; Palacios, N.; Cross, J.; Selbig, J.; Mark, S.M. A Robot-based platform to measure multiple enzyme activities in arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 2004, 16, 3304–3325. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Han, J.N.; Wang, R.F.; Li, X.X. Down-regulation of nitrogen/carbon metabolism coupled with coordinative hormone modulation contributes to developmental inhibition of the maize ear under nitrogen limitation. Planta 2016, 244, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Liseron-Monfils, C.; Bi, Y.M.; Downs, G.; Wu, W.Q.; Signorelli, T.; Lu, G.W.; Chen, X.; Bondo, E.; Zhu, T.; Lukens, L.N.; et al. Nitrogen transporter and assimilation genes exhibit developmental stage-selective expression in maize (Zea mays L.) associated with distinct cis-acting promoter motifs. Plant Signal. Behav. 2013, 8, e26056. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, M.L.; Zhang, F.X.; Xu, Y.D.; Chen, X.H.; Qin, X.L.; Wen, X.X. Effect of post-silking drought on nitrogen partitioning and gene expression patterns of glutamine synthetase and asparagine synthetase in two maize (Zea mays L.) varieties. Plant Physiol. Biochem. 2016, 102, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Maranville, J.W.; Chetima, M.M. Deficit irrigation and nitrogen effects on maize in a Sahelian environment II. Shoot growth, nitrogen uptake and water extraction. Agric. Water Manag. 2000, 46, 15–27. [Google Scholar] [CrossRef]
- Weligama, C.; Tang, C.; Sale, P.W.G.; Conyers, M.K.; Liu, D.L. Application of nitrogen in NO3- form increases rhizosphere alkalisation in the subsurface soil layers in an acid soil. Plant Soil 2010, 333, 403–416. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Danalatos, N.G.; Archontoulis, S.V. Growth and biomass productivity of kenaf (Hibiscus cannabinus L.) under different agricultural inputs and management practices incentral Greece. Ind. Crops Prod. 2010, 32, 231–240. [Google Scholar] [CrossRef]
- Valentinuz, O.R.; Tollenaar, M. Effect of genotype, nitrogen, plant density, and row spacing on the area-per-leaf profile in maize. Agron. J. 2006, 98, 94–99. [Google Scholar] [CrossRef]
- Qiao, J.; Yang, L.Z.; Yan, T.M.; Xue, F.; Zhao, D. Rice dry matter and nitrogen accumulation, soil mineral Naround root and N leaching, with increasing application rates of fertilizer. Eur. J. Agron. 2013, 49, 93–103. [Google Scholar] [CrossRef]
- Chen, B.M.; Wang, Z.H.; Li, S.X.; Wan, G.X.; Song, H.X.; Wang, X.N. Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Sci. 2004, 167, 635–643. [Google Scholar] [CrossRef]
- Ferrari, T.E.; Yoder, O.C.; Filner, D. Anaerobic nitrite production by plant cells and tissues: Evidence for two nitrate pools. Plant Physiol. 1973, 51, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Fernández, I.; De Diego, N.; Brzobohatý, B.; Muñoz-Ruedad, A.; Lacuesta, M. The imbalance between C and N metabolism during high nitrate supply inhibits photosynthesis and overall growth in maize (Zea mays L.). Plant Physiol. Biochem. 2017, 120, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Conn, V.; Plett, D.; Conn, S.; Zanghellini, J.; Mackenzie, N.; Enju, A.; Francis, K.; Holtham, L.; Roessner, U.; et al. The response of the maize nitrate transport system to nitrogen demand and supply across the lifecycle. New Phytol. 2013, 198, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Sabermanesh, K.; Holtham, L.R.; George, J.; Roessner, U.; Boughton, B.A.; Heuer, S.; Tester, M.; Plett, D.C.; Garnett, T.P. Transition from a maternal to external nitrogen source in maize seedlings. J. Integr. Plant Biol. 2017, 59, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Cren, M.; Hirel, B. Glutamine synthetase in higher plants: Regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol. 1999, 40, 1187–1193. [Google Scholar] [CrossRef]
- Silva, I.T.; Abbaraju, H.K.R.; Fallis, L.P.; Liu, H.J.; Lee, M.; Dhugga, K.S. Biochemical and genetic analyses of N metabolism in maize testcross seedlings: 1. Leaves. Theor. Appl. Genet. 2017, 130, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Y.; Gibon, Y.; Gur, A.; Chen, C.; Lepak, N.; Hohne, M.; Zhang, Z.W.; Kroon, D.; Tschoep, H.; Stitt, M.; et al. Fine quantitative trait loci mapping of carbon and nitrogen metabolism enzyme activities and seedling biomass in the maize IBM mapping population. Plant Physiol. 2010, 154, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. Transcription factors involved in controlling the expression of nitrate reductase genes in higher plants. Plant Sci. 2014, 229, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C.; Meyer, C.; Lea, U.S.; Provan, F.; Oltedal, S. Mechanism and importance of post-translational regulation of nitrate reductase. J. Exp. Bot. 2004, 55, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Amiour, N.; Imbaud, S.; Clement, G.; Agier, N.; Zivy, M.; Valot, B.; Balliau, T.; Armengaud, P.; Quillere, I.; Canas, R.; et al. The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps involved in the control of nitrogen metabolism in crops such as maize. J. Exp. Bot. 2012, 63, 5017–5033. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: Coping with increasing complexity in logic, chemistry, and network interactions. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.M.; Weiner, H.; Kandlbinder, A.; Tsai, C.B.; Rockel, P.; Sonoda, M.; Planchet, E. Modulation of nitrate reductase: Some new insights, an unusual case and a potentially important side reaction. J. Exp. Bot. 2002, 53, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.M.; Huber, S.C. Post-translational regulation of nitrate reductase: Mechanism, physiological relevance and environmental triggers. J. Exp. Bot. 2001, 52, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.J.; Dong, Z.Q.; Lan, H.L.; Pei, Z.C.; Gao, J.; Xie, Z.X. Effects of PASP-KT-NAA on photosynthesis and antioxidantenzyme activities of maize seedlings under low temperature stress. Acta Agron. Sin. 2012, 38, 352–359, (In Chinese with English Abstract). [Google Scholar] [CrossRef]



| Gene | Gene ID | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| ZmNR1 | GRMZM2G568636 | ATGATCCAGTTCGCCATCTC | GTCCGTGGTACGTCGTAGGT |
| ZmNR2 | GRMZM2G428027 | AGCAAGTCTTGAGGGAGCAC | CGCCTTGCATGACATTCGTT [41] |
| ZmNR3 | GRMZM5G878558 | ACTGGTGCTGGTGCTTCTGGTCC | ATGCCGATCTCGCCCTTGTGC [42] |
| ZmNR4 | GRMZM2G076723 | GCGTGCAGTTTCAATTCGGT | AGCTATTCCCCGTTGCCATC |
| actin | XM_008656735 | GATTCCTGGGATTGCCGAT | TCTGCTGCTGAAAAGTGCTGAG [43] |
| Parts | Source of Variation | Days after PASP Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1d | 3d | 7d | |||||||||||
| NR | GS | AspAT | AlaAT | NR | GS | AspAT | AlaAT | NR | GS | AspAT | AlaAT | ||
| Leaves | NO3− | *** | ** | ** | * | *** | ** | ns | ns | *** | *** | ** | * |
| PASP | *** | ns | ** | ns | ** | ns | ns | ns | ** | ns | ns | ns | |
| NO3− × PASP | *** | ns | ns | ns | ** | ns | * | * | *** | ns | ns | ns | |
| Roots | NO3− | *** | *** | ns | ** | *** | *** | * | ns | *** | *** | *** | *** |
| PASP | *** | ns | ns | * | ns | ns | ns | ns | *** | ** | ns | * | |
| NO3− × PASP | *** | ns | ns | ns | ns | ns | ns | ** | *** | ns | ns | * | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Tang, H.; Li, G.; Dong, H.; Dong, X.; Xu, Y.; Dong, Z. Polyaspartic Acid Improves Maize (Zea mays L.) Seedling Nitrogen Assimilation Mainly by Enhancing Nitrate Reductase Activity. Agronomy 2018, 8, 188. https://doi.org/10.3390/agronomy8090188
Wang Q, Tang H, Li G, Dong H, Dong X, Xu Y, Dong Z. Polyaspartic Acid Improves Maize (Zea mays L.) Seedling Nitrogen Assimilation Mainly by Enhancing Nitrate Reductase Activity. Agronomy. 2018; 8(9):188. https://doi.org/10.3390/agronomy8090188
Chicago/Turabian StyleWang, Qingyan, Huihui Tang, Guangyan Li, Hui Dong, Xuerui Dong, Yanli Xu, and Zhiqiang Dong. 2018. "Polyaspartic Acid Improves Maize (Zea mays L.) Seedling Nitrogen Assimilation Mainly by Enhancing Nitrate Reductase Activity" Agronomy 8, no. 9: 188. https://doi.org/10.3390/agronomy8090188
APA StyleWang, Q., Tang, H., Li, G., Dong, H., Dong, X., Xu, Y., & Dong, Z. (2018). Polyaspartic Acid Improves Maize (Zea mays L.) Seedling Nitrogen Assimilation Mainly by Enhancing Nitrate Reductase Activity. Agronomy, 8(9), 188. https://doi.org/10.3390/agronomy8090188





