Exploiting Genetic and Genomic Resources to Enhance Heat-Tolerance in Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Plant Growth
2.2. Traits Evaluation and Statistical Analysis
2.3. Association Mapping Analysis
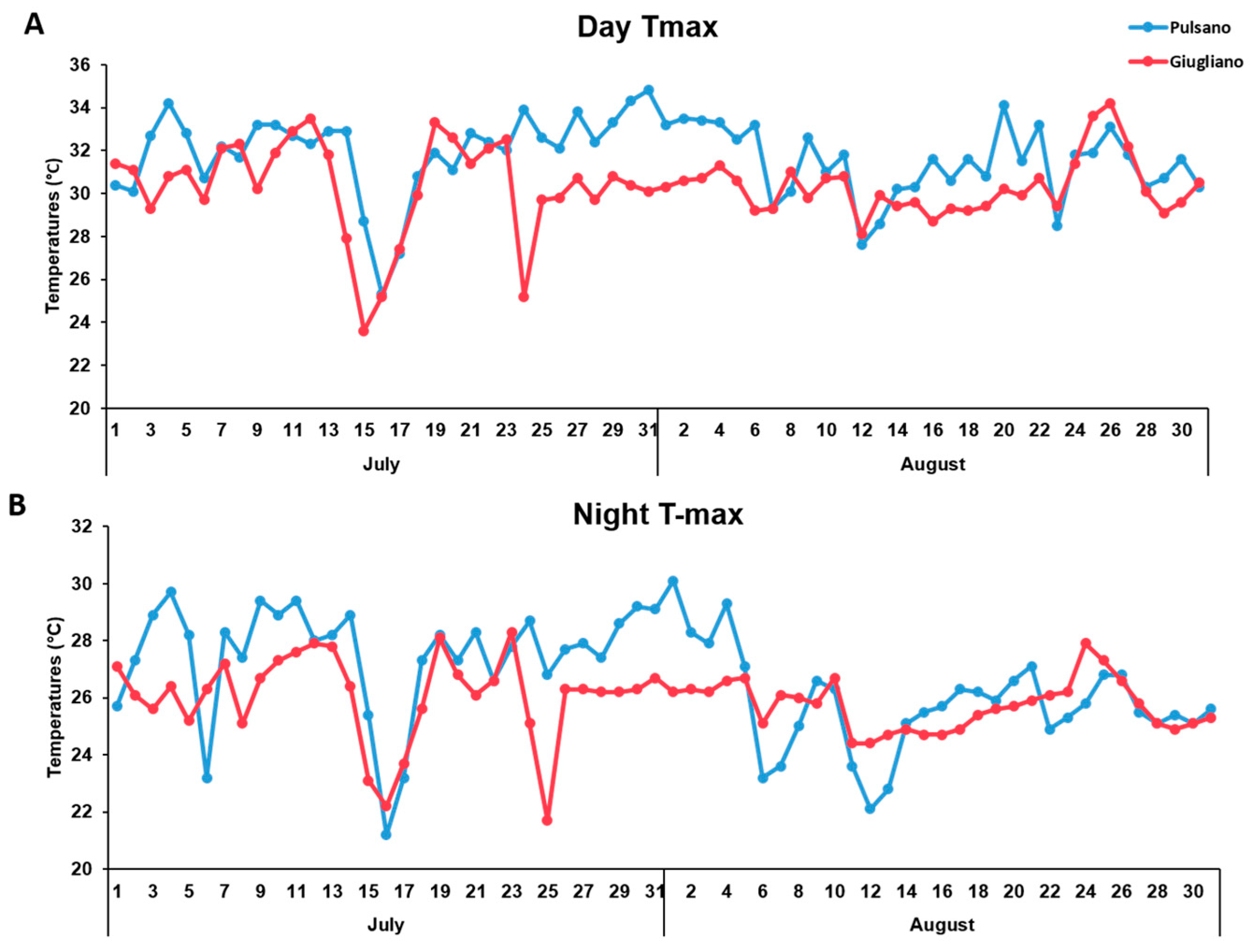
3. Results and Discussion
3.1. Phenotypic Evaluation
3.2. Association Mapping
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ainsworth, E.A.; Ort, D.R. How do we improve crop production in a warming world? Plant Physiol. 2010, 154, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Gourdji, S.M.; Sibley, A.M.; Lobell, D.B. Global crop exposure to critical high temperatures in the reproductive period: Historical trends and future projections. Environ. Res. Lett. 2013, 8, 024041. [Google Scholar] [CrossRef]
- Bokszczanin, K.L. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Warland, J.; McKeown, A.W.; McDonald, M.R. Impact of high air temperatures on Brassicacae crops in southern Ontario. Can. J. Plant Sci. 2006, 86, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Gardner, R.G. Altered flower retention and developmental patterns in nine tomato cultivars under elevated temperature. Sci. Hortic. 2004, 101, 95–101. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. CRC Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Grilli, G.V.G.; Braz, L.T.; Lemos, E.G.M. QTL identification for tolerance to fruit set in tomato by FAFLP markers. Crop Breed. Appl. Biotechnol. 2007, 7, 234–241. [Google Scholar] [CrossRef]
- Xiang-yang, X.; Dong-mei, W.; Li-gong, K.; Jing-fu, A.L. Selection of SSR and RAPD Markers Related to Tomato Heat Tolerance. Acta Hortic. 2008, 35, 47–52. [Google Scholar]
- Xu, J.; Driedonks, N.; Rutten, M.J.M.; Vriezen, W.H.; de Boer, G.J.; Rieu, I. Mapping quantitative trait loci for heat tolerance of reproductive traits in tomato (Solanum lycopersicum). Mol. Breed. New Strateg. Plant Improv. 2017, 37, 58. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.R.; Argayoso, M.A.; Redona, E.D.; Sierra, S.N.; Laza, M.A.; Dilla, C.J.; Mo, Y.; Thomson, M.J.; Chin, J.; Delavina, C.B.; et al. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 2012, 131, 33–41. [Google Scholar] [CrossRef]
- Lucas, M.R.; Ehlers, J.D.; Huynh, B.L.; Diop, N.N.; Roberts, P.A.; Close, T.J. Markers for breeding heat-tolerant cowpea. Mol. Breed. 2013, 31, 529–536. [Google Scholar] [CrossRef]
- Bergelson, J.; Roux, F. Towards identifying genes underlying ecologically relevant traits in Arabidopsis thaliana. Nat. Rev. Genet. 2010, 11, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, V.; Francese, G.; Sacco, A.; D’Alessandro, A.; Rigano, M.M.; Parisi, M.; Milone, M.; Cardi, T.; Mennella, G.; Barone, A. An association mapping approach to identify favourable alleles for tomato fruit quality breeding. BMC Plant Biol. 2014, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a Tomato Landraces Collection for Fruit-Related Traits by the Aid of a High-Throughput Genomic Platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggieri, V.; Anzar, I.; Paytuvi, A.; Calafiore, R.; Cigliano, R.A.; Sanseverino, W.; Barone, A. Exploiting the great potential of Sequence Capture data by a new tool, SUPER-CAP. DNA Res. 2016, 24, 81–91. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A.; Vonholdt, B.M. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Bouche, F.; Lobet, G.; Tocquin, P.; Perilleux, C. FLOR-ID: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2016, 44, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Naika, M.; Shameer, K.; Mathew, O.K.; Gowda, R.; Sowdhamini, R. STIFDB2: An updated version of plant stress-responsive transcription factor database with additional stress signals, stress-responsive transcription factor binding sites and stress-responsive genes in Arabidopsis and rice. Plant Cell Physiol. 2013, 54, e8. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Bautista, A.; Lobato-Ortiz, R.; Cruz-Izquierdo, S.; Garcia-Zavala, J.J.; Chavez-Servia, J.L.; Hernandez-Leal, E.; Bonilla-Barrientos, O. Fruit size QTLs affect in a major proportion the yield in tomato. Chil. J. Agric. Res. 2015, 75, 402–409. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Harel, D.; Fadida, H.; Slepoy, A.; Gantz, S.; Shilo, K. The Effect of Mean Daily Temperature and Relative Humidity on Pollen, Fruit Set and Yield of Tomato Grown in Commercial Protected Cultivation. Agronomy 2014, 4, 167. [Google Scholar] [CrossRef]
- Golam, F.; Prodhan, Z.H.; Nezhadahmadi, A.; Rahman, M. Heat tolerance in tomato. Life Sci. J. 2012, 9, 1936–1950. [Google Scholar]
- Shivanna, K.R.; Linskens, H.F.; Cresti, M. Responses of tobacco pollen to high humidity and heat stress: Viability and germinability in vitro and in vivo. Sex. Plant Reprod. 1991, 4, 104–109. [Google Scholar] [CrossRef]
- Erickson, A.N.; Markhart, A.H. Flower production, fruit set, and physiology of bell pepper during elevated temperature and vapor pressure deficit. J. Am. Soc. Hortic. Sci. 2001, 126, 697–702. [Google Scholar] [CrossRef]
- Saha, S.; Hossain, M.; Rahman, M.; Kuo, C.; Abdullah, S. Effect of high temperature stress on the performance of twelve sweet pepper genotypes. Bangladesh J. Agric. Res. 2010, 35, 525–534. [Google Scholar] [CrossRef]
- Mazzucato, A.; Papa, R.; Bitocchi, E.; Mosconi, P.; Nanni, L.; Negri, V.; Picarella, M.E.; Siligato, F.; Soressi, G.P.; Tiranti, B.; et al. Genetic diversity, structure and marker-trait associations in a collection of Italian tomato (Solanum lycopersicum L.) landraces. TAG Theor. Appl. Genet. 2008, 116, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.C.; Durstewitz, G.; Plieske, J.; Wieseke, R.; Ganal, M.W.; Van Deynze, A.; Hamilton, J.P.; Buell, C.R.; Causse, M.; Wijeratne, S.; et al. Development of a large SNP genotyping array and generation of high-density genetic maps in tomato. PLoS ONE 2012, 7, e40563. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Seo, Y.S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, J.; Pan, C.; Guan, X.; Wang, Y.; Liu, S.; He, Y.; Chen, J.; Chen, L.; Lu, G. Genome-wide identification of MAPKK and MAPKKK gene families in tomato and transcriptional profiling analysis during development and stress response. PLoS ONE 2014, 9, e103032. [Google Scholar] [CrossRef]
- Erickson, A.N.; Markhart, A.H. Flower developmental stage and organ sensitivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell Environ. 2002, 25, 123–130. [Google Scholar] [CrossRef]
- Giorno, F.; Wolters-Arts, M.; Mariani, C.; Rieu, I. Ensuring reproduction at high temperatures: The heat stress response during anther and pollen development. Plants 2013, 2, 489–506. [Google Scholar] [CrossRef]
- Chen, K.Y.; Cong, B.; Wing, R.; Vrebalov, J.; Tanksley, S.D. Changes in regulation of a transcription factor lead to autogamy in cultivated tomatoes. Science 2007, 318, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Tanksley, S.D. High-resolution mapping and functional analysis of se2.1: A major stigma exsertion quantitative trait locus associated with the evolution from allogamy to autogamy in the genus Lycopersicon. Genetics 2004, 168, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Causse, M.; Duffe, P.; Gomez, M.C.; Buret, M.; Damidaux, R.; Zamir, D.; Gur, A.; Chevalier, C.; Lemaire-Chamley, M.; Rothan, C. A genetic map of candidate genes and QTLs involved in tomato fruit size and composition. J. Exp. Bot. 2004, 55, 1671–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandillo, S.; Ku, H.M.; Tanksley, S.D. Identifying the loci responsible for natural variation in fruit size and shape in tomato. Theor. Appl. Genet. 1999, 99, 978–987. [Google Scholar] [CrossRef]
- Ariizumi, T.; Shinozaki, Y.; Ezura, H. Genes that influence yield in tomato. Breed. Sci. 2013, 63, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Trait a | Giugliano | Pulsano | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| FRL | 56.0 | 72.3 | 61.1 | 4.4 | 63.0 | 75.0 | 68.9 | 2.7 |
| NFL | 5.9 | 43.5 | 12.2 | 6.2 | 3.8 | 92.7 | 7.9 | 9.6 |
| FS | 13.7 | 78.4 | 48.5 | 14.8 | 14.3 | 95.3 | 51.4 | 18.8 |
| TNF | 0.3 | 89.2 | 13.4 | 13.8 | 7.6 | 175.1 | 60.1 | 40.6 |
| FW | 7.4 | 225.2 | 49.8 | 39.1 | 6.0 | 328.7 | 61.4 | 56.8 |
| YP | 0.02 | 1.48 | 0.45 | 0.37 | 0.54 | 4.63 | 2.30 | 0.81 |
| Trait | Associated Markers (No.) | Chr. | |||
|---|---|---|---|---|---|
| Total | Common | Giugliano Specific | Pulsano Specific | ||
| FRL | 9 | 0 | 6 | 3 | 1,3 |
| NFL | 3 | 1 | 2 | 0 | 3,4,8 |
| FS | 3 | 2 | 2 | 0 | 2,8 |
| TNF | 8 | 3 | 2 | 4 | 1,2,5,6,8 |
| FW | 9 | 8 | 0 | 1 | 3,5,6,12 |
| YP | 3 | 1 | 0 | 2 | 3,5,7 |
| Trait | Common Marker | Ch. | Marker Position (SL3.0) | Associated QTL | Associated CGs | CG/QTL Distance from Marker | CG Predicted Function |
|---|---|---|---|---|---|---|---|
| NFL | solcap_snp_sl_18195 | 8 | 56,317,554 | Solyc08g067050 | 285 Kbp | protein arginine methyltransferase | |
| FS | solcap_snp_sl_10567 | 2 | 40,605,496 | Solyc02g070430 | 121 Kbp | Gibberellic acid oxidase | |
| FS/TNF | solcap_snp_sl_20344 | 2 | 38,133,120 | Solyc02g067380 | 17.4 Kbp | Style2.1 | |
| TNF | solcap_snp_sl_8655 | 1 | 2,381,257 | ||||
| solcap_snp_sl_8656 | 1 | 2,381,153 | Solyc01g008350 | 22 Kbp | DNAJ-heat shock | ||
| FW | solcap_snp_sl_33830 | 3 | 71,244,368 | Fw3.2 | Solyc03g114940 | 5 Mbp | Cytochrome P450 |
| Fw3.3 | |||||||
| solcap_snp_sl_33829 | 3 | 71,244,962 | |||||
| solcap_snp_sl_33828 | 3 | 71,245,034 | Solyc03g120190 | 1.1 Mbp | SlOFP7 member of a gene family involved in fruit shape | ||
| solcap_snp_sl_33827 | 3 | 71,245,238 | Solyc03g120790 | 697 Kbp | SlOFP8 member of a gene family involved in fruit shape | ||
| solcap_snp_sl_33822 | 3 | 71,293,803 | Solyc03g121760 | 23 Kbp | SlSUN11 is part of the 34 members SUN-like gene family | ||
| solcap_snp_sl_36532 | 3 | 72,029,476 | Solyc03g123800 | 0 Kbp | SlMAPKK2 | ||
| solcap_snp_sl_25253 | 6 | 1,453,932 | |||||
| solcap_snp_sl_59728 | 12 | 6,426,351 | Solyc12g009580 | 3.6 Mbp | SlYABBY5b is a part of the YABBY gene family | ||
| Solyc12g014130 | 1.5 Mbp | SlSUN33 is part of the 34 members SUN-like gene family | |||||
| YP | solcap_snp_sl_70992 | 7 | 65,761,751 | fd7.1/ty7.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggieri, V.; Calafiore, R.; Schettini, C.; Rigano, M.M.; Olivieri, F.; Frusciante, L.; Barone, A. Exploiting Genetic and Genomic Resources to Enhance Heat-Tolerance in Tomatoes. Agronomy 2019, 9, 22. https://doi.org/10.3390/agronomy9010022
Ruggieri V, Calafiore R, Schettini C, Rigano MM, Olivieri F, Frusciante L, Barone A. Exploiting Genetic and Genomic Resources to Enhance Heat-Tolerance in Tomatoes. Agronomy. 2019; 9(1):22. https://doi.org/10.3390/agronomy9010022
Chicago/Turabian StyleRuggieri, Valentino, Roberta Calafiore, Carlo Schettini, Maria Manuela Rigano, Fabrizio Olivieri, Luigi Frusciante, and Amalia Barone. 2019. "Exploiting Genetic and Genomic Resources to Enhance Heat-Tolerance in Tomatoes" Agronomy 9, no. 1: 22. https://doi.org/10.3390/agronomy9010022






