Biochar Type and Ratio as a Peat Additive/Partial Peat Replacement in Growing Media for Cabbage Seedling Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochars and Plant Material
2.2. Preparation of Growing Media
2.3. Seed Emergence
2.4. Vegetative Growth and Mineral Content
2.5. Total Phenolics and Antioxidant Capacity
2.6. Lipid Peroxidation, Hydrogen Peroxide, and Enzymes Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Growing Media Properties
3.2. Experiment I
3.2.1. Seed Emergence
3.2.2. Plant Development
3.3. Experiment II
3.3.1. Seed Emergence
3.3.2. Plant Growth and Physiology
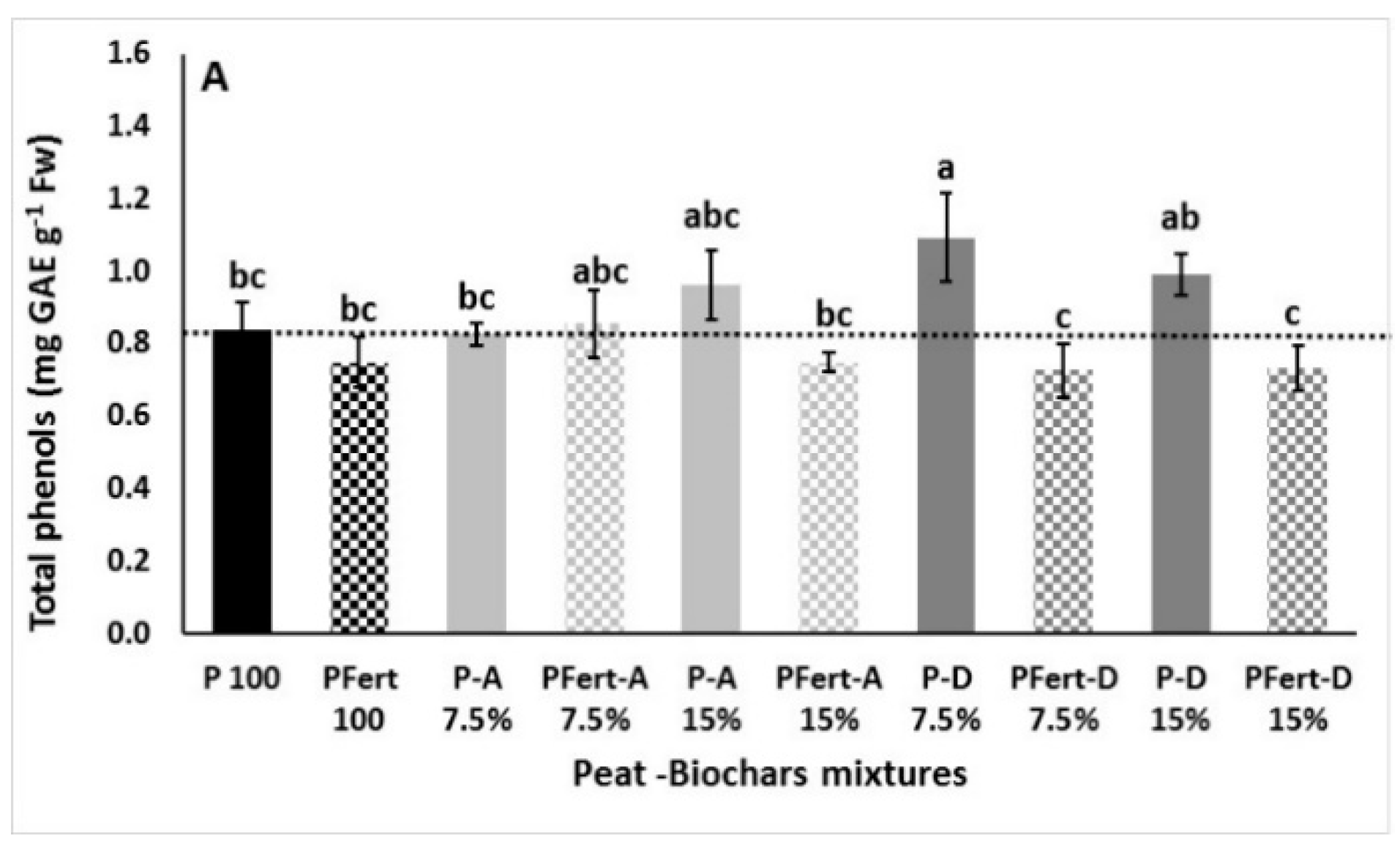
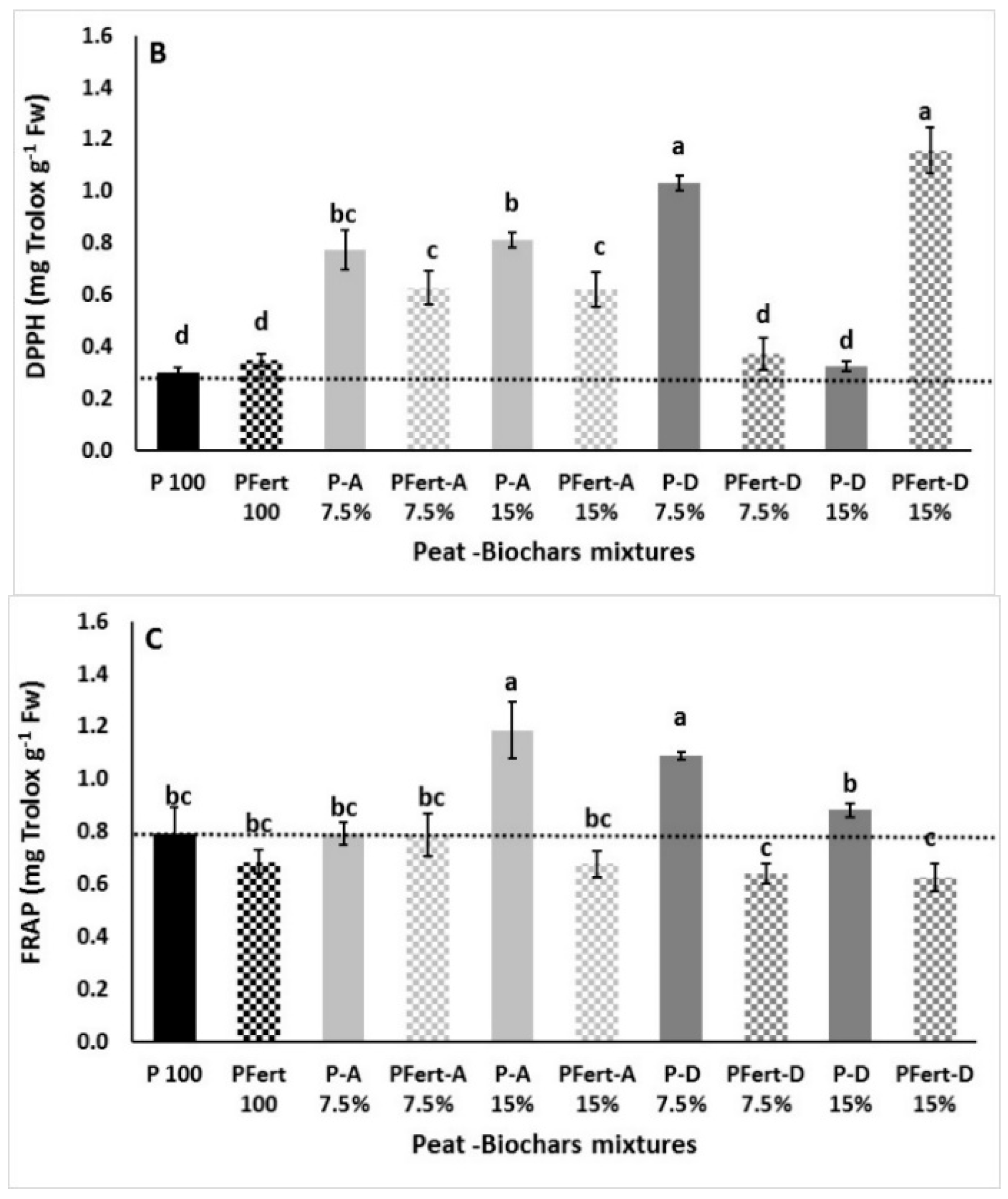
3.3.3. Total Phenolics and Antioxidant Activity
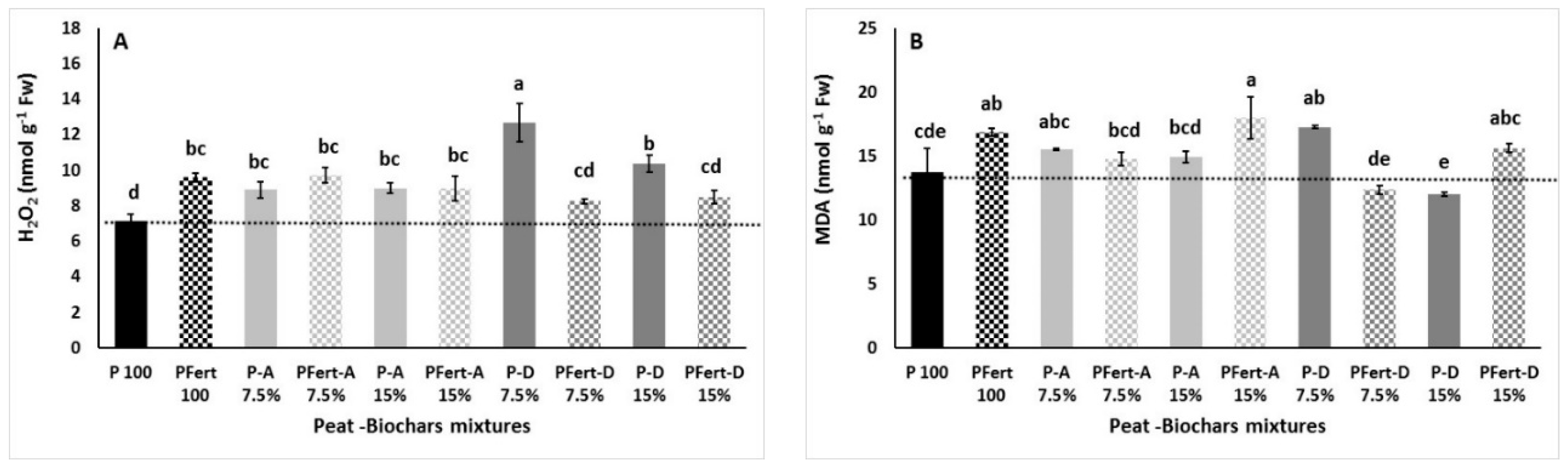
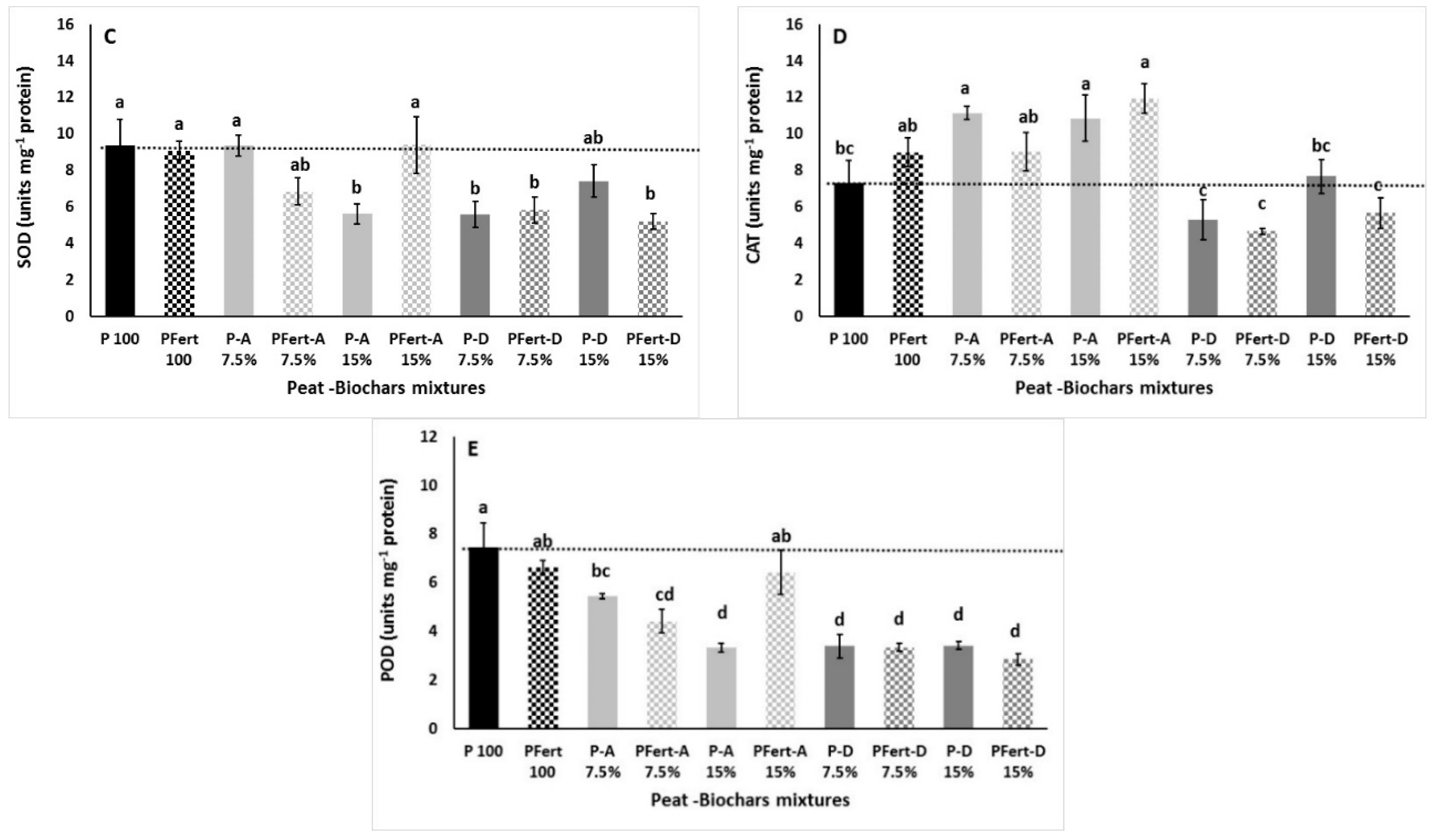
3.3.4. Lipid Peroxidation, Hydrogen Peroxide, and Enzymes Antioxidant Activity
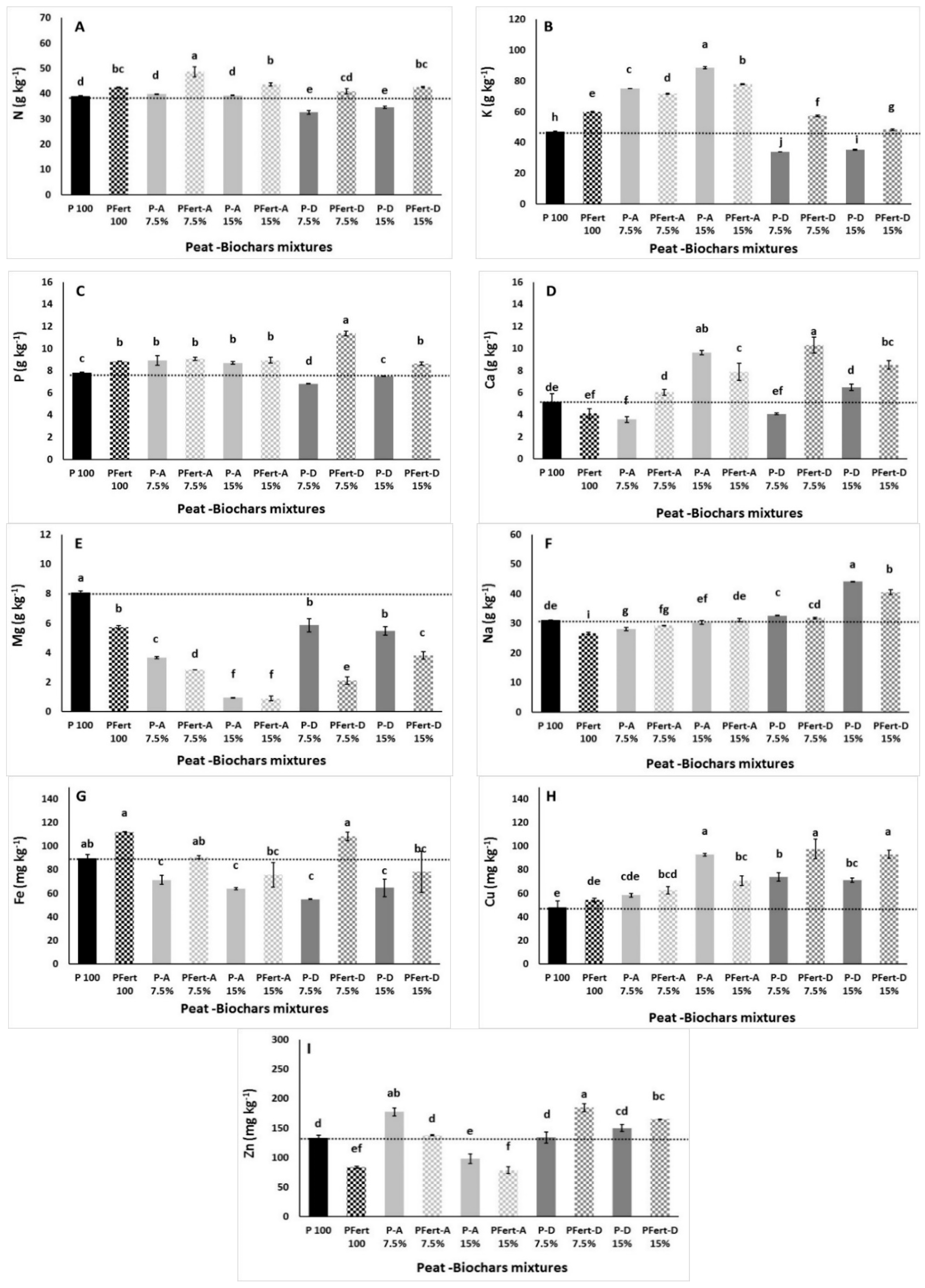
3.3.5. Mineral Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Laghari, M.; Hu, Z.; Mirjat, M.S.; Xiao, B.; Tagar, A.A.; Hu, M. Fast pyrolysis biochar from sawdust improves the quality of desert soils and enhances plant growth. J. Sci. Food Agric. 2016, 96, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.A.M.; Nelson, P.N.P.N.; Bird, M.I.M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crops Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Belda, R.M.; Lidón, A.; Fornes, F. Biochars and hydrochars as substrate constituents for soilless growth of myrtle and mastic. Ind. Crops Prod. 2016, 94, 132–142. [Google Scholar] [CrossRef]
- Calamai, A.; Palchetti, E.; Masoni, A.; Marini, L.; Chiaramonti, D.; Dibari, C.; Brilli, L. The influence of biochar and solid digestate on rose-scented geranium (Pelargonium graveolens L’Hér.) productivity and essential oil quality. Agronomy 2019, 9, 260. [Google Scholar] [CrossRef]
- Huang, L.; Niu, G.; Feagley, S.E.; Gu, M. Evaluation of a hardwood biochar and two composts mixes as replacements for a peat-based commercial substrate. Ind. Crops Prod. 2019, 129, 549–560. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, R.; Ma, F.; Li, Y.; Wang, L. Effects of biochars derived from chicken manure and rape straw on speciation and phytoavailability of Cd to maize in artificially contaminated loess soil. J. Environ. Manag. 2016, 184, 569–574. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar-manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Lehmann, J.; Kern, D.C.; German, L.A.; McCann, J.; Martins, G.C.; Moreira, A. Soil fertility and production potential. In Amazonian Dark Earths: Origin, Properties, Management; Lehmann, J., Kern, D.C., Glaser, B., Woods, W.I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 105–124. [Google Scholar]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Sadaf, J.; Shah, G.A.; Shahzad, K.; Ali, N.; Shahid, M.; Ali, S.; Hussain, R.A.; Ahmed, Z.I.; Traore, B.; Ismail, I.M.I.; et al. Improvements in wheat productivity and soil quality can accomplish by co-application of biochars and chemical fertilizers. Sci. Total Environ. 2017, 607–608, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Kenar, J.A.; Tisserat, B.; Jackson, M.A.; Joshee, N.; Vaidya, B.N.; Peterson, S.C. Chemical and physical properties of Paulownia elongata biochar modified with oxidants for horticultural applications. Ind. Crops Prod. 2017, 97, 260–267. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Sohi, S.P. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef]
- Gregory, S.J.; Anderson, C.W.N.; Camps Arbestain, M.; McManus, M.T. Response of plant and soil microbes to biochar amendment of an arsenic-contaminated soil. Agric. Ecosyst. Environ. 2014, 191, 133–141. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Tsechansky, L.; Elad, Y.; Graber, E.R. Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: A possible mechanism involved in soilborne disease suppression. Soil Biol. Biochem. 2018, 121, 59–66. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Nieto, A.; Gascó, G.; Paz-Ferreiro, J.; Fernández, J.M.; Plaza, C.; Méndez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Wagner, A.; Kaupenjohann, M. Suitability of biochars (pyro- and hydrochars) for metal immobilization on former sewage-field soils. Eur. J. Soil Sci. 2014, 65, 139–148. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Dispenza, V.; De Pasquale, C.; Fascella, G.; Mammano, M.M.; Alonzo, G. Use of biochar as peat substitute for growing substrates of Euphorbia × lomi potted plants. Span. J. Agric. Res. 2016, 14, 21. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, G.; Starman, T.; Volder, A.; Gu, M. Poinsettia growth and development response to container root substrate with Biochar. Horticulturae 2018, 4, 1. [Google Scholar] [CrossRef]
- Choi, H.; Son, H.; Kim, C. Predicting financial distress of contractors in the construction industry using ensemble learning. Expert Syst. Appl. 2018, 110, 1–10. [Google Scholar] [CrossRef]
- Judd, L.A.; Jackson, B.E.; Fonteno, W.C. Advancements in root growth measurement technologies and observation capabilities for container-grown plants. Plants 2015, 4, 369–392. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Athinodorou, F.; Vassiliou, R.; Papadaki, A.; Tzortzakis, N. Deployment of olive-stone waste as a substitute growing medium component for Brassica seedling production in nurseries. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Stavrinides, M.; Moustakas, K.; Tzortzakis, N. Utilization of paper waste as growing media for potted ornamental plants. Clean Technol. Environ. Policy 2018. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Imran, M.; Naveed, M.; Khan, M.Y.; Ahmad, M.; Zahir, Z.A.; Crowley, D.E. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J. Sci. Food Agric. 2017, 97, 5139–5145. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Pasian, C.; Lal, R.; Lopez, R.; Fernandez, M. Vermicompost and Biochar as growing media replacement for ornamental plant production. J. Appl. Hortic. 2017, 19, 205–214. [Google Scholar]
- Álvarez, J.M.; Pasian, C.; Lal, R.; López, R.; Díaz, M.J.; Fernández, M. Morpho-physiological plant quality when biochar and vermicompost are used as growing media replacement in urban horticulture. Urban For. Urban Green. 2018, 34, 175–180. [Google Scholar] [CrossRef]
- Landis, T.D.; Morgan, N. Growing media alternatives for forest and native plant nurseries. Natl. Proc. For. Conserv. Nurs. Assoc. 2008 2009, 5814, 26–31. [Google Scholar]
- Li, Q.; Chen, J.; Caldwell, R.D.; Deng, M. Cowpeat as a substitute for peat in container substrates for foliage plant propagation. Horttechnology 2009, 19, 340–345. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- British Standards Institution (BSI). EN 13037, Soil Improvers and Growing Media-Determination of pH; BSI: London, UK, 2002. [Google Scholar]
- British Standards Institution (BSI). EN 13038, Soil Improvers and Growing Media-Determination of Electrical Conductivity; BSI: London, UK, 2002. [Google Scholar]
- British Standards Institution (BSI). EN 13651, Soil Improvers and Growing Media-Extraction of Calcium Chloride/DTPA (CAT); BSI: London, UK, 2002. [Google Scholar]
- Kelepesi, S.; Tzortzakis, N.G. Olive mill wastes: A growing medium component for seedling and crop production of lettuce and chicory. Int. J. Veg. Sci. 2009, 15, 325–339. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Tzanakaki, K.; Conomakis, C.D. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assessment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J. Sci. Food Agric. 2017, 97, 3675–3684. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Shackley, S.; Ruysschaert, G.; Zwart, K.; Glaser, B. Biochar in European Soils and Agriculture: Science and Practice; Routledge: Oxon, UK, 2016; p. 324. [Google Scholar]
- Ronga, D.; Francia, E.; Allesina, G.; Pedrazzi, S.; Zaccardelli, M.; Pane, C.; Tava, A.; Bignami, C. Valorization of vineyard by-products to obtain composted digestate and biochar suitable for nursery grapevine (Vitis vinifera L.) production. Agronomy 2019, 9, 420. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217–230. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Dunlop, S.J.; Arbestain, M.C.; Bishop, P.A.; Wargent, J.J. Closing the loop: Use of biochar produced from tomato crop green waste as a substrate for soilless, hydroponic tomato production. HortScience 2015, 50, 1572–1581. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Chen, J. Biochar or biochar-compost amendment to a peat-based substrate improves growth of Syngonium podophyllum. Agronomy 2019, 9, 460. [Google Scholar] [CrossRef]
- Nair, A.; Carpenter, B. Biochar rate and transplant tray cell number have implications on pepper growth during transplant production. Horttechnology 2016, 26, 713–719. [Google Scholar] [CrossRef]
- Sun, L.; Li, L.; Chen, Z.; Wang, J.; Xiong, Z. Combined effects of nitrogen deposition and biochar application on emissions of N2O, CO2 and NH3 from agricultural and forest soils. Soil Sci. Plant Nutr. 2014, 60, 254–265. [Google Scholar] [CrossRef]
- Bedussi, F.; Zaccheo, P.; Crippa, L. Pattern of pore water nutrients in planted and non-planted soilless substrates as affected by the addition of biochars from wood gasification. Biol. Fertil. Soils 2015, 51, 625–635. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Altland, J.E.; Locke, J.C. Effect of biochar type on macronutrient retention and release from soilless substrate. HortScience 2013, 48, 1397–1402. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Kim, W.I.; Kunhikrishnan, A.; Kim, K.H. Amelioration of horticultural growing media properties through rice hull biochar incorporation. Waste Biomass Valoriz. 2017, 8, 483–492. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Gupta, D.D.; Palma, J.M.; Corpas, F.J. Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Springer International Publishing Switzerland: Cham, Switzerland, 2015; ISBN 9783319204208. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P.; Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry-A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]

| pH | EC (μS cm−1) | NO3-N (mg kg−1) | NH4-N (mg kg−1) | K (mg kg−1) | P (mg kg−1) | |
|---|---|---|---|---|---|---|
| P 100 | 4.97 ± 0.06 ijk | 203.37 ± 2.37 a | 50.13 ± 0.58 a | 47.35 ± 0.55 bc | 53.40 ± 1.62 k | 49.71 ± 0.58 c |
| P-A 5% | 5.01 ± 0.06 hij | 143.67 ± 1.67 g | 21.51 ± 1.25 e | 42.37 ± 0.49 efg | 84.33 ± 2.98 h | 41.04 ± 1.48 f |
| P-A 10% | 5.19 ± 0.07 gh | 96.12 ± 1.12 j | 16.63 ± 0.19 g | 44.12 ± 1.11 de | 110.85 ± 2.39 g | 46.53 ± 0.54 d |
| P-A 15% | 5.53 ± 0.11 de | 96.15 ± 2.14 j | 14.65 ± 0.17 i | 43.82 ± 0.51 de | 147.58 ± 3.57 e | 38.25 ± 1.44 g |
| P-A 20% | 5.89 ± 0.09 c | 93.08 ± 1.09 j | 13.84 ± 0.21 j | 43.91 ± 0.53 de | 213.67 ± 4.49 d | 44.36 ± 1.23 e |
| P-B 5% | 5.16 ± 0.06 ghi | 167.95 ± 1.95 c | 30.38 ± 1.35 b | 44.28 ± 0.65 d | 65.52 ± 0.76 j | 44 ± 39 ± 0.51 e |
| P-B 10% | 5.70 ± 0.05 cd | 141.65 ± 1.65 gh | 17.85 ± 0.26 f | 41.03 ± 0.48 g | 78.09 ± 1.90 i | 39.89 ± 1.14 f |
| P-B 15% | 6.46 ± 0.11 b | 137.61 ± 1.60 h | 17.42 ± 0.20 f | 29.35 ± 0.34 h | 77.63 ± 1.94 i | 31.49 ± 1.45 i |
| P-B 20% | 7.06 ± 0.13 a | 149.74 ± 1.74 f | 12.85 ± 0.15 k | 20.45 ± 0.24 i | 78.57 ± 1.35 hi | 18.57 ± 0.21 j |
| P-C 5% | 4.77 ± 0.05 k | 201.34 ± 2.34 a | 31.88 ± 0.37 b | 48.61 ± 1.56 b | 132.21 ± 2.98 f | 50.49 ± 1.59 c |
| P-C 10% | 4.92 ± 0.05 jk | 181.11 ± 2.11 b | 15.52 ± 0.18 h | 43.04 ± 1.52 def | 221.07 ± 4.57 c | 43.44 ± 1.78 e |
| P-C 15% | 5.16 ± 0.08 ghi | 148.73 ± 1.73 f | 10.50 ± 0.12 l | 46.78 ± 0.63 c | 317.57 ± 5.78 b | 56.38 ± 1.65 b |
| P-C 20% | 5.40 ± 0.07 ef | 160.87 ± 1.87 d | 29.06 ± 0.34 d | 72.82 ± 2.82 a | 367.38 ± 4.28 a | 86.35 ± 3.15 a |
| P-D 5% | 4.76 ± 0.05 k | 155.87 ± 2.65 e | 22.22 ± 0.26 e | 43.28 ± 1.51 def | 54.98 ± 1.64 k | 46.26 ± 0.54 d |
| P-D 10% | 4.87 ± 0.05 jk | 121.41 ± 1.59 i | 11.03 ± 0.13 l | 44.71 ± 0.52 d | 54.46 ± 1.21 k | 43.17 ± 0.89 e |
| P-D 15% | 5.06 ± 0.08 hij | 93.08 ± 1.08 j | 6.85 ± 0.28 m | 43.07 ± 063 def | 55.24 ± 1.76 k | 37.45 ± 1.23 g |
| P-D 20% | 5.32 ± 0.09 fg | 98.14 ± 1.14 j | 6.02 ± 0.07 m | 41.87 ± 0.49 fg | 79.97 ± 2.81 hi | 34.09 ± 0.89 h |
| P 100 | PFert 100 | P-A 7.5% | PFert-A 7.5% | P-A 15% | PFert-A 15% | P-D 7.5% | PFert-D 7.5% | P-D 15% | PFert-D 15% | |
|---|---|---|---|---|---|---|---|---|---|---|
| NO3-N (mg kg−1) | 63.38 ± 1.38 c | 88.42 ± 1.92 a | 78.20 ± 1.71 b | 81.26 ± 1.76 b | 37.82 ± 0.82 f | 48.04 ± 1.04 e | 47.53 ± 1.03 e | 58.78 ± 1.28 d | 36.29 ± 0.89 f | 31.69 ± 0.69 g |
| NH4-N (mg kg−1) | 20.95 ± 0.45 g | 67.98 ± 1.48 b | 18.51 ± 2.03 i | 43.95 ± 0.95 d | 33.22 ± 0.72 f | 64.16 ± 1.40 c | 36.80 ± 0.85 e | 71.55 ± 1.55 a | 32.12 ± 1.56 h | 68.49 ± 1.49 ab |
| K (mg kg−1) | 117.55 ± 3.55 f | 178.89 ± 3.89 d | 230.01 ± 5.02 c | 301.55 ± 6.55 b | 378.22 ± 8.23 a | 373.10 ± 8.24 a | 102.25 ± 2.26 f | 153.34 ± 3.38 e | 102.26 ± 2.21 f | 148.27 ± 3.25 e |
| P (mg kg−1) | 35.78 ± 0.78 g | 65.93 ± 1.43 c | 44.46 ± 1.96 e | 71.04 ± 1.63 ab | 40.38 ± 0.88 f | 57.75 ± 1.82 d | 44.46 ± 1.32 e | 68.79 ± 1.49 bc | 36.81 ± 0.98 fg | 74.11 ± 1.61 a |
| Ca (mg kg−1) | 695.11 ± 15.11 a | 710.95 ± 15.45 a | 695.12 ± 15.12 a | 721.17 ± 15.67 a | 718.62 ± 16.51 a | 712.48 ± 15.48 a | 516.73 ± 11.23 c | 519.80 ± 11.35 c | 481.46 ± 10.46 c | 609.24 ± 13.24 b |
| Mg (mg kg−1) | 482.13 ± 10.48 a | 474.82 ± 12.36 a | 326 ± 55 ± 7.10 b | 327.72 ± 7.12 b | 237.71 ± 5.16 d | 234.19 ± 5.09 d | 307.79 ± 6.69 b | 314.64 ± 6.84 b | 239.21 ± 5.23 d | 274.72 ± 5.97 c |
| Na (mg kg−1) | 45.49 ± 0.99 cd | 46.03 ± 1.02 c | 41.40 ± 0.90 de | 45.49 ± 0.99 cd | 42.42 ± 0.92 cde | 40.89 ± 0.89 e | 63.89 ± 1.39 b | 62.86 ± 1.36 b | 88.42 ± 1.92 a | 84.85 ± 1.86 a |
| Fe (mg kg−1) | 9.15 ± 0.22 bc | 8.18 ± 0.18 ef | 9.45 ± 0.21 b | 8.43 ± 0.18 de | 8.07 ± 0.17 ef | 7.72 ± 0.17 f | 8.28 ± 0.18 def | 8.58 ± 0.18 cde | 8.94 ± 0.19 bcd | 14.26 ± 0.31 a |
| Cu (mg kg−1) | 0.10 ± 0.00 e | 0.10 ± 0.00 e | 0.05 ± 0.00 f | 0.05 ± 0.00 f | 0.05 ± 0.00 f | 0.05 ± 0.00 f | 0.20 ± 0.01 c | 0.25 ± 0.01 b | 0.16 ± 0.01 d | 0.31 ± 0.01 a |
| Zn (mg kg−1) | 1.07 ± 0.02 e | 0.97 ± 0.02 e | 1.02 ± 0.02 e | 1.17 ± 0.02 d | 1.02 ± 0.02 e | 0.97 ± 0.02 e | 1.53 ± 0.03 c | 1.58 ± 0.04 c | 2.09 ± 0.05 b | 2.21 ± 0.05 a |
| Mn (mg kg−1) | 2.40 ± 0.05 e | 2.40 ± 0.05 e | 8.89 ± 0.19 b | 8.88 ± 0.019 b | 12.83 ± 0.28 a | 12.62 ± 0.27 a | 2.60 ± 0.05 de | 2.66 ± 0.06 de | 2.96 ± 0.07 cd | 3.37 ± 0.07 c |
| B (mg kg−1) | 0.61 ± 0.01 c | 0.61 ± 0.01 c | 0.66 ± 0.01 c | 0.66 ± 0.01 c | 0.68 ± 0.01 c | 0.67 ± 0.01 c | 0.82 ± 0.02 b | 0.83 ± 0.02 b | 0.97 ± 0.02 a | 0.87 ± 0.02 b |
| Height | Upper Fresh Weight | Upper Dry Weight | Root Length | Chlorophyll Fluorescence | SPAD | N | K | P | |
|---|---|---|---|---|---|---|---|---|---|
| P 100 | 2.46 ± 0.11 bcd | 2.62 ± 0.10 ef | 0.261 ± 0.011 a | 6.15 ± 0.23 abc | 0.832 ± 0.042 a | 27.92 ± 2.84 a | 22.19 ± 0.90 g | 14.70 ± 1.74 k | 7.03 ± 0.19 f |
| P-A 5% | 2.91 ± 0.07 a | 3.59 ± 0.12 a | 0.181 ± 0.005 cd | 5.38 ± 0.41 bcd | 0.701 ± 0.103 ab | 24.82 ± 1.95 abc | 39.11 ± 0.86 de | 35.58 ± 1.19 hi | 9.43 ± 0.10 de |
| P-A 10% | 2.76 ± 0.13 ab | 3.45 ± 0.11 a | 0.170 ± 0.009 de | 4.88 ± 0.21 bcd | 0.773 ± 0.022 a | 15.75 ± 1.12 ef | 39.83 ± 0.28 cde | 49.60 ± 0.18 de | 10.01 ± 0.02 cd |
| P-A 15% | 2.65 ± 0.11 abc | 2.95 ± 0.06 cdef | 0.147 ± 0.009 ef | 6.13 ± 0.25 abc | 0.753 ± 0.046 a | 21.87 ± 2.88 abcde | 34.09 ± 0.09 f | 45.07 ± 2.07 efg | 9.02 ± 0.11 e |
| P-A 20% | 2.26 ± 0.08 cde | 2.70 ± 0.17 ef | 0.165 ± 0.012 de | 6.03 ± 0.32 abcd | 0.559 ± 0.049 b | 27.77 ± 1.63 a | 40.10 ± 1.13 cde | 56.51 ± 1.09 c | 9.87 ± 0.25 cde |
| P-B 5% | 2.96 ± 0.15 a | 3.35 ± 0.13 ab | 0.208 ± 0.013 bc | 5.75 ± 0.81 abcd | 0.701 ± 0.033 ab | 25.95 ± 2.36 ab | 32.37 ± 0.23 f | 26.45 ± 1.88 j | 9.42 ± 0.32 de |
| P-B 10% | 2.71 ± 0.16 ab | 3.30 ± 0.14 abc | 0.170 ± 0.013 de | 5.53 ± 0.70 abcd | 0.551 ± 0.074 b | 18.30 ± 0.78 def | 37.44 ± 2.28 e | 41.22 ± 2.33 gh | 10.57 ± 0.42 bc |
| P-B 15% | 2.41 ± 0.11 bcd | 2.99 ± 0.10 bcde | 0.144 ± 0.007 efg | 6.13 ± 0.63 abc | 0.541 ± 0.077 b | 18.62 ± 1.38 cdef | 42.45 ± 1.87 bcd | 48.15 ± 1.59 ef | 10.19 ± 0.30 cd |
| P-B 20% | 1.96 ± 0.11 e | 2.14 ± 0.17 g | 0.113 ± 0.009 ghi | 6.36 ± 0.26 abc | 0.492 ± 0.065 b | 22.72 ± 1.55 abcd | 38.88 ± 1.19 de | 55.35 ± 2.16 cd | 11.23 ± 0.05 ab |
| P-C 5% | 2.20 ± 0.14 de | 2.66 ± 0.08 ef | 0.131 ± 0.005 fgh | 5.30 ± 0.45 bcd | 0.529 ± 0.024 b | 14.47 ± 1.53 f | 39.95 ± 0.49 cde | 60.15 ± 5.13 c | 10.63 ± 0.16 bc |
| P-C 10% | 2.28 ± 0.11 cde | 1.74 ± 0.12 h | 0.091 ± 0.006 i | 4.84 ± 0.75 cd | 0.569 ± 0.021 b | 16.97 ± 1.09 def | 43.31 ± 0.67 bc | 75.95 ± 1.44 b | 11.63 ± 0.34 a |
| P-C 15% | 2.65 ± 0.15 abc | 2.96 ± 0.17 bcdef | 0.156 ± 0.010 def | 4.42 ± 0.69 d | 0.561 ± 0.098 b | 19.42 ± 1.97 cdef | 41.37 ± 0.06 cd | 74.89 ± 0.86 b | 9.39 ± 0.15d e |
| P-C 20% | 2.40 ± 0.13 bcd | 2.83 ± 0.13 def | 0.152 ± 0.009 def | 4.50 ± 0.15 d | 0.725 ± 0.070 ab | 19.62 ± 1.49 cdef | 45.08 ± 1.29 ab | 82.17 ± 1.10 a | 10.64 ± 0.66 bc |
| P-D 5% | 2.20 ± 0.09 de | 3.22 ± 0.07 abcd | 0.215 ± 0.005 b | 7.01 ± 0.22 a | 0.751 ± 0.084 a | 23.30 ± 2.03 abcd | 33.21 ± 0.46 f | 33.17 ± 1.42 i | 9.55 ± 0.26 de |
| P-D 10% | 2.50 ± 0.13 bcd | 3.14 ± 0.04 abcd | 0.164 ± 0.013 de | 5.10 ± 0.50 bcd | 0.584 ± 0.062 ab | 17.35 ± 1.16 def | 41.08 ± 1.35 cd | 35.68 ± 2.01 hi | 10.65 ± 0.02 bc |
| P-D 15% | 2.65 ± 0.08 abc | 2.55 ± 0.15 f | 0.141 ± 0.007 efg | 4.76 ± 0.31 cd | 0.509 ± 0.076 b | 20.35 ± 3.62 bcdef | 42.74 ± 0.07 bc | 42.51 ± 0.64 fg | 11.83 ± 0.12 a |
| P-D 20% | 2.73 ± 0.07 ab | 2.11 ± 0.08 g | 0.104 ± 0.002 hi | 4.45 ± 0.29 d | 0.429 ± 0.016 c | 17.45 ± 1.09 def | 48.09 ± 1.35 a | 49.21 ± 2.21 e | 10.60 ± 0.18 bc |
| Height | Leaf Number | Upper Fresh Weight | Upper Dry Weight | |
|---|---|---|---|---|
| P 100 | 5.40 ± 0.32 abc | 3.33 ± 0.21 ab | 1.962 ± 0.065 de | 0.098 ± 0.005 c |
| PFert 100 | 4.98 ± 0.25 bcd | 3.00 ± 0.00 b | 2.230 ± 0.218 bcde | 0.098 ± 0.014 c |
| P-A 7.5% | 4.83 ± 0.31 bcd | 3.00 ± 0.00 b | 2.210 ± 0.074 bcde | 0.124 ± 0.012 bc |
| PFert-A 7.5% | 4.83 ± 0.23 bcd | 3.50 ± 0.22 a | 2.834 ± 0.147 a | 0.177 ± 0.006 a |
| P-A 15% | 4.77 ± 0.26 bcd | 3.00 ± 0.00 b | 2.033 ± 0.240 cde | 0.107 ± 0.012 c |
| PFert-A 15% | 4.15 ± 0.32 d | 3.17 ± 0.17 ab | 1.724 ± 0.180 e | 0.095 ± 0.008 c |
| P-D 7.5% | 4.20 ± 0.19 d | 3.00 ± 0.00 b | 2.091 ± 0.201 bcde | 0.121 ± 0.009 bc |
| PFert-D 7.5% | 4.40 ± 0.26 cd | 3.00 ± 0.00 b | 2.387 ± 0.301 abcd | 0.110 ± 0.008 c |
| P-D 15% | 5.52 ± 0.14 ab | 3.00 ± 0.00 b | 2.633 ± 0.218 abc | 0.144 ± 0.007 b |
| PFert-D 15% | 6.15 ± 0.64 a | 3.50 ± 0.22 a | 2.681 ± 0.0.091 ab | 0.121 ± 0.005 bc |
| Stomatal Conductance | SPAD | Chl a | Chl b | Total Chls | |
|---|---|---|---|---|---|
| P 100 | 2.69 ± 0.29 ab | 18.02 ± 1.99 ab | 0.322 ± 0.025 abcd | 0.177 ± 0.010 ab | 0.439 ± 0.035 abcd |
| PFert 100 | 3.08 ± 0.65 a | 18.75 ± 1.96 ab | 0.311 ± 0.003 bcd | 0.112 ± 0.003 b | 0.422 ± 0.003 bcd |
| P-A 7.5% | 2.08 ± 0.29 abc | 15.05 ± 1.00 b | 0.362 ± 0.020 ab | 0.125 ± 0.006 ab | 0.487 ± 0.026 ab |
| PFert-A 7.5% | 1.11 ± 0.23 c | 18.50 ± 2.55 ab | 0.359 ± 0.034 ab | 0.114 ± 0.009 ab | 0.474 ± 0.043 abc |
| P-A 15% | 2.07 ± 0.42 abc | 15.43 ± 1.26 ab | 0.274 ± 0.024 cd | 0.097 ± 0.011 bc | 0.371 ± 0.035 cd |
| PFert-A 15% | 2.73 ± 0.47 ab | 15.25 ± 0.53 ab | 0.260 ± 0.006 d | 0.081 ± 0.002 c | 0.342 ± 0.008 d |
| P-D 7.5% | 1.89 ± 0.06 bc | 20.02 ± 1.25 ab | 0.345 ± 0.049 abc | 0.117 ± 0.015 ab | 0.461 ± 0.063 abc |
| PFert-D 7.5% | 2.00 ± 0.11 abc | 18.68 ± 1.78 ab | 0.319 ± 0.016 abcd | 0.116 ± 0.008 ab | 0.435 ± 0.025 abcd |
| P-D 15% | 2.04 ± 0.37 abc | 20.37 ± 1.21 a | 0.340 ± 0.018 abcd | 0.110 ± 0.004 b | 0.450 ± 0.022 abcd |
| PFert-D 15% | 1.60 ± 0.22 bc | 18.18 ± 0.75 ab | 0.397 ± 0.029 a | 0.142 ± 0.012 a | 0.539 ± 0.041 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar Type and Ratio as a Peat Additive/Partial Peat Replacement in Growing Media for Cabbage Seedling Production. Agronomy 2019, 9, 693. https://doi.org/10.3390/agronomy9110693
Chrysargyris A, Prasad M, Kavanagh A, Tzortzakis N. Biochar Type and Ratio as a Peat Additive/Partial Peat Replacement in Growing Media for Cabbage Seedling Production. Agronomy. 2019; 9(11):693. https://doi.org/10.3390/agronomy9110693
Chicago/Turabian StyleChrysargyris, Antonios, Munoo Prasad, Anna Kavanagh, and Nikos Tzortzakis. 2019. "Biochar Type and Ratio as a Peat Additive/Partial Peat Replacement in Growing Media for Cabbage Seedling Production" Agronomy 9, no. 11: 693. https://doi.org/10.3390/agronomy9110693





