Screening of Provitamin-A Maize Inbred Lines for Drought Tolerance: Beta-Carotene Content and Secondary Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Study Sites
2.2. Experimental Design and Crop Establishment
2.3. Plant Characteristics
2.3.1. Morphophysiological Traits
2.3.2. Biochemical Traits
2.4. Data Analysis
2.4.1. Analysis of Variance, Mean Performance and Stress-Tolerant Index
2.4.2. Variance Components and Heritability
2.4.3. Principal Component Biplot, Trait Correlations and Relative Selection Efficiency
3. Results
3.1. Analysis of Variance, Mean Performances and Stress-Tolerant Index
3.2. Heritability and Variance Components
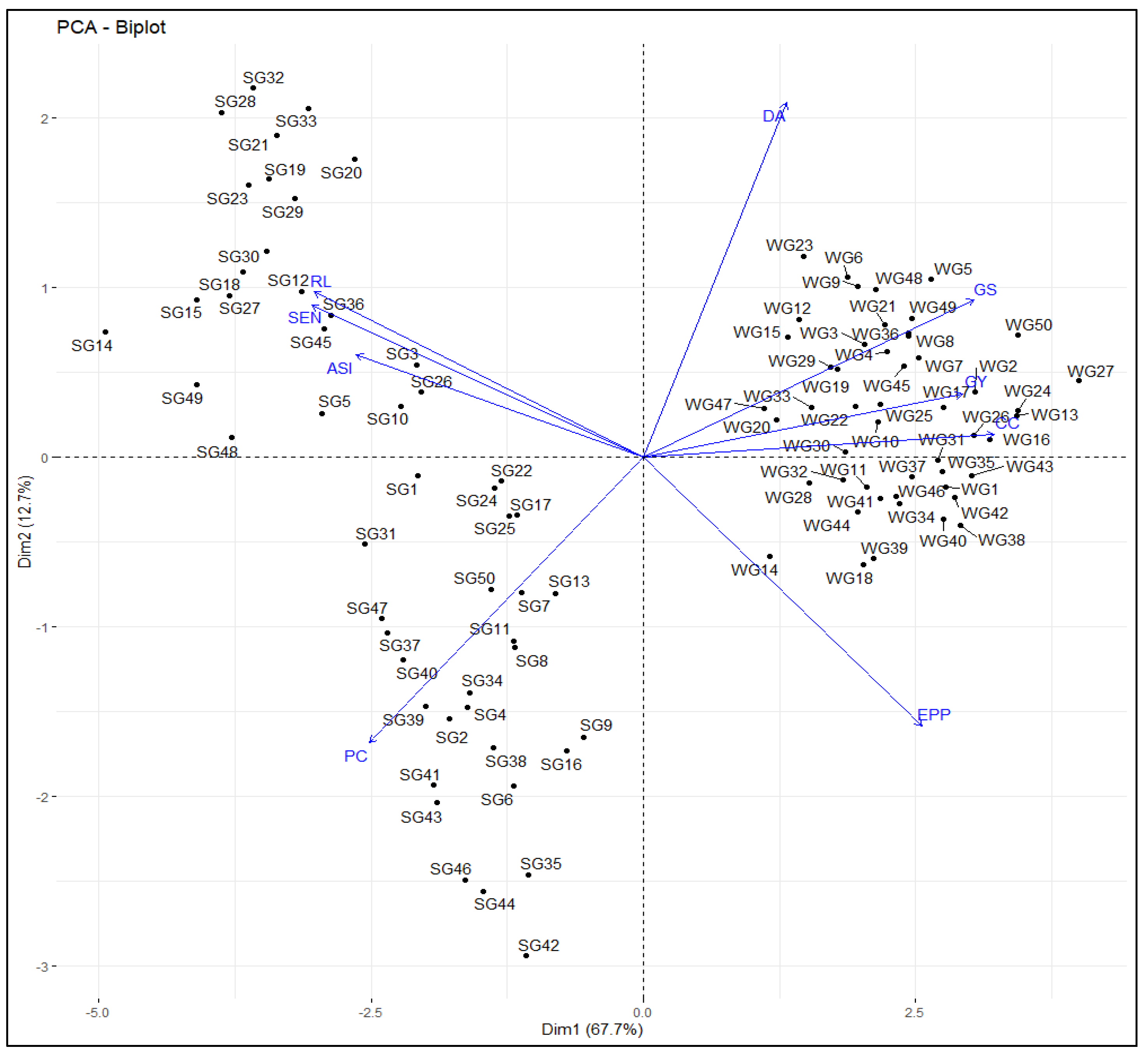
3.3. Principal Component Biplot Analysis
3.4. Phenotypic Correlation Analysis
3.5. Relative Efficiency of Indirect Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thurnham, D. Vitamin A and carotenoids. In Essentials of Human Nutrition; Mann, J., Truswell, A.S., Eds.; Oxfrd University Press: Oxford, UK, 2012; pp. 191–216. [Google Scholar]
- Harjes, C.E.; Rocheford, T.R.; Bai, L.; Brutnell, T.P.; Kandianis, C.B.; Sowinski, S.G.; Stapleton, A.E.; Vallabhaneni, R.; Williams, M.; Wurtzel, E.T.; et al. Natural Genetic Variation in Lycopene Epsilon Cyclase Tapped for Maize Biofortification. Science 2008, 319, 330–333. [Google Scholar] [CrossRef]
- Andersson, M.; Saltzman, A.; Singh Virk, P.; Pfeiffer, W. Progress update: Crop development of biofortified staple food crops under HarvestPlus. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11905–11935. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Magorokosho, C.; Tongooona, P. Selection for Drought Tolerance in Two Tropical Maize Populations. Afr. Crop Sci. J. 2003, 11, 151–161. [Google Scholar] [CrossRef]
- DAFF. Trends in the Agricultural Sector; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2013. [Google Scholar]
- FAO. Southern Africa el Niño Response Plan (2016/2017); Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Lu, Y.; Hao, Z.; Xie, C.; Crossa, J.; Araus, J.-L.; Gao, S.; Vivek, B.S.; Magorokosho, C.; Mugo, S.; Makumbi, D.; et al. Large-scale screening for maize drought resistance using multiple selection criteria evaluated under water-stressed and well-watered environments. Field Crop. Res. 2011, 124, 37–45. [Google Scholar] [CrossRef]
- Almeida, G.D.; Nair, S.; Borém, A.; Cairns, J.; Trachsel, S.; Ribaut, J.-M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. Molecular mapping across three populations reveals a QTL hotspot region on chromosome 3 for secondary traits associated with drought tolerance in tropical maize. Mol. Breed. 2014, 34, 701–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A. Phenotyping and Selection. In Plant Breeding for Water-Limited Environments; Springer: New York, NY, USA, 2011; pp. 153–216. [Google Scholar]
- Bänziger, M.; Edmeades, G.; Lafitte, H. Selection for drought tolerance increases maize yields across a range of nitrogen levels. Crop Sci. 1999, 39, 1035–1040. [Google Scholar] [CrossRef]
- Betrán, F.J.; Beck, D.; Bänziger, M.; Edmeades, G.O. Secondary traits in parental inbreds and hybrids under stress and non-stress environments in tropical maize. Field Crop. Res. 2003, 83, 51–65. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics, 2nd edn London; Longman: London, UK, 1981. [Google Scholar]
- Edmeades, G. Progress in Achieving and Delivering Drought Tolerance in Maize—An Update; ISAAA: Ithaca, NY, USA, 2013; p. 130. [Google Scholar]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing Down the Targets: Towards Successful Genetic Engineering of Drought-Tolerant Crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Helander, J.D.M.; Vaidya, A.S.; Cutler, S.R. Chemical manipulation of plant water use. Bioorganic Med. Chem. 2016, 24, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.-B.; Chen, X.-Y.; Chu, L.-Y.; Zhao, X.-N.; Wu, G.; Yuan, Y.-B.; Zhao, C.-X.; Hu, Z.-M. Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Colloids Surf. B Biointerfaces 2006, 53, 113–119. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, L.-F.; Zhang, K.; Liu, F.-Z.; Wan, Y.-S. Drought-induced responses of organic osmolytes and proline metabolism during pre-flowering stage in leaves of peanut (Arachis hypogaea L.). J. Integr. Agric. 2017, 16, 2197–2205. [Google Scholar] [CrossRef]
- Bänzinger, M.; Edmeades, G.O.; Beck, D.; Bellon, M. Breeding for Drought and Nitrogen Stress Tolerance in Maize: From Theory to Practice; CIMMYT: Mexico City, Mexico, 2000. [Google Scholar]
- Menkir, A.; Liu, W.; White, W.S.; Maziya-Dixon, B.; Rocheford, T. Carotenoid diversity in tropical-adapted yellow maize inbred lines. Food Chem. 2008, 109, 521–529. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Fernandez, G.C. Effective Selection Criteria for Assessing Plant Stress Tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Allard, R. Principles of Plant Breeding; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Robinson, H.F.; Comstock, R.E.; Harvey, P.H. Estimates of heritability and the degree of dominance in corn. Agron. J. 1949, 41, 353–359. [Google Scholar] [CrossRef]
- Alvarado, G.; López, M.; Vargas, M.; Pacheco, Á; Rodríguez, F.; Burgueño, J.; Crossa, J. META-R (Multi Environment Trail Analysis with R for Windows) Version 6.04 CIMMYT Research Data & Software Repository Network: 2019. Available online: https://data.cimmyt.org/dataset.xhtml?persistentId=hdl:11529/10201 (accessed on 20 August 2019).
- Mukaka, M.M. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Pixley, K.; Rojas, N.P.; Babu, R.; Mutale, R.; Surles, R.; Simpungwe, E. Biofortification of maize with provitamin A carotenoids. In Carotenoids in Human Nutrition and Health; Tanumihardjo, S.A., Ed.; Springer Science and Business Media: New York, NY, USA, 2013. [Google Scholar]
- Wen, W.; Araus, J.L.; Shah, T.; Cairns, J.; Mahuku, G.; Bänziger, M.; Torres, J.L.; Sánchez, C.; Yan, J. Molecular Characterization of a Diverse Maize Inbred Line Collection and its Potential Utilization for Stress Tolerance Improvement. Crop Sci. 2011, 51, 2569. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crop. Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Araus, J.L.; Serret, M.D.; Edmeades, G. Phenotyping maize for adaptation to drought. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Bänziger, M.; Cooper, M. Breeding for low input conditions and consequences for participatory plant breeding examples from tropical maize and wheat. Euphytica 2001, 122, 503–519. [Google Scholar] [CrossRef]
- Betrán, F.J.; Beck, D.; Bänziger, M.; Edmeades, G.O. Genetic Analysis of Inbred and Hybrid Grain Yield under Stress and Nonstress Environments in Tropical Maize. Crop Sci. 2003, 43, 807–817. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting Maize Productivity: Ideotypes Associated with Grain Yield under Drought Stress and Well-watered Conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, M.T.; Grzesiak, S.; Skoczowski, A. Changes of leaf water potential and gas exchange during and after drought in triticale and maize genotypes differing in drought tolerance. Photosynthetica 2006, 44, 561–568. [Google Scholar] [CrossRef]
- Campos, H.; Cooper, M.; Edmeades, G.O.; Löffler, C.; Schussler, J.R.; Ibañez, M. Changes in drought tolerance in maize associated with fifty years of breeding for yield in the U.S. corn belt. Maydica 2006, 51, 369–381. [Google Scholar]
- Ziyomo, C.; Bernardo, R. Drought tolerance in maize: Indirect selection through secondary traits versus genomewide selection. Crop Sci. 2013, 53, 1269–1275. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Nogués, S.; Araus, J.L. Water and nitrogen conditions affect the relationships of Δ13C and Δ18O to gas exchange and growth in durum wheat. J. Exp. Bot. 2009, 60, 1633–1644. [Google Scholar] [CrossRef]
- Vadez, V. Root hydraulics: The forgotten side of roots in drought adaptation. Field Crop. Res. 2014, 165, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.J.; Wu, A.Z.; Zheng, C.C.; Wang, Y.F.; Cai, R.; Shen, X.F.; Xu, R.R.; Liu, P.; Kong, L.J.; Dong, S.T. QTL mapping of maize (Zea mays) stay-green traits and their relationship to yield. Plant Breed. 2009, 128, 54–62. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Moayedi, A.A.; Nasrulhaq-Boyce, A.; Tavakoli, H. Application of physiological and biochemical indices for screening and assessment of drought tolerance in durum wheat genotypes. Aust. J. Crop Sci. 2011, 5, 1014–1018. [Google Scholar]
- Menkir, A.; Maziya-Dixon, B. Influence of genotype and environment on beta-carotene content of tropical yellow-endosperm maize genotypes [Zea mays L.; Nigeria]. Maydica 2004, 49, 313–318. [Google Scholar]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding Crops for Better Nutrition. Crop Sci. 2007, 47, S-88–S-105. [Google Scholar] [CrossRef]
- Suwarno, W.B.; Pixley, K.V.; Palacios-Rojas, N.; Kaeppler, S.M.; Babu, R. Formation of Heterotic Groups and Understanding Genetic Effects in a Provitamin A Biofortified Maize Breeding Program. Crop Sci. 2014, 54, 14. [Google Scholar] [CrossRef]
- Egesel, C.O.; Wong, J.C.; Lambert, R.J.; Rocheford, T.R. Combining Ability of Maize Inbreds for Carotenoids and Tocopherols. Crop Sci. 2003, 43, 818–823. [Google Scholar] [CrossRef]
- Menkir, A.; Rocheford, O.; Maziya-Dixon, B.; Tanumihardjo, S. Exploiting natural variation in exotic germplasm for increasing provitamin-A carotenoids in tropical maize. Euphytica 2015, 205, 203–217. [Google Scholar] [CrossRef]
| SOV | DF | GY | ASI | DA | EPP | RL | Gs | SEN | CC | PC | SOV | DF | BCC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rep | 1 | 0.33 *** | 0.02 * | 1.62 | 0.24 | 5.28 | 3370.51 | 28.13 | 11.05 | 570.12 | Gen | 45 | 2.758 *** |
| Rep.Bloc | 8 | 0.37 *** | 9.20 ns | 156.74 *** | 0.19 | 346.60 *** | 10,845.54 *** | 239.00 *** | 35.05 ** | 2076.57 | Error | 92 | 0.006 |
| Gen | 49 | 1.70 *** | 124.35 *** | 487.55 *** | 2.23 *** | 1737.56 *** | 17,231.90 *** | 1993.14 *** | 131.57 *** | 6212.13 *** | |||
| Env | 3 | 24.22 *** | 10.71 *** | 54.82 *** | 5.04 *** | 516.00 *** | 13,750.30 * | 1472.46 *** | 495.93 *** | 889.32 | |||
| WR | 1 | 142.85 *** | 8685.62 *** | 6339.38 *** | 63.85 *** | 287,471.53 *** | 20,372,674.06 *** | 297,606.12 *** | 88,914.06 *** | 2,756,772.93 *** | |||
| Gen.Env | 147 | 0.53 *** | 23.21 *** | 117.80 *** | 0.48 *** | 357.14 *** | 10,469.86 *** | 231.03 *** | 31.12 *** | 1517.61 *** | |||
| Gen.WR | 49 | 0.28 *** | 102.51 *** | 281.83 *** | 0.92 *** | 1715.20 *** | 21,625.12 *** | 1847.35 *** | 94.69 *** | 6224.34 *** | |||
| Env.WR | 3 | 13.07 *** | 97.87 *** | 89.21 | 0.47 | 670.90 *** | 11,839.37 *** | 1964.46 *** | 284.66 *** | 106.78 | |||
| Gen.Env.WR | 147 | 0.22 *** | 18.13 *** | 119.03 *** | 0.43 *** | 341.42 *** | 10,995.21 *** | 215.09 *** | 33.01 *** | 1506.32 *** | |||
| Error | 391 | 0.01 | 1.99 | 9.36 | 0.20 | 10.23 | 1789.44 | 29.28 | 6.92 | 468.59 |
| GY | ASI | DA | EPP | RL | Gs | SG | CC | PC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | STI | BCC | S | W | S | W | S | W | S | W | S | W | S | W | S | W | S | W | S | W |
| Top 10 | ||||||||||||||||||||
| 24 | 0.77 | 2.73 | 1.00 | 2.27 | 6.88 | 0.75 | 77.63 | 79.00 | 1.88 | 2.75 | 27.50 | 2.00 | 26.03 | 420.63 | 31.25 | 11.25 | 19.18 | 44.45 | 148.93 | 33.20 |
| 1 | 0.75 | 4.22 | 1.00 | 2.23 | 12.75 | 2.38 | 74.88 | 71.25 | 1.63 | 2.50 | 27.50 | 1.63 | 26.60 | 330.11 | 37.50 | 10.00 | 22.21 | 41.93 | 175.82 | 24.88 |
| 8 | 0.70 | 2.26 | 1.00 | 2.08 | 4.75 | 2.13 | 73.63 | 78.38 | 2.00 | 2.13 | 12.50 | 1.88 | 35.53 | 353.66 | 33.75 | 13.75 | 20.99 | 37.17 | 193.53 | 25.57 |
| 17 | 0.69 | 1.59 | 0.99 | 2.07 | 4.00 | 2.25 | 80.13 | 74.50 | 1.88 | 2.25 | 26.25 | 3.25 | 40.64 | 413.58 | 26.25 | 10.00 | 19.23 | 44.53 | 178.11 | 40.96 |
| 42 | 0.68 | 2.64 | 0.95 | 2.12 | 5.25 | 0.25 | 53.88 | 71.75 | 2.50 | 2.63 | 13.75 | 3.50 | 24.12 | 406.85 | 27.50 | 13.75 | 24.33 | 39.06 | 165.51 | 34.52 |
| 38 | 0.64 | 2.76 | 0.91 | 2.08 | 0.50 | 1.38 | 63.50 | 71.38 | 2.38 | 2.75 | 30.00 | 2.50 | 20.14 | 347.01 | 47.50 | 12.50 | 16.73 | 43.74 | 140.98 | 28.30 |
| 44 | 0.60 | 2.53 | 0.94 | 1.88 | 5.50 | 1.38 | 58.00 | 64.88 | 2.13 | 2.00 | 16.25 | 3.00 | 37.85 | 361.02 | 30.00 | 10.00 | 25.19 | 35.24 | 213.15 | 25.96 |
| 7 | 0.58 | 2.61 | 1.00 | 1.73 | 4.63 | 2.25 | 79.25 | 80.75 | 2.00 | 2.38 | 18.75 | 3.88 | 34.79 | 376.43 | 25.00 | 10.00 | 19.79 | 40.66 | 205.98 | 35.33 |
| 39 | 0.58 | 1.69 | 0.91 | 1.90 | 6.88 | 1.75 | 62.00 | 64.63 | 1.75 | 2.25 | 21.25 | 4.00 | 17.52 | 292.60 | 43.75 | 10.00 | 21.73 | 39.95 | 175.97 | 23.09 |
| 25 | 0.58 | 1.67 | 0.85 | 2.01 | 4.75 | 2.63 | 79.50 | 75.13 | 1.75 | 2.13 | 22.50 | 2.88 | 44.85 | 273.27 | 27.50 | 11.25 | 23.01 | 38.75 | 184.89 | 28.27 |
| Bottom 5 | ||||||||||||||||||||
| 21 | 0.34 | 1.97 | 0.75 | 1.32 | 11.38 | 1.13 | 78.63 | 81.25 | 1.25 | 2.13 | 78.75 | 4.00 | 66.66 | 384.13 | 71.25 | 12.50 | 16.32 | 41.94 | 103.21 | 31.60 |
| 32 | 0.31 | 2.92 | 0.81 | 1.15 | 12.13 | 2.88 | 77.50 | 74.13 | 1.00 | 2.50 | 66.25 | 3.88 | 92.69 | 376.40 | 85.00 | 12.50 | 12.19 | 36.62 | 89.56 | 35.81 |
| 19 | 0.31 | 1.81 | 0.75 | 1.21 | 12.38 | 3.50 | 74.88 | 78.00 | 1.13 | 2.13 | 58.75 | 2.50 | 73.76 | 349.29 | 81.25 | 11.25 | 15.14 | 38.61 | 100.77 | 27.59 |
| 12 | 0.27 | 2.88 | 0.70 | 1.16 | 10.63 | 1.63 | 75.63 | 77.88 | 1.38 | 1.63 | 58.75 | 2.13 | 66.85 | 314.55 | 66.25 | 13.75 | 16.04 | 38.04 | 139.47 | 28.92 |
| 20 | 0.23 | 3.39 | 0.69 | 0.97 | -9.88 | 0.88 | 80.88 | 72.00 | 1.13 | 1.75 | 87.50 | 3.50 | 68.86 | 338.51 | 88.75 | 12.50 | 10.07 | 34.20 | 95.98 | 28.24 |
| Mean | 2.05 | 0.88 | 1.72 | 8.56 | 1.97 | 69.40 | 75.03 | 1.68 | 2.24 | 40.95 | 3.04 | 49.78 | 368.94 | 50.18 | 11.60 | 18.80 | 39.88 | 149.23 | 31.83 | |
| CV | 3.65 | 7.87 | 5.33 | 17.96 | 63.75 | 1.93 | 5.51 | 20.69 | 23.40 | 10.85 | 27.36 | 1.38 | 16.11 | 13.26 | 26.78 | 4.28 | 9.09 | 20.24 | 13.45 | |
| LSD | 0.12 | 0.07 | 0.09 | 1.52 | 1.24 | 1.32 | 4.08 | 0.34 | 0.52 | 4.38 | 0.82 | 0.68 | 58.60 | 6.56 | 3.06 | 0.79 | 3.58 | 29.79 | 4.22 | |
| Variance Components | Traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GY | ASI | DA | EPP | RL | Gs | SEN | CC | PC | |
| Non-stressed conditions (W) | |||||||||
| Gen () | 0.098 | 0.189 | 73.122 | 0.189 | 0.074 | 506.778 | 0.656 | 18.235 | 7.753 |
| Env () | 0.238 | 0.025 | 0.000 | 0.025 | 0.214 | 0.385 | 0.323 | 1.471 | 0.000 |
| Gen.Env () | 0.232 | 1.052 | 51.808 | 0.138 | 0.910 | 3.887 | 3.094 | 16.008 | 78.695 |
| Error () | 0.012 | 0.683 | 2.580 | 0.123 | 1.618 | 0.525 | 9.500 | 0.714 | 16.873 |
| Phenotypic () | 0.157 | 0.537 | 86.396 | 0.239 | 0.504 | 507.815 | 2.617 | 22.326 | 29.536 |
| Heritability (H) | 0.621 | 0.352 | 0.846 | 0.792 | 0.147 | 0.998 | 0.251 | 0.817 | 0.263 |
| Stressed conditions (S) | |||||||||
| Gen () | 0.091 | 23.487 | 12.852 | 0.055 | 345.544 | 405.282 | 424.332 | 2.265 | 1200.237 |
| Env | 0.021 | 0.463 | 2.244 | 0.127 | 4.748 | 0.036 | 29.510 | 5.685 | 0.000 |
| Gen.Env | 0.417 | 17.578 | 69.455 | 0.143 | 343.666 | 7.164 | 194.567 | 9.653 | 977.106 |
| Error | 0.273 | 2.328 | 30.552 | 0.005 | 19.500 | 26.971 | 48.751 | 12.963 | 917.053 |
| Phenotypic () | 0.193 | 28.173 | 34.034 | 0.091 | 433.898 | 410.444 | 479.067 | 6.298 | 1559.145 |
| Heritability (H) | 0.398 | 0.834 | 0.378 | 0.602 | 0.796 | 0.987 | 0.886 | 0.360 | 0.770 |
| Non-Stressed Condition (W) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water Stressed (S) | GY | ASI | DA | EPP | RL | Gs | SEN | CC | PC | |
| GY | 1 | −0.593 *** | 0.462 *** | 0.502 *** | 0.187 *** | 0.496 *** | −0.205 ** | 0.612 *** | 0.109 * | |
| ASI | −0.694 *** | 1 | 0.446 *** | −0.520 *** | −0.013 | −0.307 | 0.166 | −0.004 | −0.067 | |
| DA | −0.444 ** | 0.510 ** | 1 | 0.363 *** | −0.308 | 0.046 | 0.106 | 0.267 | 0.068 | |
| EPP | 0.774 *** | −0.711 *** | −0.563 *** | 1 | 0.096 *** | 0.626 *** | −0.338 *** | 0.324 *** | 0.088 | |
| RL | 0.446 * | 0.458 | 0.207 | 0.513 ** | 1 | 0.152 | 0.128 | −0.061 | 0.262 | |
| Gs | −0.566 * | 0.333 * | 0.232 | −0.573 *** | 0.538*** | 1 | −0.120 *** | 0.246 * | 0.492 | |
| SEN | −0.423 *** | 0.365 | 0.041 | −0.486 ** | 0.334 ** | 0.584 ** | 1 | −0.250 ** | 0.038 * | |
| CC | 0.406 *** | −0.209 | −0.24 | 0.455 *** | −0.466 * | −0.390 * | −0.396 *** | 1 | −0.188 * | |
| PC | 0.317 *** | 0.374 | −0.076 | 0.415 *** | 0.472 *** | −0.235 | 0.489 *** | −0.356 * | 1 | |
| Secondary Traits | Genetic Correlation (rg) with GY | Relative Efficiency of Indirect Selection | ||
|---|---|---|---|---|
| Stressed | Non-Stressed | Stressed | Non-Stressed | |
| Anthesis silking interval | 0.352 * | 0.19 ns | 0.510 | 0.143 |
| Days to anthesis | 0.146 *** | 0.163 ns | 0.142 | 0.190 |
| Ears per plant | 0.909 *** | 0.912 *** | 1.118 | 1.030 |
| Leaf Rolling | 0.934 *** | 0.334 ns | 1.321 | 0.163 |
| Stomatal conductance | 0.715 *** | 0.974 *** | 1.126 | 1.235 |
| Leaf senescence | 0.918 *** | 0.842 *** | 1.370 | 0.535 |
| Chlorophyll content | 0.738 *** | 0.996 *** | 0.702 | 1.142 |
| Proline content | 0.829 *** | 0.018 ns | 1.153 | 0.012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondwakwenda, A.; Sibiya, J.; Zengeni, R.; Musvosvi, C.; Tesfay, S. Screening of Provitamin-A Maize Inbred Lines for Drought Tolerance: Beta-Carotene Content and Secondary Traits. Agronomy 2019, 9, 692. https://doi.org/10.3390/agronomy9110692
Kondwakwenda A, Sibiya J, Zengeni R, Musvosvi C, Tesfay S. Screening of Provitamin-A Maize Inbred Lines for Drought Tolerance: Beta-Carotene Content and Secondary Traits. Agronomy. 2019; 9(11):692. https://doi.org/10.3390/agronomy9110692
Chicago/Turabian StyleKondwakwenda, Aleck, Julia Sibiya, Rebecca Zengeni, Cousin Musvosvi, and Samson Tesfay. 2019. "Screening of Provitamin-A Maize Inbred Lines for Drought Tolerance: Beta-Carotene Content and Secondary Traits" Agronomy 9, no. 11: 692. https://doi.org/10.3390/agronomy9110692





