Unraveling Molecular and Genetic Studies of Wheat (Triticum aestivum L.) Resistance against Factors Causing Pre-Harvest Sprouting
Abstract
:1. Introduction
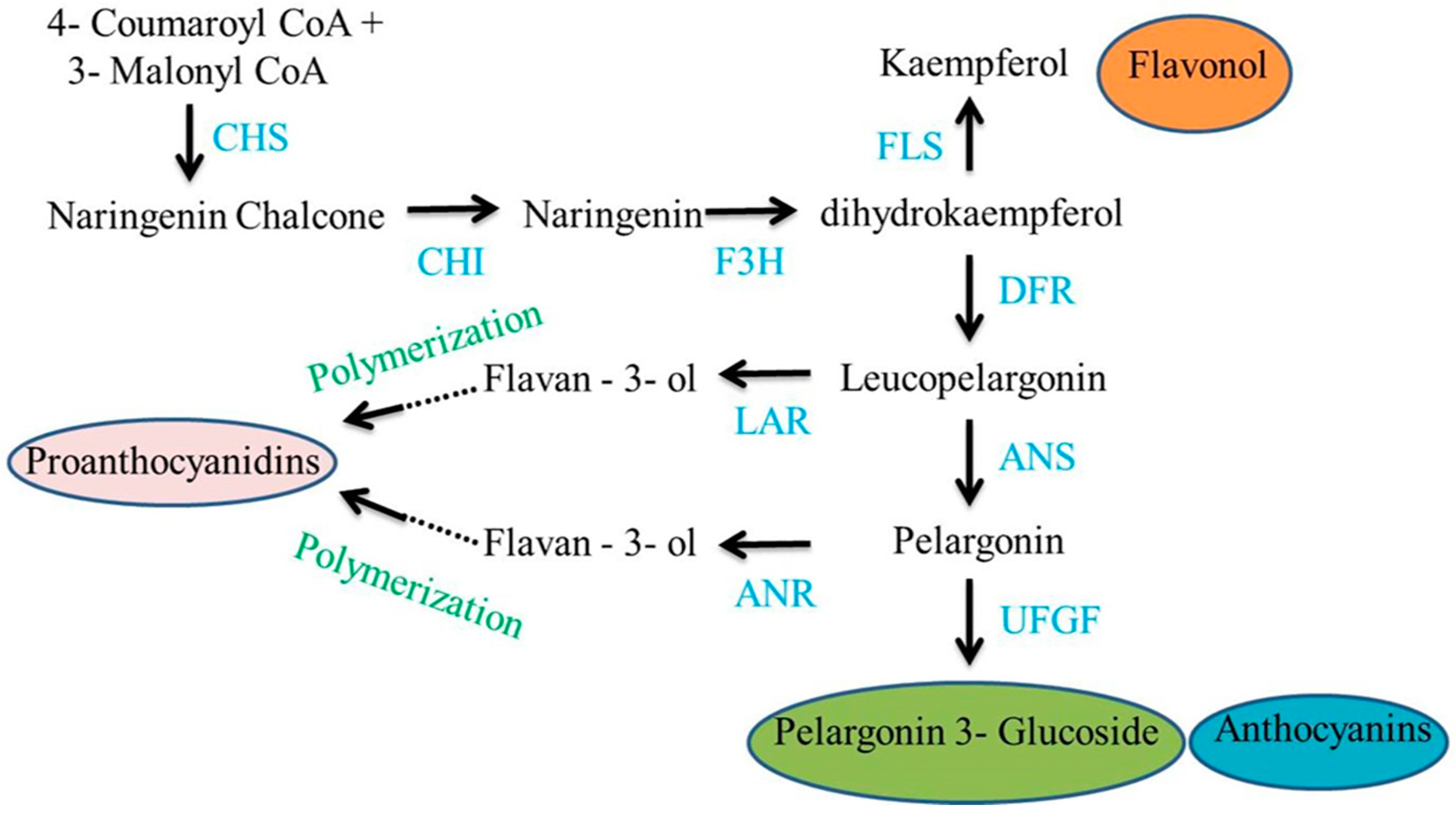
1.1. Grain Color
1.2. Seed Dormancy
1.3. α-Amylase Activity
1.4. Plant Growth Hormones
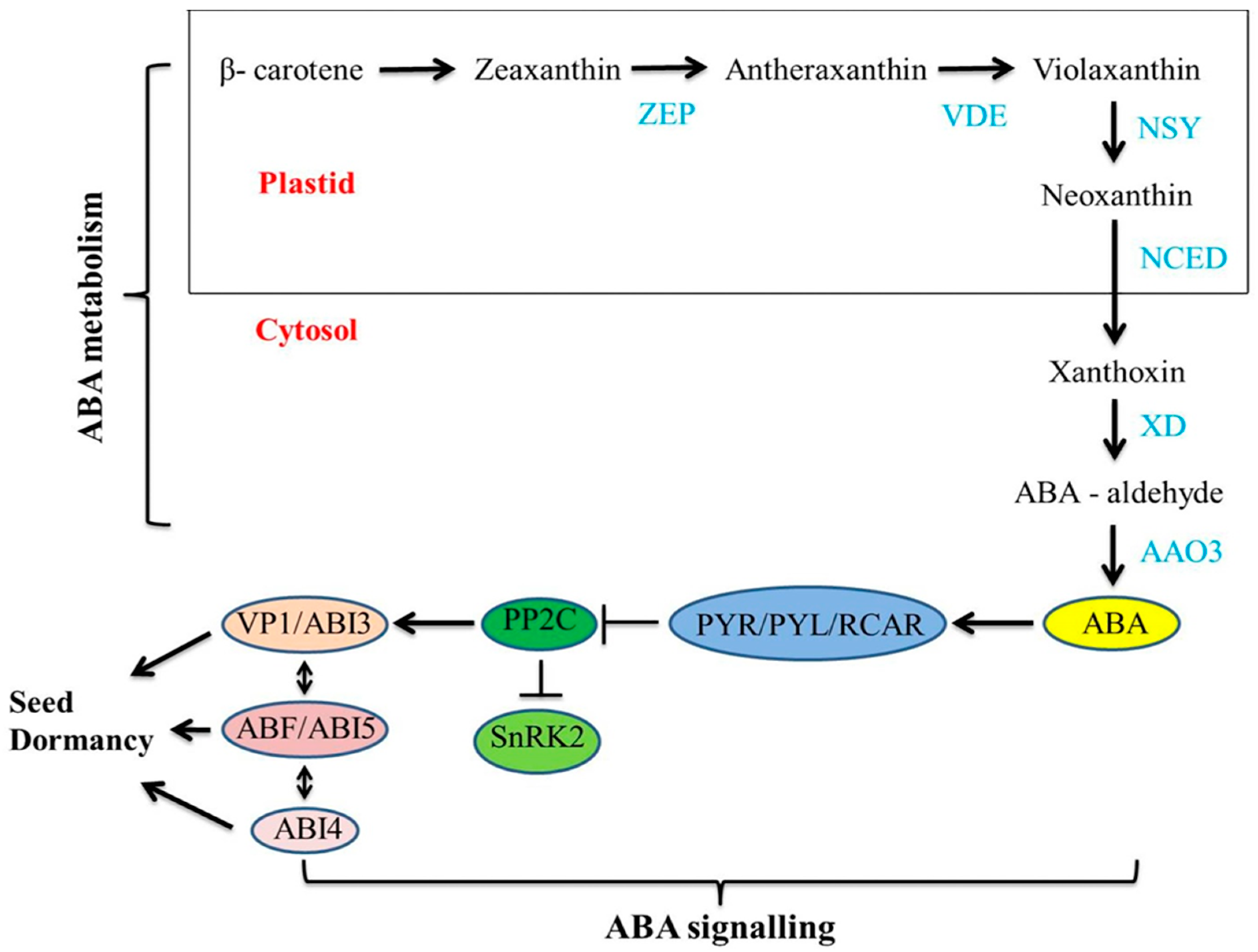
1.4.1. Abscisic Acid
1.4.2. Gibberellin
1.4.3. Other Plant Hormones
1.5. Environmental Factors Affecting PHS Resistance
1.6. QTL/genes Identified for PHS Resistance
2. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Groos, C.; Gay, G.; Perretant, M.-R.; Gervais, L.; Bernard, M.; Dedryver, F.; Charmet, G. Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor. Appl. Genet. 2002, 104, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kulwal, P.; Ishikawa, G.; Benscher, D.; Feng, Z.; Yu, L.X.; Jadhav, A.; Mehetre, S.; Sorrells, M.E. Association mapping for pre-harvest sprouting resistance in white winter wheat. Theor. Appl. Genet. 2012, 125, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.; Mrva, K.; Cheong, J.; Williams, K.; Watson, B.; Storlie, E.; Sutherland, M.; Zou, Y. A QTL located on chromosome 4A associated with dormancy in white- and red-grained wheats of diverse origin. Theor. Appl. Genet. 2005, 111, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, M.; Sherman, J. Facts: Pre—Harvest Sprouting. MAS Wheat, 2007. Available online: https://maswheat.ucdavis.edu/ (accessed on 2 January 2019).
- Andreoli, C.; Bassoi, M.C.; Brunetta, D. Genetic control of seed dormancy and pre-harvest sprouting in wheat. Sci. Agric. 2006, 63, 564–566. [Google Scholar] [CrossRef] [Green Version]
- Simsek, S.; Ohm, J.B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-Harvest sprouting on physicochemical properties of starch in wheat. Foods 2014, 3, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M.; Halmer, P. The encyclopedia of seeds science. In Technology and Uses; CABI Publishing: Oxfordshire, UK, 2006; p. 528. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-H.; Zhang, X.-Y.; Yan, C.-S.; Lin, H. Germplasm improvement for preharvest sprouting resistance in Chinese white-grained wheat: An overview of the current strategy. Euphytica 2002, 126, 35–38. [Google Scholar] [CrossRef]
- Kruger, J.E. Biochemistry of preharvest sprouting in cereals. In Preharvest Field Sprouting in Cereals; Derera, N.F., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1989; pp. 61–84. [Google Scholar]
- Wahl, T.I.; O’Rourke, A.D. The economics of sprout damage in wheat. In Preharvest Field Sprouting in Cereals; Walker-Simmonds, M.K., Ried, J.L., Eds.; CRC Press: Boca Raton, FL, USA; American Association of Cereal Chemists: St. Paul, MN, USA, 1993; pp. 10–17. [Google Scholar]
- Lan, X.; Zheng, Y.; Ren, X.; Liu, D.; Wei, Y.; Yan, Z. Utilization of preharvest sprouting tolerance gene of synthetic wheat RSP. J. Plant Genet. Resour. 2005, 6, 204–209. [Google Scholar]
- Lin, R.; Horsley, R.D.; Schwarz, P.B. Associations between caryopsis dormancy, α-amylase activity, and pre-harvest sprouting in barley. J. Cereal Sci. 2008, 48, 446–456. [Google Scholar] [CrossRef]
- Munkvold, J.D.; Tanaka, J.; Benscher, D.; Sorrells, M.E. Mapping quantitative trait loci for preharvest sprouting resistance in white wheat. Theor. Appl. Genet. 2009, 119, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Yu, Y.X.; Cheng, J.; Tan, X.L.; Shen, W.P. Study on the Pre-harvest Sprouting Tolerance in Triticum aestivum ssp. Yunnanense King. J. Triticeae Crops 2011, 31, 747–752. [Google Scholar]
- Liu, S.; Sehgal, S.K.; Li, J.; Lin, M.; Trick, H.N.; Yu, J.; Gill, B.S.; Bai, G. Cloning and characterization of a critical regulator for preharvest sprouting in wheat. Genetics 2013, 195, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ayele, B.T. Functional genomics of seed dormancy in wheat: Advances and prospects. Front. Plant Sci. 2014, 5, 458. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Mrva, K. Wheat grain preharvest sprouting and late maturity alpha-amylase. Planta 2014, 240, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- King, R.W.; von Wettstein-Knowles, P. Epicuticular waxes and regulation of ear wetting and pre-harvest sprouting in barley and wheat. Euphytica 2000, 112, 157–166. [Google Scholar] [CrossRef]
- Gatford, K.T.; Eastwood, R.F.; Halloran, G.M. Germination inhibitors in bracts surrounding the grain of Triticum tauschii. Funct. Plant Biol. 2002, 29, 881–890. [Google Scholar] [CrossRef]
- Kato, K.; Nakamura, W.; Tabiki, T.; Miura, H.; Sawada, S. Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theor. Appl. Genet. 2001, 102, 980–985. [Google Scholar] [CrossRef]
- Flintham, J.; Adlam, R.; Bassoi, M.; Holdsworth, M.; Gale, M. Mapping genes for resistance to sprouting damage in wheat. Euphytica 2002, 126, 39–45. [Google Scholar] [CrossRef]
- Osa, M.; Kato, K.; Mori, M.; Shindo, C.; Torada, A.; Miura, H. Mapping QTLs for seed dormancy and the Vp1 homologue on chromosome 3A in wheat. Theor. Appl. Genet. 2003, 106, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Kulwal, P.L.; Kumar, N.; Gaur, A.; Khurana, P.; Khurana, J.P.; Tyagi, A.K.; Balyan, H.S.; Gupta, P.K. Mapping of a major QTL for pre-harvest sprouting tolerance on chromosome 3A in bread wheat. Theor. Appl. Genet. 2005, 111, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Uchino, N.; Chono, M.; Kato, K.; Miura, H. Mapping QTLs for grain dormancy on wheat chromosome 3A and the group 4 chromosomes, and their combined effect. Theor. Appl. Genet. 2005, 110, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottearachchi, N.S.; Uchino, N.; Kato, K.; Miura, H. Increased grain dormancy in white-grained wheat by introgression of preharvest sprouting tolerance QTLs. Euphytica 2006, 152, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Ogbonnaya, F.C.; Imtiaz, M.; Ye, G.; Hearnden, P.R.; Hernandez, E.; Eastwood, R.F.; van Ginkel, M.; Shorter, S.C.; Winchester, J.M. Genetic and QTL analyses of seed dormancy and preharvest sprouting resistance in the wheat germplasm CN10955. Theor. Appl. Genet. 2008, 116, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.B.; Lan, X.J.; Liu, D.C.; Wang, J.L.; Zheng, Y.L. Mapping QTLs for pre-harvest sprouting tolerance on chromosome 2D in a synthetic hexaploid wheat x common wheat cross. J. Appl. Genet. 2008, 49, 333–341. [Google Scholar] [PubMed]
- Fofana, B.; Humphreys, D.G.; Rasul, G.; Cloutier, S.; Brûlé-Babel, A.; Woods, S.; Lukow, O.M.; Somers, D.J. Mapping quantitative trait loci controlling pre-harvest sprouting resistance in a red × white seeded spring wheat cross. Euphytica 2009, 165, 509–521. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Singh, R.; Garg, T.; Chhuneja, P.; Balyan, H.S.; Gupta, P.K. QTL analysis for grain colour and pre-harvest sprouting in bread wheat. Plant Sci. 2009, 177, 114–122. [Google Scholar] [CrossRef]
- Mares, D.; Rathjen, J.; Mrva, K.; Cheong, J. Genetic and environmental control of dormancy in white-grained wheat (Triticum aestivum L.). Euphytica 2009, 168, 311–318. [Google Scholar] [CrossRef]
- Mohan, A.; Kulwal, P.; Singh, R.; Kumar, V.; Mir, R.R.; Kumar, J.; Prasad, M.; Balyan, H.S.; Gupta, P.K. Genome-wide QTL analysis for pre-harvest sprouting tolerance in bread wheat. Euphytica 2009, 168, 319–329. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Tian, B.; Liu, B.; Xie, Q.G.; Tian, J.C. Quantitative Trait Loci Analysis for Pre-harvest Sprouting Using Intact Spikes in Wheat (Triticum aestivum L.). Shandong Agric. Sci. 2010, 6, 19–23. [Google Scholar]
- Zhang, H.P.; Feng, J.M.; Chang, C. Investigation of main loci contributing to strong seed dormancy of Chinese wheat landrace. J. Agric. Biotechnol. 2011, 19, 270–277. [Google Scholar]
- Knox, R.E.; Clarke, F.R.; Clarke, J.M.; Fox, S.L.; DePauw, R.M.; Singh, A.K. Enhancing the identification of genetic loci and transgressive segregants for preharvest sprouting resistance in a durum wheat population. Euphytica 2012, 186, 193–206. [Google Scholar] [CrossRef]
- Kumar, S.; Knox, R.E.; Clarke, F.R.; Pozniak, C.J.; DePauw, R.M.; Cuthbert, R.D.; Fox, S. Maximizing the identification of QTL for pre-harvest sprouting resistance using seed dormancy measures in a white-grained hexaploid wheat population. Euphytica 2015, 205, 287–309. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tang, H.; Cheng, M.-P.; Dankwa, K.O.; Chen, Z.-X.; Li, Z.-Y.; Gao, S.; Liu, Y.-X.; Jiang, Q.-T.; Lan, X.-J.; et al. Genome-Wide Association Study for Pre-harvest Sprouting Resistance in a Large Germplasm Collection of Chinese Wheat Landraces. Front. Plant Sci. 2017, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.L.; Xia, L.Q.; Chen, X.M.; Xia, X.C.; Yu, Z.; He, Z.H.; Roder, M. Development and validation of a Viviparous-1 STS marker for pre-harvest sprouting tolerance in Chinese wheats. Theor. Appl. Genet. 2007, 115, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Feng, J.M.; Si, H.Q.; Yin, B.; Zhang, H.P.; Ma, C.X. Validating a novel allele of viviparous-1 (Vp-1Bf) associated with high seed dormancy of Chinese wheat landrace, Wanxianbaimaizi. Mol. Breed. 2010, 25, 517–525. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Abe, F.; Kawahigashi, H.; Nakazono, K.; Tagiri, A.; Matsumoto, T.; Utsugi, S.; Ogawa, T.; Handa, H.; Ishida, H.; et al. Wheat Homolog of MOTHER OF FT AND TFL1 Acts in the Regulation of Germination. Plant Cell 2011, 23, 3215–3229. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sehgal, S.K.; Lin, M.; Li, J.; Trick, H.N.; Gill, B.S.; Bai, G. Independent mis-splicing mutations in TaPHS1 causing loss of preharvest sprouting (PHS) resistance during wheat domestication. New Phytol. 2015, 208, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Gao, F.; Kanno, Y.; Jordan, M.C.; Kamiya, Y.; Seo, M.; Ayele, B.T. Regulation of Wheat Seed Dormancy by After-Ripening Is Mediated by Specific Transcriptional Switches That Induce Changes in Seed Hormone Metabolism and Signaling. PLoS ONE 2013, 8, e56570. [Google Scholar] [CrossRef] [PubMed]
- Barrero, J.M.; Cavanagh, C.; Verbyla, K.L.; Tibbits, J.F.G.; Verbyla, A.P.; Huang, B.E.; Rosewarne, G.M.; Stephen, S.; Wang, P.; Whan, A.; et al. Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genome Biol. 2015, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Torada, A.; Koike, M.; Ogawa, T.; Takenouchi, Y.; Tadamura, K.; Wu, J.; Matsumoto, T.; Kawaura, K.; Ogihara, Y. A Causal Gene for Seed Dormancy on Wheat Chromosome 4A Encodes a MAP Kinase Kinase. Curr. Biol. 2016, 26, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, X.; He, Z. The seed dormancy allele TaSdr-A1a associated with pre-harvest sprouting tolerance is mainly present in Chinese wheat landraces. Theor. Appl. Genet. 2017, 130, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Metzger, R.J.; Silbaugh, B.A. Location of genes for seed coat color in hexaploid wheat (Triticum aestivum L.). Crop Sci. 1970, 10, 495–496. [Google Scholar] [CrossRef]
- Wang, D.; Dowell, F.E.; Lacey, R.E. Predicting the Number of Dominant R Alleles in Single Wheat Kernels Using Visible and Near-Infrared Reflectance Spectra. Cereal Chem. 1999, 76, 6–8. [Google Scholar] [CrossRef]
- Himi, E.; Noda, K. Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 2005, 143, 239–242. [Google Scholar] [CrossRef]
- Chopra, S.; Athma, P.; Peterson, T. Alleles of the maize P gene with distinct tissue specificities encode Myb-homologous proteins with C-terminal replacements. Plant Cell 1996, 8, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Kohyama, N.; Chono, M.; Nakagawa, H.; Matsuo, Y.; Ono, H.; Matsunaka, H. Flavonoid compounds related to seed coat color of wheat. Biosci. Biotechnol. Biochem. 2017, 81, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Himi, E.; Mares, D.J.; Yanagisawa, A.; Noda, K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J. Exp. Bot. 2002, 53, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.L.; Kudrna, D.A.; Spaeth, S.C.; Jones, S.S. Dormancy in white-grain mutants of Chinese Spring wheat (Triticum aestivum L.). Seed Sci. Res. 2007, 10, 51–60. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, D.; Liu, S.; Zhang, G.; Yu, J.; Fritz, A.K.; Bai, G. Genome-wide association analysis on pre-harvest sprouting resistance and grain color in U.S. winter wheat. BMC Genom. 2016, 17, 794. [Google Scholar] [CrossRef] [PubMed]
- Chouard, P. Vernalization and its Relations to Dormancy. Annu. Rev. Plant Physiol. 1960, 11, 191–238. [Google Scholar] [CrossRef]
- Belderok, B. Seed dormancy problems in cereals. Field Crop Abstr. 1968, 21, 203–211. [Google Scholar]
- Marzougui, S.; Sugimoto, K.; Yamanouchi, U.; Shimono, M.; Hoshino, T.; Hori, K.; Kobayashi, M.; Ishiyama, K.; Yano, M. Mapping and characterization of seed dormancy QTLs using chromosome segment substitution lines in rice. Theor. Appl. Genet. 2012, 124, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Gubler, F.; Millar, A.A.; Jacobsen, J.V. Dormancy release, ABA and pre-harvest sprouting. Curr. Opin. Plant Biol. 2005, 8, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.W.; Jones, H.D.; Yang, Y.; Dreisigacker, S.; Li, S.M.; Chen, X.M.; Shewry, P.R.; Xia, L.Q. Haplotype analysis of Viviparous-1 gene in CIMMYT elite bread wheat germplasm. Euphytica 2012, 186, 25–43. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.L.; Liu, S.X.; Sun, Y.Q.; Meng, J.Y.; Xia, L.Q. Characterization of the rich haplotypes of Viviparous-1A in Chinese wheats and development of a novel sequence-tagged site marker for pre-harvest sprouting resistance. Mol. Breed. 2014, 33, 75–88. [Google Scholar] [CrossRef]
- Belderok, B. Physiological-biochemical aspects of dormancy in wheat. Cereal Res. Commun. 1976, 4, 133–137. [Google Scholar]
- Freed, R.D.; Everson, E.H.; Ringlund, K.; Gullord, M. Seed coat color in wheat and the relationship to seed dormancy at maturity. Cereal Res. Commun. 1976, 4, 147–149. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.-Q.; Luan, S.; Li, J.; He, Z.-H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Van Staden, J.; Bell, W.E. Seed coat structure and dormancy. Plant Growth Regul. 1992, 11, 201–209. [Google Scholar] [CrossRef]
- Gfeller, F.; Svejda, F. Inheritance of post-harvest seed dormancy and kernal colour in spring wheat lines. Can. J. Plant Sci. 1960, 40, 1–6. [Google Scholar] [CrossRef]
- Everson, E. Varietal variation for dormancy in mature wheat. Q. Bull. Mich. St. Univ. Agric. Exp. Stn. 1961, 43, 820–829. [Google Scholar]
- McEwan, J.M. The sprouting reaction of stocks with single genes for red grain colour derived from hilgendorf 61 wheat. Cereal Res. Commun. 1980, 8, 261–264. [Google Scholar]
- Jacobsen, J.V.; Pearce, D.W.; Poole, A.T.; Pharis, R.P.; Mander, L.N. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol. Plant. 2002, 115, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Zhu, X.; Wang, S.; Zhu, M.; Carver, B.F.; Yan, L. TaMFT-A1 Is Associated with Seed Germination Sensitive to Temperature in Winter Wheat. PLoS ONE 2013, 8, e73330. [Google Scholar] [CrossRef] [PubMed]
- Ashikawa, I.; Mori, M.; Nakamura, S.; Abe, F. A transgenic approach to controlling wheat seed dormancy level by using Triticeae DOG1-like genes. Transgen. Res. 2014, 23, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.; Kovach, K.; Elnacash, T.; He, H.; Bentsink, L.; Donohue, K. DOG1-imposed dormancy mediates germination responses to temperature cues. Environ. Exp. Bot. 2015, 112, 33–43. [Google Scholar] [CrossRef]
- Sugimoto, K.; Takeuchi, Y.; Ebana, K.; Miyao, A.; Hirochika, H.; Hara, N.; Ishiyama, K.; Kobayashi, M.; Ban, Y.; Hattori, T.; et al. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 2010, 107, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, X.; Xia, X.; He, Z. Cloning of seed dormancy genes (TaSdr) associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker. Theor. Appl. Genet. 2014, 127, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Shorinola, O.; Bird, N.; Simmonds, J.; Berry, S.; Henriksson, T.; Jack, P.; Werner, P.; Gerjets, T.; Scholefield, D.; Balcárková, B.; et al. The wheat Phs-A1 pre-harvest sprouting resistance locus delays the rate of seed dormancy loss and maps 0.3 cM distal to the PM-19 genes in UK germplasm. J. Exp. Bot. 2016, 67, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, F.C.; Imtiaz, M.; Depauw, R.M. Haplotype diversity of preharvest sprouting QTLs in wheat. Genome Biol. 2007, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Torada, A.; Koike, M.; Ikeguchi, S.; Tsutsui, I. Mapping of a major locus controlling seed dormancy using backcrossed progenies in wheat (Triticum aestivum L.). Genome 2008, 51, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Cabral, A.L.; Jordan, M.C.; McCartney, C.A.; You, F.M.; Humphreys, D.G.; MacLachlan, R.; Pozniak, C.J. Identification of candidate genes, regions and markers for pre-harvest sprouting resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Sydenham, S.L.; Barnard, A. Targeted Haplotype Comparisons between South African Wheat Cultivars Appear Predictive of Pre-harvest Sprouting Tolerance. Front. Plant Sci. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Shorinola, O.; Balcárková, B.; Hyles, J.; Tibbits, J.F.G.; Hayden, M.J.; Holušova, K.; Valárik, M.; Distelfeld, A.; Torada, A.; Barrero, J.M.; et al. Haplotype Analysis of the Pre-harvest Sprouting Resistance Locus Phs-A1 Reveals a Causal Role of TaMKK3-A in Global Germplasm. Front. Plant Sci. 2017, 8, 1555. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.R.; Hattori, T.; Carson, C.B.; Vasil, V.; Lazar, M.; Vasil, I.K. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 1991, 66, 895–905. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.-P.; Zhao, Q.-X.; Feng, J.-M.; Si, H.-Q.; Lu, J.; Ma, C.-X. Rich allelic variations of Viviparous-1A and their associations with seed dormancy/pre-harvest sprouting of common wheat. Euphytica 2011, 179, 343–353. [Google Scholar] [CrossRef]
- Hattori, T.; Vasil, V.; Rosenkrans, L.; Hannah, L.C.; McCarty, D.R.; Vasil, I.K. The Viviparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Genes Dev. 1992, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.C.; McKibbin, R.S.; Lenton, J.R.; Holdsworth, M.J.; Flintham, J.E.; Gale, M.D. Genetic map locations for orthologous Vp1 genes in wheat and rice. Theor. Appl. Genet. 1999, 98, 281–284. [Google Scholar] [CrossRef]
- Nakamura, S.; Toyama, T. Isolation of a VP1 homologue from wheat and analysis of its expression in embryos of dormant and non-dormant cultivars. J. Exp. Bot. 2001, 52, 875–876. [Google Scholar] [CrossRef] [PubMed]
- McKibbin, R.S.; Wilkinson, M.D.; Bailey, P.C.; Flintham, J.E.; Andrew, L.M.; Lazzeri, P.A.; Gale, M.D.; Lenton, J.R.; Holdsworth, M.J. Transcripts of Vp-1 homeologues are misspliced in modern wheat and ancestral species. Proc. Natl. Acad. Sci. USA 2002, 99, 10203–10208. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.Q.; Ganal, M.W.; Shewry, P.R.; He, Z.H.; Yang, Y.; Röder, M.S. Exploiting the diversity of Viviparous-1 gene associated with pre-harvest sprouting tolerance in European wheat varieties. Euphytica 2008, 159, 411–417. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; He, Z.; Röder, M.; Xia, L. Distribution of Vp-1 alleles in Chinese white-grained landraces, historical and current wheat cultivars. Cereal Res. Commun. 2009, 37, 169–177. [Google Scholar] [CrossRef]
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination. Am. Soc. Plant Biol. 2008, 6, e0119. [Google Scholar] [CrossRef] [PubMed]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, R. Abscisic acid synthesis and response. Arab Book 2013, 11, e0166. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed dormancy and germination-emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Bentsink, L.; Jowett, J.; Hanhart, C.J.; Koornneef, M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc. Natl Acad. Sci. USA 2006, 103, 17042–17047. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Bartsch, M.; Xiang, Y.; Miatton, E.; Pellengahr, S.; Yano, R.; Seo, M.; Soppe, W.J. The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 2012, 24, 2826–2838. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Tsuchiya, W.; Moresco, J.J.; Hayashi, Y.; Satoh, K.; Kaiwa, N.; Irisa, T.; Kinoshita, T.; Schroeder, J.I.; Yates, J.R.; et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme. Nat. Commun. 2018, 9, 2132. [Google Scholar] [CrossRef] [PubMed]
- International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Mascher, M.; Buluç, A.; Barry, K.; Georganas, E.; Session, A.; Strnadova, V.; Jenkins, J.; Sehgal, S.; Oliker, L.; et al. A whole-genome shotgun approach for assembling and anchoring the hexaploid bread wheat genome. Genome Biol. 2015, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, B.J.; Venturini, L.; Schudoma, C.; Accinelli, G.G.; Kaithakottil, G.; Wright, J.; Borrill, P.; Kettleborough, G.; Heavens, D.; Chapman, H.; et al. An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Res. 2017, 27, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, R.H.; Borrill, P.; Lang, D.; Harrington, S.A.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; van Ex, F.; Pasha, A.; et al. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.; Simoinen, T.; Suortti, T.; Salmenkallio-Marttila, M.; Lassila, K.; Wilhelmson, A. Structural and Enzymic Changes in Germinated Barley and Rye. J. Inst. Brew. 2001, 107, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Masojć, P.; Milczarski, P. Relationship between QTLs for preharvest sprouting and alpha-amylase activity in rye grain. Mol. Breed. 2009, 23, 75–84. [Google Scholar] [CrossRef]
- Barrero, J.M.; Mrva, K.; Talbot, M.J.; White, R.G.; Taylor, J.; Gubler, F.; Mares, D.J. Genetic, hormonal and physiological analysis of late maturity alpha-amylase (LMA) in wheat. Plant Physiol. 2013, 161, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Woodger, F.; Jacobsen, J.V.; Gubler, F. Gibberellin action in germinated cereal grains. In Plant Hormones: Biosynthesis, Signal Transduction, Action; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 221–240. [Google Scholar]
- Wu, Y.; Hu, H.; Wang, G.; Zhang, Y.; Ji, J. Relationship between alpha amylase activity and resistance of pre-harvest sprouting in spring wheat. J. Jilin Agric. Univ. 2002, 24, 22–25. [Google Scholar]
- Wang, X.G.; Ren, J.P.; Yin, J. The mechanism on wheat pre-harvest resistant sprouting. China Agric. Sci. 2008, 24, 243–250. [Google Scholar]
- Paterson, A.H.; Sorrells, M.E.; Obendorf, R.L. Methods of evalution for pre-harvest sprouting resistance in wheat breeding programs. Can. J. Plant Sci. 1989, 69, 681–689. [Google Scholar] [CrossRef]
- Humphreys, D.G.; Noll, J. Methods for characterization of preharvest sprouting resistance in a wheat breeding program. Euphytica 2002, 126, 61–65. [Google Scholar] [CrossRef]
- Gale, M.D.; Ainsworth, C.C. The relationship between alpha-amylase species found in developing and germinating wheat grain. Biochem. Genet. 1984, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C. Comparisons of Copy Number, Genomic Structure, and Conserved Motifs for α-Amylase Genes from Barley, Rice, and Wheat. Front. Plant Sci. 2017, 8, 1727. [Google Scholar] [CrossRef] [PubMed]
- Marchylo, B.A.; Kruger, J.E.; Macgregor, A.W. Production of multiple forms of α-amylase in germinated, incubated, whole, de-embryonated wheat kernels. Cereal Chem. 1983, 61, 305–310. [Google Scholar]
- Mundy, J.; Hejgaard, J.; Svendsen, I. Characterization of a bifunctional wheat inhibitor of endogenous α-amylase and subtilisin. FEBS Lett. 1984, 167, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Henry, R.J.; Battershell, V.G.; Brennan, P.S.; Oono, K. Control of wheat α-amylase using inhibitors from cereals. J. Sci. Food Agric. 1992, 58, 281–284. [Google Scholar] [CrossRef]
- Macgregor, A.W.; Marchylo, B.A.; Kruger, J.E. Multiple α-amylase components in germinated cereal grains determined by isoelectric focusing and chromatofocusing. Cereal Chem. 1988, 65, 326–333. [Google Scholar]
- Masojc, P.; Zawistowski, J.; Howes, N.K.; Aung, T.; Gale, M.D. Polymorphism and chromosomal location of endogenous alpha-amylase inhibitor genes in common wheat. Theor. Appl. Genet. 1993, 85, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.P.; Chen, X.; Xiao, S.H.; Zhang, W. Extraction and identification of barley α-amylase/subtilisin inhibitor. J. Triticeae Crops 2005, 25, 40–43. [Google Scholar]
- Chitnis, V.R.; Gao, F.; Yao, Z.; Jordan, M.C.; Park, S.; Ayele, B.T. After-ripening induced transcriptional changes of hormonal genes in wheat seeds: The cases of brassinosteroids, ethylene, cytokinin and salicylic acid. PLoS ONE 2014, 9, e87543. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Ann. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matilla, A.J.; Matilla-Vázquez, M.A. Involvement of ethylene in seed physiology. Plant Sci. 2008, 175, 87–97. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Lzydorczyk, C.; Nguyen, T.N.; Jo, S.; Son, S.; Tuan, P.A.; Ayele, B.T. Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signaling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum L.). Plant Cell Environ. 2017, 41, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.V.; Mendiondo, G.M.; Maskin, L.; Gudesblat, G.E.; Iusem, N.D.; Benech-Arnold, R.L. Expression of ABA signalling genes and ABI5 protein levels in imbibed Sorghum bicolor caryopses with contrasting dormancy and at different developmental stages. Ann. Bot. 2009, 104, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Ried, J.L.; Walker-Simmons, M.K. Synthesis of abscisic Acid-responsive, heat-stable proteins in embryonic axes of dormant wheat grain. Plant Physiol. 1990, 93, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 55–67. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.J.; Krochko, J.E. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef]
- Saito, S.; Hirai, N.; Matsumoto, C.; Ohigashi, H.; Ohta, D.; Sakata, K.; Mizutani, M. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 2004, 134, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Matsuura, T.; Kawakami, N.; Noda, K. Accumulation and leakage of abscisic acid during embryo development and seed dormancy in wheat. J. Plant Growth Regul. 2000, 30, 253–260. [Google Scholar] [CrossRef]
- Chono, M.; Honda, I.; Shinoda, S.; Kushiro, T.; Kamiya, Y.; Nambara, E.; Kawakami, N.; Kaneko, S.; Watanabe, Y. Field studies on the regulation of abscisic acid content and germinability during grain development of barley: Molecular and chemical analysis of pre-harvest sprouting. J. Exp. Bot. 2006, 57, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Garello, G.; Le Page-Degivry, M.T. Evidence for the role of abscisic acid in the genetic and environmental control of dormancy in wheat (Triticum aestivum L.). Seed Sci. Res. 1999, 9, 219–226. [Google Scholar] [CrossRef]
- Son, S.; Chitnis, V.R.; Liu, A.; Gao, F.; Nguyen, T.N.; Ayele, B.T. Abscisic acid metabolic genes of wheat (Triticum aestivum L.): Identification and insights into their functionality in seed dormancy and dehydration tolerance. Planta 2016, 244, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Chono, M.; Matsunak, H.; Seki, M.; Fujita, M.; Kiribuchi-Otobe, C.; Oda, S.; Kojima, H.; Kobayashi, D.; Kawakami, N. Isolation of a wheat (Triticum aestivum L.) mutant in ABA 8′-hydroxylase gene: Effect of reduced ABA catabolism on germination inhibition under field condition. Breed. Sci. 2013, 63, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, M.; Kurup, S.; Mkibbin, R. Molecular and genetic mechanisms regulating the transition from embryo development to germination. Trends Plant Sci. 1999, 4, 275–280. [Google Scholar] [CrossRef]
- Ksenia, V.K.; Vasquez-Gross, H.A.; Howell, T.; Bailey, P.; Paraiso, F.; Clissold, L.; Simmonds, J.; Ramirez-Gonzalez, R.H.; Wang, X.; Borrill, P.; et al. Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. USA 2017, 114, 913–921. [Google Scholar] [CrossRef]
- Marin, E.; Nussaume, L.; Quesada, A.; Gonneau, M.; Sotta, B.; Hugueney, P.; Frey, A.; Marion-Poll, A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996, 15, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- North, H.M.; De Almeida, A.; Boutin, J.P.; Frey, A.; To, A.; Botran, L.; Sotta, B.; Marion-Poll, A. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007, 50, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.; McCarty, D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–12240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbidge, A.; Grieve, T.M.; Jackson, A.; Thompson, A.; McCarty, D.R.; Taylor, I.B. Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. Plant J. 1999, 17, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Leon-Kloosterziel, K.M.; Koornneef, M.; Zeevaart, J.A. Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol. 1997, 114, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Scazzocchio, C.; Fluhr, R. The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J. 2002, 31, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Hwang, H.; Hong, J.W.; Lee, Y.N.; Ahn, I.P.; Yoon, I.S.; Yoo, S.D.; Lee, S.; Lee, S.C.; Kim, B.G. A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth. J. Exp. Bot. 2012, 63, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Matsuura, T.; Maekawa, M.; Taketa, S. Chromosomes responsible for sensitivity of embryo to abscisic acid and dormancy in wheat. Euphytica 2002, 123, 203–209. [Google Scholar] [CrossRef]
- Schramm, E.C.; Nelson, S.K.; Kidwell, K.K.; Steber, C.M. Increased ABA sensitivity results in higher seed dormancy in soft white spring wheat cultivar ‘Zak’. Theor. Appl. Genet. 2013, 126, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F.; Moffatt, J.M.; Sears, R.G.; Paulsen, G.M. Seed dormancy and responses of caryopses, embryos, and calli to abscisic Acid in wheat. Plant Physiol. 1989, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Corbineau, F.B.A.; Come, D. Changes in sensitivity to abscisic acid of the developing and maturing embryo of two wheat cultivars with different sprouting susceptibility. Isr. J. Plant Sci. 2000, 48, 189–197. [Google Scholar] [CrossRef]
- De Laethauwer, S.; Reheul, D.; De Riek, J.; Haesaert, G. Vp1 expression profiles during kernel development in six genotypes of wheat, triticale and rye. Euphytica 2012, 188, 61–70. [Google Scholar] [CrossRef]
- Fan, J.; Niu, X.; Wang, Y.; Ren, G.; Zhuo, T.; Yang, Y.; Lu, B.R.; Liu, Y. Short, direct repeats (SDRs)-mediated post-transcriptional processing of a transcription factor gene OsVP1 in rice (Oryza sativa). J. Exp. Bot. 2007, 58, 3811–3817. [Google Scholar] [CrossRef] [PubMed]
- Giraudat, J.; Hauge, B.M.; Valon, C.; Smalle, J.; Parcy, F.; Goodman, H.M. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 1992, 4, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14, s15–s45. [Google Scholar] [CrossRef] [PubMed]
- McCrate, A.J.; Nielsen, M.T.; Paulsen, G.M.; Heyne, E.G. Relationship between sprouting in wheat and embryo response to endogenous inhibition. Euphytica 1982, 31, 193–200. [Google Scholar] [CrossRef]
- Curaba, J.; Moritz, T.; Blervaque, R.; Parcy, F.; Raz, V.; Herzog, M.; Vachon, G. AtGA3ox2, a Key Gene Responsible for Bioactive Gibberellin Biosynthesis, Is Regulated during Embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol. 2004, 136, 3660–3669. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.S.; Paz, J.D.; Pathmanathan, A.; Chiu, R.S.; Tsai, A.Y.; Gazzarrini, S. The C-terminal domain of FUSCA3 negatively regulates mRNA and protein levels, and mediates sensitivity to the hormones abscisic acid and gibberellic acid in Arabidopsis. Plant J. 2010, 64, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Ann. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Huttly, A.K.; Prosser, I.M.; Li, Y.D.; Vaughan, S.P.; Gallova, B.; Patil, A.; Coghill, J.A.; Dubcovsky, J.; Hedden, P.; et al. Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biol. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Kashiwakura, Y.-I.; Kobayashi, D.; Jikumaru, Y.; Takebayashi, Y.; Nambara, E.; Seo, M.; Kamiya, Y.; Kushiro, T.; Kawakami, N. Highly Sprouting-Tolerant Wheat Grain Exhibits Extreme Dormancy and Cold Imbibition-Resistant Accumulation of Abscisic Acid. Plant Cell Physiol. 2016, 57, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.V.; Mendiondo, G.M.; Cantoro, R.; Auge, G.A.; Luna, V.; Masciarelli, O.; Benech-Arnold, R.L. Expression of seed dormancy in grain sorghum lines with contrasting pre-harvest sprouting behavior involves differential regulation of gibberellin metabolism genes. Plant Cell Physiol. 2012, 53, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Feng, J.; Zhang, L.; Zhang, J.; Mispan, M.S.; Cao, Z.; Beighley, D.H.; Yang, J.; Gu, X. Map-based cloning of Seed Dormancy1-2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiol. 2015, 169, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Magwa, R.A.; Zhao, H.; Xing, Y. Genome-wide association mapping revealed a diverse genetic basis of seed dormancy across subpopulations in rice (Oryza sativa L.). BMC Genet. 2016, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yang, W.; Li, S.; Liu, D.; Guo, X.; Sun, J.; Zhang, A. Molecular characterization of three GIBBERELLIN-INSENSITIVE DWARF1 homologous genes in hexaploid wheat. J. Plant Physiol. 2013, 170, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.P. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr. Biol. 2011, 21, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Itoh, H.; Gomi, K.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Jeong, D.H.; An, G.; Kitano, H.; Ashikari, M.; et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 2003, 299, 1896–1898. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, K.M.; Thomas, S.G.; Soule, J.D.; Strader, L.C.; Zale, J.M.; Sun, T.P.; Steber, C.M. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 2003, 15, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Hirano, Y.; Sun, T.P.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, L.; Thomas, S.G.; Hu, J.; Dill, A.; Alonso, J.M.; Ecker, J.R.; Sun, T.P. Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004, 135, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Ueguchi-Tanaka, M.; Sonoda, Y.; Kitano, H.; Koshioka, M.; Futsuhara, Y.; Matsuoka, M.; Yamaguchi, J. Slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 2001, 13, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.M.; Marion-Poll, A.; Ellis, M.; Gubler, F. Mutants at the Slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiol. 2002, 129, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.M. Hormonal regulation of gene expression in the “slender” mutant of barley (Hordeum vulgare L.). Planta 1988, 175, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Gubler, F.; Raventos, D.; Keys, M.; Watts, R.; Mundy, J.; Jacobsen, J.V. Target genes and regulatory domains of the GAMYB transcriptional activator in cereal aleurone. Plant J. 1999, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodger, F.J.; Gubler, F.; Pogson, B.J.; Jacobsen, J.V. A Mak-like kinase is a repressor of GAMYB in barley aleurone. Plant J. 2003, 33, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, M.; Inukai, Y.; Ueguchi-Tanaka, M.; Itoh, H.; Izawa, T.; Kobayashi, Y.; Hattori, T.; Miyao, A.; Hirochika, H.; Ashikari, M.; et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell 2004, 16, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L.). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Truong, T.T.; Barrero, J.M.; Jacobsen, J.V.; Hocart, C.H.; Gubler, F. A role for jasmonates in the release of dormancy by cold stratification in wheat. J. Exp. Bot. 2016, 67, 3497–3508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrichsen, D.M.; Nemhauser, J.; Muramitsu, T.; Maloof, J.N.; Alonso, J.; Ecker, J.R.; Furuya, M.; Chory, J. Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 2002, 162, 1445–1456. [Google Scholar] [PubMed]
- Zhang, L.Y.; Bai, M.Y.; Wu, J.; Zhu, J.Y.; Wang, H.; Zhang, Z.; Wang, W.; Sun, Y.; Zhao, J.; Sun, X.; et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.W.; Ross, J.D. Studies in Wild Oat Seed Dormancy: The role of ethylene in dormancy breakage and germination of wild oat seeds (Avena fatua L.). Plant Physiol. 1981, 67, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Steber, C.M.; McCourt, P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Divi, U.K.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene AtDWF4 in Arabidopsis seeds overcomes abscisic acid-induced inhibition of germination and increases cold tolerance in transgenic seedlings. J. Plant Growth Regul. 2010, 29, 385–393. [Google Scholar] [CrossRef]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009, 57, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locke, J.M.; Bryce, J.H.; Morris, P.C. Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J. Exp. Bot. 2000, 51, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Gianinetti, A.; Laarhoven, L.J.; Persijn, S.T.; Harren, F.J.; Petruzzelli, L. Ethylene production is associated with germination but not seed dormancy in red rice. Ann. Bot. 2007, 99, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F.; Mueller, D.D.; Faubion, J.M.; Paulsen, G.M. Identification of l-Tryptophan as an Endogenous Inhibitor of Embryo Germination in White Wheat. Plant Physiol. 1988, 88, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ramaih, S.; Guedira, M.; Paulsen, G.M. Relationship of indoleacetic acid and tryptophan to dormancy and preharvest sprouting of wheat. Funct. Plant Biol. 2003, 30, 939–945. [Google Scholar] [CrossRef]
- Brady, S.M.; Sarkar, S.F.; Bonetta, D.; McCourt, P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 2003, 34, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.A.; Tuttle, K.M.; Takebayashi, Y.; Seo, M.; Campbell, K.G.; Steber, C.M. The wheat ABA hypersensitive ERA8 mutant is associated with increased preharvest sprouting tolerance and altered hormone accumulation. Euphytica 2016, 212, 229–245. [Google Scholar] [CrossRef]
- Das, A.; Kim, D.; Khadka, P.; Rakwal, R.; Rohila, J.S. Unraveling Key Metabolomic Alterations in Wheat Embryos Derived from Freshly Harvested and Water-Imbibed Seeds of Two Wheat Cultivars with Contrasting Dormancy Status. Front. Plant Sci. 2017, 8, 1203. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.J.; Estelle, M. Mechanism of auxin-regulated gene expression in plants. Ann. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Estelle, M. Auxin receptors and plant development: A new signaling paradigm. Ann. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Ooms, J.; Leon-Kloosterziel, K.M.; Bartels, D.; Koornneef, M.; Karssen, C.M. Acquisition of Desiccation Tolerance and Longevity in Seeds of Arabidopsis thaliana (A Comparative Study Using Abscisic Acid-Insensitive abi3 Mutants). Plant Physiol. 1993, 102, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 1994, 5, 765–771. [Google Scholar] [CrossRef]
- Brocard-Gifford, I.M.; Lynch, T.J.; Finkelstein, R.R. Regulatory Networks in Seeds Integrating Developmental, Abscisic Acid, Sugar, and Light Signaling. Plant Physiol. 2003, 131, 78–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kendall, S.; Penfield, S. Maternal and zygotic temperature signalling in the control of seed dormancy and germination. Seed Sci. Res. 2012, 22, S23–S29. [Google Scholar] [CrossRef]
- Reddy, L.V.; Metzger, R.J.; Ching, T.M. Effect of Temperature on Seed Dormancy of Wheat. Crop Sci. 1985, 25, 455–458. [Google Scholar] [CrossRef]
- Nyachiro, J.; Clarke, F.R.; Depauw, R.; Knox, R.; Armstrong, K.C. Temperature effects on seed germination and expression of seed dormancy in wheat. Euphytica 2002, 126, 123–127. [Google Scholar] [CrossRef]
- Mares, D. Pre-harvest sprouting in wheat. I. Influence of cultivar, rainfall and temperature during grain ripening. Aust. J. Agric. Res. 1993, 44, 1259–1272. [Google Scholar] [CrossRef]
- Lunn, G.D.; Kettlewell, P.; Major, B.J.; Scott, R.K. Variation in dormancy duration of the U.K. wheat cultivar Hornet due to environmental conditions during grain development. Euphytica 2002, 126, 89–97. [Google Scholar] [CrossRef]
- Biddulph, T.; Mares, D.; Plummer, J.A.; Setter, T. Drought and high temperature increases preharvest sprouting tolerance in a genotype without grain dormancy. Euphytica 2005, 143, 277–283. [Google Scholar] [CrossRef]
- Footitt, S.; Douterelo-Soler, I.; Clay, H.; Finch-Savage, W.E. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 20236–20241. [Google Scholar] [CrossRef] [PubMed]
- Kulwal, P.L.; Singh, R.; Balyan, H.S.; Gupta, P.K. Genetic basis of pre-harvest sprouting tolerance using single-locus and two-locus QTL analyses in bread wheat. Funct. Integr. Genom. 2004, 4, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Ogbonnaya, F.C.; Oman, J.; van Ginkel, M. Characterization of quantitative trait loci controlling genetic variation for preharvest sprouting in synthetic backcross-derived wheat lines. Genetics 2008, 178, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bai, G. Dissection and fine mapping of a major QTL for preharvest sprouting resistance in white wheat Rio Blanco. Theor. Appl. Genet. 2010, 121, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Flintham, J. Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci. Res. 2000, 10, 43–50. [Google Scholar] [CrossRef]
- Lohwasser, U.; Röder, M.S.; Börner, A. QTL mapping of the domestication traits pre-harvest sprouting and dormancy in wheat (Triticum aestivum L.). Euphytica 2005, 143, 247–249. [Google Scholar] [CrossRef]
- Chao, S.; Xu, S.; Elias, E.; Faris, J.; Sorrells, M. Identification of Chromosome Locations of Genes Affecting Preharvest Sprouting and Seed Dormancy Using Chromosome Substitution Lines in Tetraploid Wheat (Triticum turgidum L.). Crop Sci. 2010, 50, 1180–1187. [Google Scholar] [CrossRef] [Green Version]
- Lohwasser, U.; Rehman, M.A.; Börner, A. Discovery of loci determining pre-harvest sprouting and dormancy in wheat and barley applying segregation and association mapping. Biol. Plant 2013, 57, 663–674. [Google Scholar] [CrossRef]
- Borner, A.; Nagel, M.; Agacka-Moldoch, M.; Gierke, P.U.; Oberforster, M.; Albrecht, T.; Mohler, V. QTL analysis of falling number and seed longevity in wheat (Triticum aestivum L.). J. Appl. Genet. 2018, 59, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, S.; Graybosch, R.; Chen, C.; Bai, G. Quantitative trait loci for resistance to pre-harvest sprouting in US hard white winter wheat Rio Blanco. Theor. Appl. Genet. 2008, 117, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Knox, R.E.; Clarke, J.M.; Clarke, F.R.; Singh, A.; DePauw, R.M.; Cuthbert, R.D. Genetics of pre-harvest sprouting resistance in a cross of Canadian adapted durum wheat genotypes. Mol. Breed. 2014, 33, 919–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Cai, S.; Wang, S.; Liu, S.; Zhang, G.; Bai, G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 2015, 128, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Hayashi, K.; Tokui, M.; Mori, M.; Miura, H.; Onishi, K. Detection of QTLs for traits associated with pre-harvest sprouting resistance in bread wheat (Triticum aestivum L.). Breed. Sci. 2016, 66, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Dale, Z.; Jie, H.; Luyu, H.; Cancan, Z.; Yun, Z.; Yarui, S.; Suoping, L. An Advanced Backcross Population through Synthetic Octaploid Wheat as a “Bridge”: Development and QTL Detection for Seed Dormancy. Front. Plant Sci. 2017, 8, 2123. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, S.; Liu, Y.; Liu, Y.; Chen, G.; Xu, J.; Deng, M.; Jiang, Q.; Wei, Y.; Lu, Y.; et al. Genome-wide association study of pre-harvest sprouting resistance in Chinese wheat founder parents. Genet. Mol. Biol. 2017, 40, 620–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torada, A.; Ikeguchi, S.; Koike, M. Mapping and validation of PCR-based markers associated with a major QTL for seed dormancy in wheat. Euphytica 2005, 143, 251–255. [Google Scholar] [CrossRef]
- Lazo, G.R.; Chao, S.; Hummel, D.D.; Edwards, H.; Crossman, C.C.; Lui, N.; Matthews, D.E.; Carollo, V.L.; Hane, D.L.; You, F.M.; et al. Development of an expressed sequence tag (EST) resource for wheat (Triticum aestivum L.): EST generation, unigene analysis, probe selection and bioinformatics for a 16,000-locus bin-delineated map. Genetics 2004, 168, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Iyer-Pascuzzi, A.S.; McCouch, S.R. Functional markers for xa5-mediated resistance in rice (Oryza sativa, L.). Mol. Breed. 2007, 19, 291–296. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.-L.; Zhang, Y.; Chen, X.-M.; He, Z.-H.; Yu, Z.; Xia, L.-Q. Evaluation and Validation of Four Molecular Markers Associated with Pre-harvest Sprouting Tolerance in Chinese Wheat. Acta Agron. Sin. 2008, 34, 17–24. [Google Scholar] [CrossRef]
- Guo, F.; Liang, W.; Fan, Q.; Huang, C.; Gao, Q.; Li, G. Distribution and evolution of allelic variation of Vp1B3 in Shandong wheat. J. Triticeae Crops 2009, 29, 575–578. [Google Scholar]
- Xia, L.Q.; Yang, Y.; Ma, Y.Z.; Chen, X.M.; He, Z.H.; Röder, M.S.; Jones, H.D.; Shewry, P.R. What can the Viviparous-1 gene tell us about wheat pre-harvest sprouting? Euphytica 2009, 168, 385–394. [Google Scholar] [CrossRef]
- Zhao, B.; Wan, Y.X.; Wang, R. Screening of wheat cultivar resources with pre-harvest sprouting resistance. J. Anhui Agric. Sci. 2010, 38, 8900–8902. [Google Scholar]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.L.; Meng, J.Y.; Zhang, Y.J.; He, Z.H.; Yang, Y. Characterization of Tamyb10 allelic variants and development of STS marker for pre-harvest sprouting resistance in Chinese bread wheat. Mol. Breed. 2016, 36, 148. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, R.; Spannagl, M.; Pfeifer, M.; Barker, G.L.A.; D’Amore, R.; Allen, A.M.; McKenzie, N.; Kramer, M.; Kerhornou, A.; Bolser, D.; et al. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 2012, 491, 705. [Google Scholar] [CrossRef] [PubMed]

| Trait | QTL | Chromosome | Nearest Marker | QTL name | Material | Reference |
|---|---|---|---|---|---|---|
| PHS and GC | 5 | 3AL 3BL 3DL 5AS | Xffb293 Xgwm403, Xbcd131 Xgwm3 Xbcd1871 | - | RILs | [1] |
| PHS and GC | 3 | 1BS 4BL 7AS | Xpsp3000 Xpsp3030-Xpsp3078 Xpsp3050 | - | RILs | [22] |
| PHS and SD | 3 | 3A 3A 3A | Xpsr394-Xgwm5 Xcdo345 Xcdo345-Xbcd141 | taVp1 QPhs.ocs-3A.1 QPhs.ocs-3A.2 | RILs | [23] |
| PHS and SD | 2 | 4AL 3AL | Xksuf8a-Xbcd402b Xpsr903b-XATPased | - | RILs | [217] |
| PHS | 1 | 3AL | Xwmc153-Xgwm155 | QPhs.ccsu3A.1 | RILs | [24] |
| SD | 1 | 4A | Xgwm397-Xgwm269-Xbarc170 | - | DHLs | [3] |
| PHS and SD | 1 | 3AL | Xbarc310-Xbcd907 | QPhs.ocs-3A.1 | RILs | [25] |
| PHS and SD | 1 | 3AS 3AS | Xbarc310 Xbarc321 | QPhs-3AS QPhs.pseru-3AS | RILs | [26] |
| PHS | 3 | 2B 2B | Xdup398-Xbarc54 Xbarc105-Xbarc334 | QPhs.pseru-2B.1 QPhs.pseru-2B.2 | RILs | [221] |
| PHS | 1 | 2DS | Xgwm261-Xgwm484 | Qphs.sau-2D | F2 and F6 | [28] |
| PHS | 4 | 2B 2D 3D 6D | Xbarc55-Xwmc474 Xwmc111-WxPt-999 7Xbarc1161 Xcfd37-Xbarc196 | QPhs.cnl-2B.1 QPhs.cnl-2D.1 QPhs.cnl-3D.1 QPhs.cnl-6D.1 | DHLs | [14] |
| PHS | 3 | 2AL 3AL 3BL | Xgwm1045-Xgwm296 Xgwm153-Xgwm155 Xgwm1005–Xgwm980 | QPhs.ccsu-2A.5 QPhs.ccsu-3A.1 QPhs.ccsu-3B.6 | RILs | [32] |
| PHS and GC | 5 | 3B 3D 3A 5D 3D | Xbarc77–Xwmc30 7Xwmc552–Xwmc533 Xcfa2193–Xwmc594 Xgwm469–Xcfd10 Xwmc11–Xcfd223 | QGi.crc-3B QGi.crc-3D QSi.crc-3A QSi.crc-5D QCl.crc-3D | DHLs | [29] |
| PHS and SD | 1 | 3BL | Xwmc527-Xgwm77 | - | DHLs | [31] |
| PHS | 1 | 5D | XCFD40-XBARC1097 | qPhs5D.1 | DHLs | [33] |
| PHS and SD | 5 | 2A 2B 3A 4A 7B | 521-2A 521-2B 521-3A 521-4A 521-7B | - | Single chromosome substitution lines | [218] |
| PHS | 3 | 1A 2A 7B | Xwmc611-Xwmc333 Xgwm515-Xgwm425 Xgwm297-Xwmc532 | QPhsd.spa.-1A.1 QPhsd.spa.-2A.1 QPhsd.spa.-7B.1 | RILs | [35] |
| PHS | 4 | 3B 4A 7B 7D | 19 SNPs flanking the QTL 12 SNPs flanking the QTL 10 SNPs flanking the QTL 04 SNPs flanking the QTL | QSi.crc-3B QGi.crc-4A QSi.crc-7B QFn.crc-7D | DHLs | [80] |
| PHS | 5 | 1A 1B 5B 7A 7B | wPt-6274 Xwmc191 wPt-6910-wPt-7400 Xcfa2174 Xwmc606 | QPhs.spa-1A QPhs.spa-1B QPhs.spa-5B QPhs.spa-7A QPhs.spa-7B | DHLs | [222] |
| PHS and SD | 1 | 2B | Xwmc477-Xbarc55 | Sdr2B | RILs | [76] |
| PHS and SD | 1 | 4A | wsnp_Ex_c66324_64493429 - CD920298 | 4A-1 | RILs | [44] |
| PHS and SD | 4 | 4A 4B 5A 5B | GBS212432-GBS10994 7Xbarc20-Xwmc238 TTM_199619-TTM_1259 7Xbarc346-2-TTM_62137_50 | Qphs.pseru-4A.1 Qphs.pseru-4B.1 Qphs.pseru-5A.1 Qphs.pseru-5B.1 | RILs | [223] |
| PHS and GC | 6 | 3AL 3AL 3AL 3DL 3DL 1A/1D/3A/5B | Xwmc559-1 Tamyb10-A1-66 Tamyb10-A1-74 BS00067163_51 Tamyb10-D1-93 Xbarc148 | - | RILs | [55] |
| PHS | 6 | 3A 4A 1B 7B 4A 6B | TaMFT cfa2256 Xbarc181 UCW99 cfa2256 Xwmc397 | QDor-3A QDor-4A QDor-1B QHt-7B QAwn-4A QAwn-6B | RILs | [224] |
| PHS and SD | 1 | 2A | Xgwm95-Xgwm372 | Sdr2A | RILs | [46] |
| PHS and SD PHS PHS | 3 2 3 | 2D 3D 3D 1B 1B 3A 3D 5D | Xwmc503 Xcfd22 Vp1-4 tPt-7980 wPt-645 7AX-111578083 3 DArT-seq and 5 SNPs AX-109028892 | QDor-2D QDor-3D TaVp1 - QTL1 QTL2 QTL3 | Back crosspopulation 86 Chinesegermplasm 717 Chinese wheat landraces | [225] [226] [37] |
| PHS | 5 | 1A 4D 5A 5D 7B | wPt-6654-wPt-7030 wPt-0710-Rht-D1 gwm186-P7560-439 P7551-267-wmc574 P7455-236-P7553-711 | - | RIL | [220] |
| Wheat Gene | Chromosomes | Gene Function | Homologs/Orthologs Gene | Experimental Methodology | References |
|---|---|---|---|---|---|
| TaSdr-A1 | 2AS | SD | Rice OsSdr4 orthologs | Comparative genomics approach | [46] |
| TaSdr-B1 | 2BS | SD | Rice OsSdr4 orthologs | homologous cloning approach | [76] |
| TaMFT | 3AS | SD | Wheat TaMFT homolog | Transcriptomic approach | [41] |
| TaPHS1 | 3AS | SD | Wheat MFT homolog | comparative fine mapping and map-based cloning | [16,42] |
| TaVp-1 | Group 3 Chromosomes | SD and PHS | Maize Vp1 and rice OsVp1 orthologs | Genomic southern analysis | [38,39,84,86] |
| Tamyb10 PM19-A1/A2 TaMKK3-A | Group 3 Chromosomes 4AL 4AL | GC SD SD | Arabidopsis TT2 and Rice OsMYB3 orthologs - - | Cloning approach Transcriptomic approach Map-based approach | [40] [44] [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Cao, J.; Jiang, H.; Chang, C.; Zhang, H.-P.; Sheikh, S.W.; Shah, L.; Ma, C. Unraveling Molecular and Genetic Studies of Wheat (Triticum aestivum L.) Resistance against Factors Causing Pre-Harvest Sprouting. Agronomy 2019, 9, 117. https://doi.org/10.3390/agronomy9030117
Ali A, Cao J, Jiang H, Chang C, Zhang H-P, Sheikh SW, Shah L, Ma C. Unraveling Molecular and Genetic Studies of Wheat (Triticum aestivum L.) Resistance against Factors Causing Pre-Harvest Sprouting. Agronomy. 2019; 9(3):117. https://doi.org/10.3390/agronomy9030117
Chicago/Turabian StyleAli, Ahmad, Jiajia Cao, Hao Jiang, Cheng Chang, Hai-Ping Zhang, Salma Waheed Sheikh, Liaqat Shah, and Chuanxi Ma. 2019. "Unraveling Molecular and Genetic Studies of Wheat (Triticum aestivum L.) Resistance against Factors Causing Pre-Harvest Sprouting" Agronomy 9, no. 3: 117. https://doi.org/10.3390/agronomy9030117




