Effect of PPO-Inhibiting Herbicides on the Growth and Sex Ratio of a Dioecious Weed Species Amaranthus palmeri (Palmer Amaranth)
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
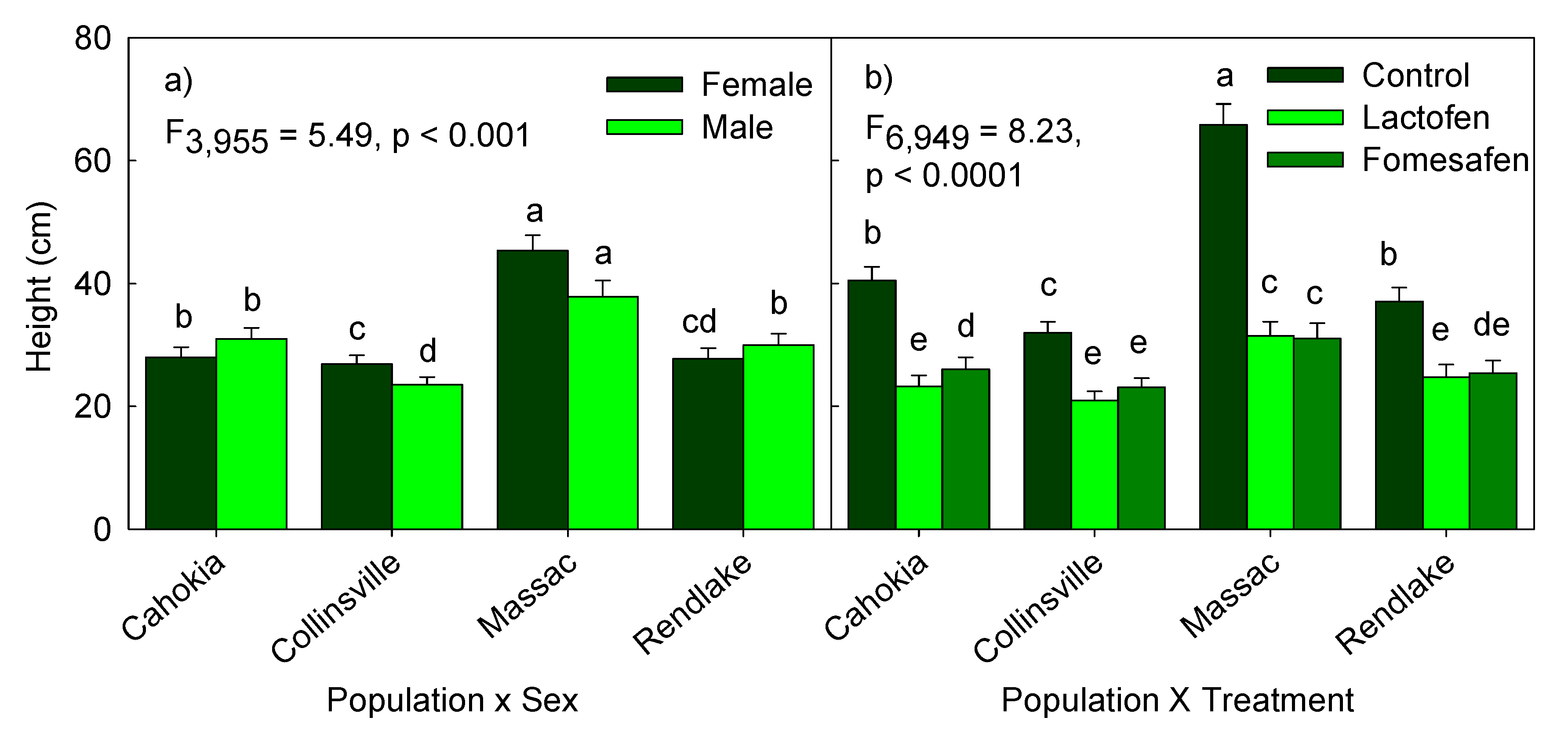
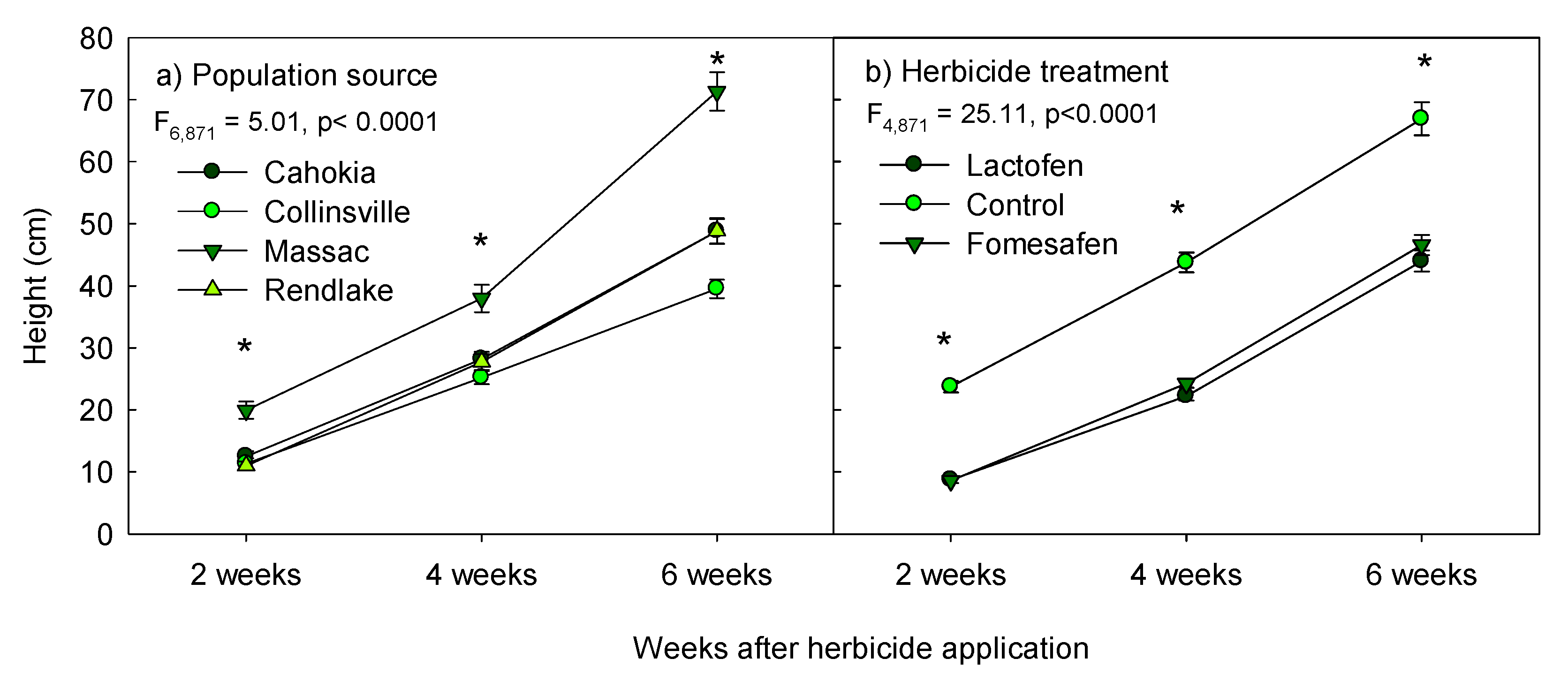
3.1. Effect of Population Sources and Herbicide Treatments on Height
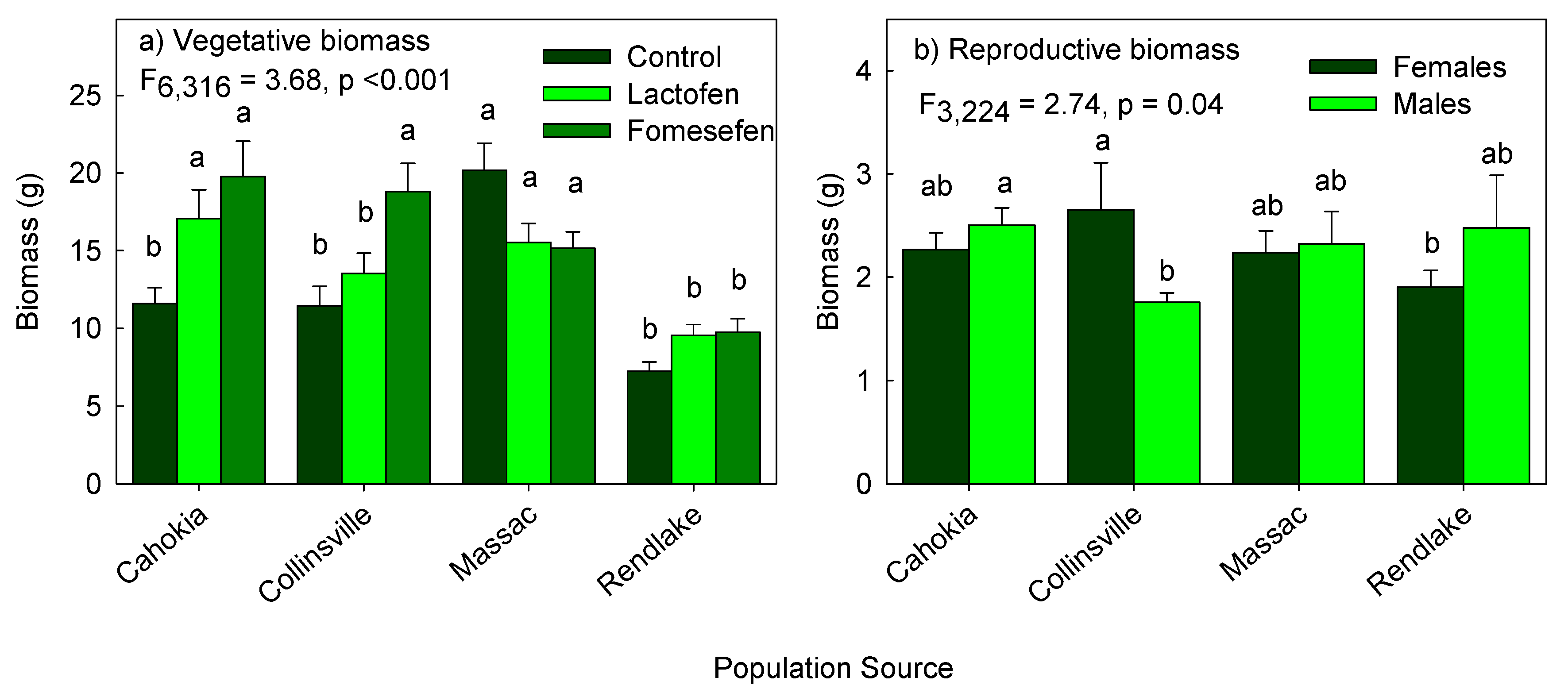
3.2. Effect of Population Sources and Herbicide Treatments on Vegetative Biomass
3.3. Effect of Population Sources and Herbicide Treatments on Reproductive Biomass
3.4. Effect of Population Sources and Herbicide Treatments on the Flowering Date
3.5. Effect of Population Sources and Herbicide Treatments on the Male-to-female Sex Ratios
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicotra, A. Sex ratio variation and spatial distribution of Siparuna grandiflora, a tropical dioecious shrub. Oecologia 1998, 115, 102–113. [Google Scholar] [CrossRef]
- Fisher, R.A.S. The Genetical Theory of Natural Selection; Dover: New York, NY, USA, 1958. [Google Scholar]
- Charlesworth, D. Plant sex chromosomes. Ann. Rev. Plant Biol. 2016, 67, 397–420. [Google Scholar] [CrossRef]
- Freeman, D.; Harper, K.; Charnov, E. Sex change in plants: Old and new observations and new hypotheses. Oecologia 1980, 47, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Liebman, M.; Menalled, F.D.; Buhler, D.D.; Richard, T.L.; Sundberg, D.N.; Cambardella, C.A.; Kohler, K.A. Impacts of composted swine manure on weed and corn nutrient uptake, growth, and seed production. Weed Sci. 2004, 52, 365–375. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. The experimental modification of sex expression in flowering plants. Biol. Rev. 1957, 32, 38–90. [Google Scholar] [CrossRef]
- Van der Werf, H.M.; Van Geel, W.; Van Gils, L.; Haverkort, A. Nitrogen fertilization and row width affect self-thinning and productivity of fibre hemp (Cannabis sativa L.). Field Crops Res. 1995, 42, 27–37. [Google Scholar] [CrossRef]
- Nigam, R.K.; Varkey, M.; Reuben, D.E. Irradiation induced changes in flower formation in Cannabis sativa L. Biol. Plant. 1981, 23, 389–391. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Studies on flowering-plant growth and organogenesis. II. The modification of sex expression in Cannabis sativa by carbon monoxide. Proc. R. Soc. Edinb. Biol. 1957, 66, 424–434. [Google Scholar]
- Culafic, L.; Neskovic, M. Effect of growth substances on flowering and sex expression in isolated apical buds of Spinacia oleracea. Physiol. Plant. 1980, 48, 588–591. [Google Scholar] [CrossRef]
- Culafic, L.; Konjevic, R.; Neskovic, M. Flowering of in vitro grown spinach shoots in the presence of the herbicide Sandoz 9789. Biol. Plant. 1983, 25, 155–157. [Google Scholar] [CrossRef]
- Lemen, C. Allocation of reproductive effort to the male and female strategies in wind-pollinated plants. Oecologia 1980, 45, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The biology of invasive alien plants in Canada. 3. Amaranthus tuberculatus (Moq.) Sauer var. rudis (Sauer) Costea & Tardif. Can. J. Plant Sci. 2005, 85, 507–522. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Webb, C. Secondary sex characters in plants. Bot. Rev. 1977, 43, 177–216. [Google Scholar] [CrossRef]
- Delph, L.F.; Meagher, T.R. Sexual dimorphism masks life history trade-offs in the dioecious plant Silene latifolia. Ecology 1995, 76, 775–785. [Google Scholar] [CrossRef]
- Bierzychudek, P.; Eckhart, V. Spatial segregation of the sexes of dioecious plants. Am. Nat. 1988, 132, 34–43. [Google Scholar] [CrossRef]
- Dawson, T.E.; Ehleringer, J.R. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder, Acer negundo. Ecology 1993, 74, 798–815. [Google Scholar] [CrossRef]
- Sauer, J.D. The grain Amaranthus: A survey of their history and classification. Ann. Mo. Bot. Gard. 1950, 37, 561–632. [Google Scholar] [CrossRef]
- Steckel, L.E. The dioecious Amaranthus spp.: Here to stay. Weed Technol. 2007, 21, 567–570. [Google Scholar] [CrossRef]
- Keeley, P.E.; Carter, C.H.; Thullen, R.J. Influence of planting date on growth of Palmer amaranth (Amaranthus palmeri). Weed Sci. 1987, 35, 199–204. [Google Scholar] [CrossRef]
- Massinga, R.A.; Currie, R.S.; Trooien, T.P. Water use and light interception under Palmer amaranth (Amaranthus palmeri) and corn competition. Weed Sci. 2003, 51, 523–531. [Google Scholar] [CrossRef]
- Moore, J.W.; Murray, D.S.; Westerman, R.B. Palmer Amaranth (Amaranthus palmeri) effects on the harvest and yield of grain sorghum (Sorghum bicolor). Weed Technol. 2004, 18, 23–29. [Google Scholar] [CrossRef]
- Klingaman, T.E.; Oliver, L.R. Palmer amaranth (Amaranthus palmeri) interference in soybeans (Glycine max). Weed Sci. 1994, 42, 523–527. [Google Scholar] [CrossRef]
- Bensch, C.N.; Horak, M.J.; Peterson, D. Interference of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in soybean. Weed Sci. 2003, 51, 37–43. [Google Scholar] [CrossRef]
- Morgan, G.D.; Baumann, P.A.; Chandler, J.M. Competitive impact of Palmer Amaranth (Amaranthus palmeri) on cotton (Gossypium hirsutum) development and yield. Weed Technol. 2001, 15, 408–412. [Google Scholar] [CrossRef]
- Burke, I.C.; Schroeder, M.; Thomas, W.E.; Wilcut, J.W. Palmer amaranth interference and seed production in peanut. Weed Technol. 2007, 21, 367–371. [Google Scholar] [CrossRef]
- Meyers, S.L.; Jennings, K.M.; Schultheis, J.R.; Monks, D.W. Evaluation of flumioxazin and s-metolachlor rate and timing for Palmer amaranth (Amaranthus palmeri) control in sweetpotato. Weed Technol. 2010, 24, 495–503. [Google Scholar] [CrossRef]
- Meyers, S.L.; Jennings, K.M.; Monks, D.W. Herbicide-based weed management programs for Palmer amaranth (Amaranthus palmeri) in sweetpotato. Weed Technol. 2013, 27, 331–340. [Google Scholar] [CrossRef]
- Menges, R.M. Weed seed population dynamics during six years of weed management systems in crop rotations on irrigated soil. Weed Sci. 1987, 35, 328–332. [Google Scholar] [CrossRef]
- Connick, W.; Bradow, J.; Legendre, M.; Vail, S.; Menges, R. Identification of volatile allelochemicals from Amaranthus palmeri S. Wats. J. Chem. Ecol. 1987, 13, 463–472. [Google Scholar] [CrossRef]
- Ward, S.M.; Webster, T.M.; Steckel, L.E. Palmer Amaranth (Amaranthus palmeri): A review. Weed Technol. 2013, 27, 12–27. [Google Scholar] [CrossRef]
- Sadeque, A.; Brown, P.J.; Tranel, P.J. Towards a novel control strategy for dioecious Amaranthus: Identification of gender-specific DNA sequences. In Proceedings of the Weed Science Society of America, Tuscon, AZ, USA, 6–9 February 2017. [Google Scholar]
- Thomas, J.T.; Michael, E.E.; Peter, T.K. Effect of moisture stress and glyphosate on adventitious shoot growth of Canada Thistle (Cirsium arvense). Weed Sci. 1998, 46, 59–64. [Google Scholar]
- Giacomini, D.; Westra, P.; Ward, S.M. Impact of genetic background in fitness cost studies: An example from glyphosate-resistant Palmer amaranth. Weed Sci. 2014, 62, 29–37. [Google Scholar] [CrossRef]
- Rumpa, M.M. Effect of PPO-Inhibiting Herbicides on Growth Characteristics and Sex Ratio of a Dioecious Weed Species Amaranthus palmeri (Palmer Amaranth). Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2017. [Google Scholar]
- Dan Hess, F. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Wuerffel, R.J.; Young, J.M.; Matthews, J.L.; Young, B.G. Characterization of PPO-inhibitor–resistant waterhemp (Amaranthus tuberculatus) response to soil-applied PPO-inhibiting herbicides. Weed Sci. 2015, 63, 511–521. [Google Scholar] [CrossRef]
- Lee, R.M.; Hager, A.G.; Tranel, P.J. Prevalence of a novel resistance mechanism to PPO-inhibiting herbicides in waterhemp (Amaranthus tuberculatus). Weed Sci. 2008, 56, 371–375. [Google Scholar] [CrossRef]
- Lermontova, I.; Grimm, B. Overexpression of plastidic protoporphyrinogen IX oxidase leads to resistance to the diphenyl-ether herbicide acifluorfen. Plant Physiol. 2000, 122, 75–84. [Google Scholar] [CrossRef]
- Schwartz-Lazaro, L.M.; Norsworthy, J.K.; Scott, R.C.; Barber, L.T. Resistance of two Arkansas Palmer amaranth populations to multiple herbicide sites of action. Crop Prot. 2017, 96, 158–163. [Google Scholar] [CrossRef]
- Salas-Perez, R.A.; Burgos, N.R.; Rangani, G.; Singh, S.; Paulo Refatti, J.; Piveta, L.; Tranel, P.J.; Mauromoustakos, A.; Scott, R.C. Frequency of Gly-210 Deletion Mutation among Protoporphyrinogen Oxidase Inhibitor–Resistant Palmer Amaranth (Amaranthus palmeri) Populations. Weed Sci. 2017, 65, 718–731. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2019. Available online: http://www.weedscience.org/in.asp (accessed on 20 May 2019).
- Jenkins, M.E.; Krausz, R.F.; Matthews, J.L.; Gage, K.L.; Walters, S.A. Control of volunteer Horseradish and Palmer Amaranth (Amaranthus palmeri) with Dicamba and Glyphosate. Weed Technol. 2017, 31, 852–862. [Google Scholar] [CrossRef]
- Anonymous. Cobra Herbicide Product Label; EPA Reg. No. 59639-34. Valent USA Corporation: Walnut Creek, CA, USA. Available online: https://s3-us-west-1.amazonaws.com/www.agrian.com/pdfs/Cobrar_Herbicide_Label1y.pdf (accessed on 20 May 2019).
- Anonymous. Flexstar Herbicide Product Label; EPA Reg. No. 100-1101. Syngenta Crop Protection, LLC: Greensboro, NC, USA. Available online: https://s3-us-west-1.amazonaws.com/www.agrian.com/pdfs/Flexstar1g_Label.pdf (accessed on 20 May 2019).
- Bond, J.A.; Oliver, L.R. Comparative growth of Palmer amaranth (Amaranthus palmeri) accessions. Weed Sci. 2006, 54, 121–126. [Google Scholar] [CrossRef]
- Bravo, W.; Leon, R.G.; Ferrell, J.A.; Mulvaney, M.J.; Wood, C.W. Differentiation of life–history traits among Palmer Amaranth populations (Amaranthus palmeri) and Its relation to cropping systems and glyphosate sensitivity. Weed Sci. 2017, 65, 339–349. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Schwinning, S.; Weiner, J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 1998, 113, 447–455. [Google Scholar] [CrossRef]
- Nagashima, H.; Hikosaka, K. Plants in a crowded stand regulate their height growth so as to maintain similar heights to neighbours even when they have potential advantages in height growth. Ann. Bot. 2011, 108, 207–214. [Google Scholar] [CrossRef]
- Schwartz, L.M.; Gibson, D.J.; Young, B.G. Do plant traits predict the competitive abilities of closely related species? AoB Plants 2016, 8, plv147. [Google Scholar] [CrossRef]
- Barrett, S.C.; Yakimowski, S.B.; Field, D.L.; Pickup, M. Ecological genetics of sex ratios in plant populations. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 2549–2557. [Google Scholar] [CrossRef] [Green Version]
- Gough, L.; Grace, J.B.; Taylor, K.L. The Relationship between species richness and community biomass: The importance of environmental variables. Oikos 1994, 70, 271–279. [Google Scholar] [CrossRef]
- Putwain, P.; Harper, J.L. Studies in the dynamics of plant populations: V. Mechanisms governing the sex ratio in Rumex acetosa and R. acetosella. J. Ecol. 1972, 60, 113–129. [Google Scholar] [CrossRef]
- Zluvova, J.; Zak, J.; Janousek, B.; Vyskot, B. Dioecious Silene latifolia plants show sexual dimorphism in the vegetative stage. BMC Plant Biol. 2010, 10, 1471–2229. [Google Scholar] [CrossRef]
- Delph, L.F.; Herlihy, C.R. Sexual, fecundity, and viability selection on flower size and number in a sexually dimorphic plant. Evol. Int. J. Org. Evol. 2012, 66, 1154–1166. [Google Scholar] [CrossRef]
- Delph, L.F.; Lu, Y.; Jayne, L.D. Patterns of resource allocation in a dioecious Carex (Cyperaceae). Am. J. Bot. 1993, 80, 607–615. [Google Scholar] [CrossRef]
- Antos, J.A.; Allen, G.A. Patterns of reproductive effort in male and female shrubs of Oemleria cerasiformis: A 6-year study. J. Ecol. 1999, 87, 77–84. [Google Scholar] [CrossRef]
- MacRae, A.; Webster, T.; Sosnoskie, L.; Culpepper, A.; Kichler, J. Cotton yield loss potential in response to length of Palmer amaranth (Amaranthus palmeri) interference. J. Cotton Sci. 2013, 17, 227–232. [Google Scholar]
- Bonduriansky, R.; Maklakov, A.; Zajitschek, F.; Brooks, R. Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 2008, 22, 443–453. [Google Scholar] [CrossRef]
- Field, D.L.; Pickup, M.; Barrett, S.C.H. Ecological context and metapopulation dynamics affect sex-ratio variation among dioecious plant populations. Ann. Bot. 2013, 111, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Field, D.L.; Pickup, M.; Barrett, S.C.H. Comparative analysis of sex-ratio variation in dioecious flowering plants. Evol. Int. J. Org. Evol. 2013, 66, 661–672. [Google Scholar] [CrossRef]
- Gibson, D.J.; Menges, E.S. Population structure and spatial pattern in the dioecious shrub Ceratiola ericoides. J. Veg. Sci. 1994, 5, 337–346. [Google Scholar] [CrossRef]
- Spaunhorst, D.J.; Devkota, P.; Johnson, W.G.; Smeda, R.J.; Meyer, C.J.; Norsworthy, J.K. Phenology of five Palmer amaranth (Amaranthus palmeri) populations grown in northern Indiana and Arkansas. Weed Sci. 2018, 66, 457–469. [Google Scholar] [CrossRef]
- Chailakhyan, M.K.; Khryanin, V.N. Effect of growth regulators and role of roots in sex expression in spinach. Planta 1978, 142, 207–210. [Google Scholar] [CrossRef]
- Louis, J.P.; Augur, C.; Teller, G. Cytokinins and differentiation processes in Mercurialis annua: Genetic regulation, relations with auxins, indoleacetic acid oxidases, and sexual expression patterns. Plant Physiol. 1990, 94, 1535–1541. [Google Scholar] [CrossRef]
- Negi, S.; Olmo, H. Induction of sex conversion in male Vitis. J. Grape Vine Res. 1971, 10, 1–19. [Google Scholar] [CrossRef]
- Fechter, I.; Hausmann, L.; Daum, M.; Sörensen, T.R.; Viehöver, P.; Weisshaar, B.; Töpfer, R. Candidate genes within a 143 kb region of the flower sex locus in Vitis. Mol. Gen. Genet. 2012, 287, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.P.; Durand, B. Studies with the dioecious angiosperm Mercurialis annua L. (2n = 16): Correlation between genic and cytoplasmic male sterility, sex segregation and feminizing hormones (cytokinins). Mol. Gen. Genet. 1978, 165, 309–322. [Google Scholar] [CrossRef]
- Janoušek, B.; Široký, J.; Vyskot, B. Epigenetic control of sexual phenotype in a dioecious plant, Melandrium album. Mol. Gen. Genet. 1996, 250, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Troadec, C.; Boualem, A.; Rajab, M.; Fernandez, R.; Morin, H.; Pitrat, M.; Dogimont, C.; Bendahmane, A. A transposon-induced epigenetic change leads to sex determination in melon. Nature 2009, 461, 1135–1138. [Google Scholar] [CrossRef]
- Davis, A.S.; Schutte, B.J.; Hager, A.G.; Young, B.G. Palmer amaranth (Amaranthus palmeri) damage niche in Illinois soybean is seed limited. Weed Sci. 2015, 63, 658–668. [Google Scholar] [CrossRef]


| Effect | F Value | Pr > F |
|---|---|---|
| pop | 76.053,960 | <0.0001 |
| treat | 83.582,54.4 | <0.0001 |
| pop × treat | 8.236,949 | <0.0001 |
| sex | <0.101,902 | 0.95 |
| pop × sex | 5.493,955 | 0.001 |
| treat × sex | 0.292,875 | 0.75 |
| pop × treat × sex | 1.726,945 | 0.11 |
| time | 1386.572,871 | <0.0001 |
| pop × time | 5.016,871 | <0.0001 |
| treat × time | 25.114,871 | <0.0001 |
| pop × treat × time | 1.2612,871 | 0.24 |
| sex × time | 0.442,871 | 0.65 |
| pop × sex × time | 0.276,871 | 0.95 |
| treat × sex × time | 0.014,871 | 0.99 |
| pop × treat × sex × time | 0.6512,871 | 0.80 |
| Vegetative Biomass | Reproductive Biomass | Flowering Date | ||||
|---|---|---|---|---|---|---|
| Effect | F value | Pr > F | F value | Pr > F | F value | Pr > F |
| P | 29.383,1 | 0.12 | 0.503,222 | 0.68 | 36.283,326 | <0.0001 |
| T | 0.542,31.6 | 0.009 | 0.382,18.5 | 0.69 | 115.202,49.9 | <0.0001 |
| P × T | 3.686,316 | 0.0015 | 1.846,215 | 0.09 | 0.106,324 | 0.99 |
| S | Infty1,1 | <.0001 | 0.291,267 | 0.59 | 26.891,333 | <0.0001 |
| P × S | 0.563,330 | 0.64 | 2.743,224 | 0.04 | 1.543,333 | 0.21 |
| T × S | 0.182,333 | 0.31 | 0.032,255 | 0.97 | 3.022,333 | 0.05 |
| P × T × S | 0.666,330 | 0.68 | 0.966,220 | 0.45 | 0.706,333 | 0.69 |
| Treatment | Population | t-Value | p-Value |
|---|---|---|---|
| lactofen | Cahokia | –3.59 | 0.070 |
| lactofen | Collinsville | –3.59 | 0.070 |
| lactofen | Massac | –4.35 | 0.049 |
| lactofen | Rendlake | –6.50 | 0.023 |
| control | Cahokia | –0.69 | 0.560 |
| control | Collinsville | –2.17 | 0.163 |
| control | Massac | –2.50 | 0.130 |
| control | Rendlake | –4.25 | 0.050 |
| fomesafen | Cahokia | –4.33 | 0.049 |
| fomesafen | Collinsville | –1.70 | 0.231 |
| fomesafen | Massac | –2.80 | 0.173 |
| fomesafen | Rendlake | –6.06 | 0.026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumpa, M.M.; Krausz, R.F.; Gibson, D.J.; Gage, K.L. Effect of PPO-Inhibiting Herbicides on the Growth and Sex Ratio of a Dioecious Weed Species Amaranthus palmeri (Palmer Amaranth). Agronomy 2019, 9, 275. https://doi.org/10.3390/agronomy9060275
Rumpa MM, Krausz RF, Gibson DJ, Gage KL. Effect of PPO-Inhibiting Herbicides on the Growth and Sex Ratio of a Dioecious Weed Species Amaranthus palmeri (Palmer Amaranth). Agronomy. 2019; 9(6):275. https://doi.org/10.3390/agronomy9060275
Chicago/Turabian StyleRumpa, Mafia M., Ronald F. Krausz, David J. Gibson, and Karla L. Gage. 2019. "Effect of PPO-Inhibiting Herbicides on the Growth and Sex Ratio of a Dioecious Weed Species Amaranthus palmeri (Palmer Amaranth)" Agronomy 9, no. 6: 275. https://doi.org/10.3390/agronomy9060275






