Optimal Thawing of Cryopreserved Peripheral Blood Mononuclear Cells for Use in High-Throughput Human Immune Monitoring Studies
Abstract
:1. Introduction
2. Results
2.1. The Effect of Temperature and Speed of the Wash Medium Addition during Thawing on the Viability and Functionality of Cryopreserved PBMC
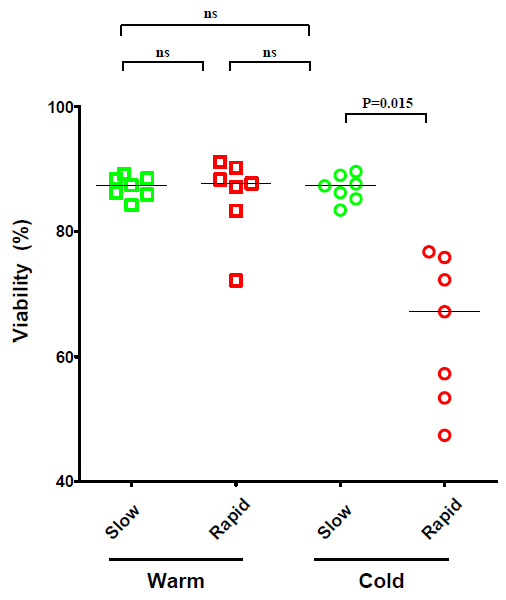
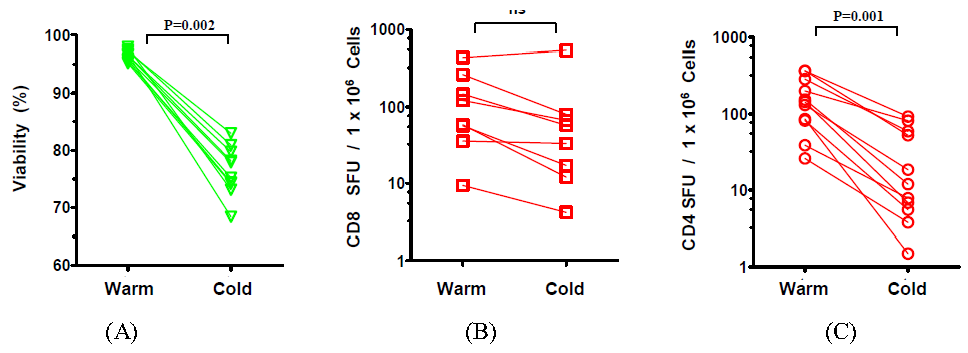
2.2. The Effect of the Number of Washing Steps during Thawing on the Viability and Functionality of Cryopreserved PBMC

2.3. The Effect of Prolonged Post-Thaw DMSO Exposure at 37 °C on the Viability and Functionality of Cryopreserved PBMC
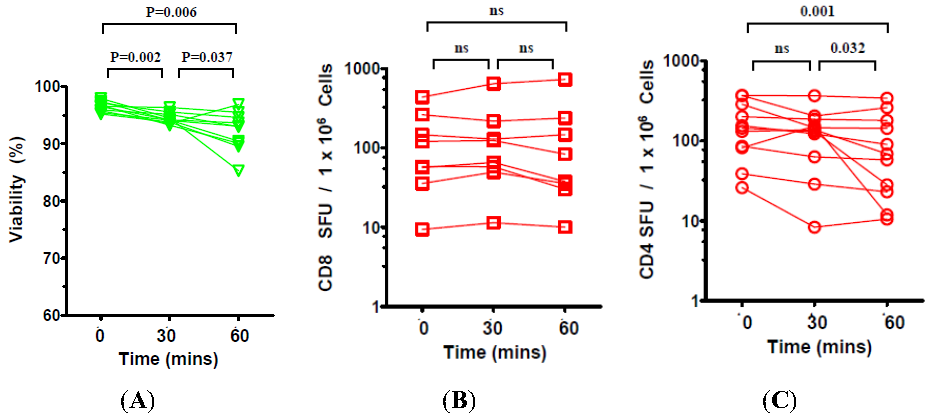
3. Discussion
4. Experimental Section
4.1. Thawing and Handling of Cryopreserved PBMC and Antigens
4.2. Human Interferon-γ ELISPOT Assay
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Kreher, C.R.; Dittrich, M.T.; Guerkov, R.; Boehm, B.O.; Tary-Lehmann, M. Cd4+ and cd8+ cells in cryopreserved human pbmc maintain full functionality in cytokine elispot assays. J. Immunol. Methods 2003, 278, 79–93. [Google Scholar] [CrossRef]
- Currier, J.R.; deSouza, M.; Chanbancherd, P.; Bernstein, W.; Birx, D.L.; Cox, J.H. Comprehensive screening for human immunodeficiency virus type 1 subtype-specific cd8 cytotoxic t lymphocytes and definition of degenerate epitopes restricted by hla-a0207 and -c(w)0304 alleles. J. Virol. 2002, 76, 4971–4986. [Google Scholar]
- Peters, B.S.; Jaoko, W.; Vardas, E.; Panayotakopoulos, G.; Fast, P.; Schmidt, C.; Gilmour, J.; Bogoshi, M.; Omosa-Manyonyi, G.; Dally, L.; et al. Studies of a prophylactic hiv-1 vaccine candidate based on modified vaccinia virus ankara (mva) with and without DNA priming: Effects of dosage and route on safety and immunogenicity. Vaccine 2007, 25, 2120–2127. [Google Scholar]
- Basha, S.; Hazenfeld, S.; Brady, R.C.; Subbramanian, R.A. Comparison of antibody and t-cell responses elicited by licensed inactivated- and live-attenuated influenza vaccines against h3n2 hemagglutinin. Hum. Immunol. 2011, 72, 463–469. [Google Scholar] [CrossRef]
- Garcia, F.; Bernaldo de Quiros, J.C.; Gomez, C.E.; Perdiguero, B.; Najera, J.L.; Jimenez, V.; Garcia-Arriaza, J.; Guardo, A.C.; Perez, I.; Diaz-Brito, V.; et al. Safety and immunogenicity of a modified pox vector-based hiv/aids vaccine candidate expressing env, gag, pol and nef proteins of hiv-1 subtype b (mva-b) in healthy hiv-1-uninfected volunteers: A phase i clinical trial (risvac02). Vaccine 2011, 29, 8309–8316. [Google Scholar]
- Yang, F.F.; Tu, Z.Q.; Fang, Y.M.; Li, Y.; Peng, Y.; Dong, T.; Wang, C.; Lin, S.X.; Zhan, N.Y.; Ma, Z.M.; et al. Monitoring of peptide-specific and interferon-gamma-productive t cells in patients with active and convalescent tuberculosis using elispot. Clin. Vaccine Immunol. 2012. [Google Scholar] [CrossRef]
- Axelsson, S.; Faresjo, M.; Hedman, M.; Ludvigsson, J.; Casas, R. Cryopreserved peripheral blood mononuclear cells are suitable for the assessment of immunological markers in type 1 diabetic children. Cryobiology 2008, 57, 201–208. [Google Scholar] [CrossRef]
- Weinberg, A.; Song, L.Y.; Wilkening, C.L.; Fenton, T.; Hural, J.; Louzao, R.; Ferrari, G.; Etter, P.E.; Berrong, M.; Canniff, J.D.; et al. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from hiv-infected and uninfected individuals for elispot assays. J. Immunol. Methods 2010, 363, 42–50. [Google Scholar] [CrossRef]
- Zhang, W.; Caspell, R.; Karulin, A.Y.; Ahmad, M.; Haicheur, N.; Abdelsalam, A.; Johannesen, K.; Vignard, V.; Dudzik, P.; Georgakopoulou, K.; et al. Elispot assays provide reproducible results among different laboratories for t-cell immune monitoring—Even in hands of elispot-inexperienced investigators. J. Immunotoxicol. 2009, 6, 227–234. [Google Scholar] [CrossRef]
- Areman, E.M.; Simonis, T.B.; Carter, C.S.; Read, E.J.; Klein, H.G. Bulk cryopreservation of lymphocytes in glycerol. Transfusion 1988, 28, 151–156. [Google Scholar] [CrossRef]
- Yokoyama, W.M. Cryopreservation of Cells. In Current Protocols in Immunology; Coligan, J.E., Kruisbeek, A.M., Margulies, D.H., Shevach, E.M., Strober, W., Eds.; Wiley: New York, NY, USA, 1997; pp. A.3.G.1–A.3.G.3. [Google Scholar]
- Birkeland, S.A. The influence of different freezing procedures and different cryoprotective agents on the immunological capacity of frozen-stored lymphocytes. Cryobiology 1976, 13, 442–447. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hsu, M.L.; Tzeng, C.H.; Hsu, H.C.; Ho, C.K. The influence of cryopreservation on cytokine production by human t lymphocytes. Cryobiology 1998, 37, 22–29. [Google Scholar] [CrossRef]
- Jeurink, P.V.; Vissers, Y.M.; Rappard, B.; Savelkoul, H.F. T cell responses in fresh and cryopreserved peripheral blood mononuclear cells: Kinetics of cell viability, cellular subsets, proliferation, and cytokine production. Cryobiology 2008, 57, 91–103. [Google Scholar] [CrossRef]
- Posavad, C.M.; Magaret, A.S.; Zhao, L.; Mueller, D.E.; Wald, A.; Corey, L. Development of an interferon-gamma elispot assay to detect human t cell responses to hsv-2. Vaccine 2011, 29, 7058–7066. [Google Scholar]
- Cox, J.H.; Ferrari, G.; Kalams, S.A.; Lopaczynski, W.; Oden, N.; D'Souza, M.P. Results of an elispot proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res. Hum. Retroviruses 2005, 21, 68–81. [Google Scholar] [CrossRef]
- Weinberg, A.; Song, L.Y.; Wilkening, C.; Sevin, A.; Blais, B.; Louzao, R.; Stein, D.; Defechereux, P.; Durand, D.; Riedel, E.; et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic t-cell characterization. Clin. Vaccine Immunol. 2009, 16, 1176–1186. [Google Scholar] [CrossRef]
- Gill, D.K.; Huang, Y.; Levine, G.L.; Sambor, A.; Carter, D.K.; Sato, A.; Kopycinski, J.; Hayes, P.; Hahn, B.; Birungi, J.; et al. Equivalence of elispot assays demonstrated between major hiv network laboratories. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Almeida, C.A.; Roberts, S.G.; Laird, R.; McKinnon, E.; Ahmed, I.; Pfafferott, K.; Turley, J.; Keane, N.M.; Lucas, A.; Rushton, B.; et al. Automation of the elispot assay for high-throughput detection of antigen-specific t-cell responses. J. Immunol. Methods 2009, 344, 1–5. [Google Scholar] [CrossRef]
- Trubiani, O.; Salvolini, E.; Staffolani, R.; di Primio, R.; Mazzanti, L. Dmso modifies structural and functional properties of rpmi-8402 cells by promoting programmed cell death. Int. J. Immunopathol. Pharmacol. 2003, 16, 253–259. [Google Scholar]
- Trubiani, O.; Ciancarelli, M.; Rapino, M.; Di Primio, R. Dimethyl sulfoxide induces programmed cell death and reversible g1 arrest in the cell cycle of human lymphoid pre-t cell line. Immunol. Lett. 1996, 50, 51–57. [Google Scholar] [CrossRef]
- Suneetha, P.V.; Schlaphoff, V.; Wang, C.; Stegmann, K.A.; Fytili, P.; Sarin, S.K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Effect of peptide pools on effector functions of antigen-specific cd8+ t cells. J. Immunol. Methods 2009, 342, 33–48. [Google Scholar] [CrossRef]
- Baust, J.M.; Van Buskirk, R.; Baust, J.G. Modulation of the cryopreservation cap: Elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology 2002, 45, 97–108. [Google Scholar] [CrossRef]
- Deng, Y.; Jing, Y.; Campbell, A.E.; Gravenstein, S. Age-related impaired type 1 t cell responses to influenza: Reduced activation ex vivo, decreased expansion in ctl culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J. Immunol. 2004, 172, 3437–3446. [Google Scholar]
- Mahnke, Y.D.; Saqr, A.; Hazenfeld, S.; Brady, R.C.; Roederer, M.; Subbramanian, R.A. Age-related changes in durability and function of vaccine-elicited influenza-specific cd4(+) t-cell responses. Vaccine 2011, 29, 8606–8614. [Google Scholar]
- Sasaki, S.; He, X.S.; Holmes, T.H.; Dekker, C.L.; Kemble, G.W.; Arvin, A.M.; Greenberg, H.B. Influence of prior influenza vaccination on antibody and b-cell responses. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Russell, N.D.; Hudgens, M.G.; Ha, R.; Havenar-Daughton, C.; McElrath, M.J. Moving to human immunodeficiency virus type 1 vaccine efficacy trials: Defining t cell responses as potential correlates of immunity. J. Infect. Dis. 2003, 187, 226–242. [Google Scholar] [CrossRef]
- Ghanekar, S.A.; Bhatia, S.; Ruitenberg, J.J.; DeLa Rosa, C.; Disis, M.L.; Maino, V.C.; Maecker, H.T.; Waters, C.A. Phenotype and in vitro function of mature mddc generated from cryopreserved pbmc of cancer patients are equivalent to those from healthy donors. J. Immune Based Ther. Vaccines 2007, 5. [Google Scholar] [CrossRef]
- Lehmann, P.V.; Zhang, W. Unique strengths of elispot for t cell diagnostics. Methods Mol. Biol. 2012, 792, 3–23. [Google Scholar] [CrossRef]
- Addo, M.M.; Yu, X.G.; Rathod, A.; Cohen, D.; Eldridge, R.L.; Strick, D.; Johnston, M.N.; Corcoran, C.; Wurcel, A.G.; Fitzpatrick, C.A.; et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (hiv-1)-specific t-cell responses directed against the entire expressed hiv-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 2003, 77, 2081–2092. [Google Scholar]
- Moss, R.B.; Diveley, J.; Jensen, F.C.; Gouveia, E.; Savary, J.; Carlo, D.J. Hiv-specific cd4(+) and cd8(+) immune responses are generated with a gp120-depleted, whole-killed hiv-1 immunogen with cpg immunostimulatory sequences of DNA. J. Interferon. Cytokine Res. 2000, 20, 1131–1137. [Google Scholar] [CrossRef]
- Subbramanian, R.A.; Basha, S.; Shata, M.T.; Brady, R.C.; Bernstein, D.I. Pandemic and seasonal h1n1 influenza hemagglutinin-specific t cell responses elicited by seasonal influenza vaccination. Vaccine 2010, 28, 8258–8267. [Google Scholar]
- Kloverpris, H.; Fomsgaard, A.; Handley, A.; Ackland, J.; Sullivan, M.; Goulder, P. Dimethyl sulfoxide (dmso) exposure to human peripheral blood mononuclear cells (pbmcs) abolish t cell responses only in high concentrations and following coincubation for more than two hours. J. Immunol. Methods 2010, 356, 70–78. [Google Scholar] [CrossRef]
- Kiecker, F.; Streitz, M.; Ay, B.; Cherepnev, G.; Volk, H.D.; Volkmer-Engert, R.; Kern, F. Analysis of antigen-specific t-cell responses with synthetic peptides—What kind of peptide for which purpose? Hum. Immunol. 2004, 65, 523–536. [Google Scholar]
- Lehmann, P.V. Image analysis and data management of elispot assay results. Methods Mol. Biol. 2005, 302, 117–132. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ramachandran, H.; Laux, J.; Moldovan, I.; Caspell, R.; Lehmann, P.V.; Subbramanian, R.A. Optimal Thawing of Cryopreserved Peripheral Blood Mononuclear Cells for Use in High-Throughput Human Immune Monitoring Studies. Cells 2012, 1, 313-324. https://doi.org/10.3390/cells1030313
Ramachandran H, Laux J, Moldovan I, Caspell R, Lehmann PV, Subbramanian RA. Optimal Thawing of Cryopreserved Peripheral Blood Mononuclear Cells for Use in High-Throughput Human Immune Monitoring Studies. Cells. 2012; 1(3):313-324. https://doi.org/10.3390/cells1030313
Chicago/Turabian StyleRamachandran, Hari, Jessica Laux, Ioana Moldovan, Richard Caspell, Paul V. Lehmann, and Ramu A. Subbramanian. 2012. "Optimal Thawing of Cryopreserved Peripheral Blood Mononuclear Cells for Use in High-Throughput Human Immune Monitoring Studies" Cells 1, no. 3: 313-324. https://doi.org/10.3390/cells1030313
APA StyleRamachandran, H., Laux, J., Moldovan, I., Caspell, R., Lehmann, P. V., & Subbramanian, R. A. (2012). Optimal Thawing of Cryopreserved Peripheral Blood Mononuclear Cells for Use in High-Throughput Human Immune Monitoring Studies. Cells, 1(3), 313-324. https://doi.org/10.3390/cells1030313




