Additive Effects of Mechanical Marrow Ablation and PTH Treatment on de Novo Bone Formation in Mature Adult Rats
Abstract
:1. Introduction
2. Results and Discussion
| Group | Body weight (g) | |
|---|---|---|
| Day 1 | Day 21 | |
| Baseline | 506.1 ± 60.4 | - |
| Control | 608.3 ± 55.5 | 631.3 ± 54.4 |
| bmx + PBS | 540.0 ± 65.4 | 573.1 ± 58.7 |
| bmx + PTH | 580.0 ± 61.5 | 602.4 ± 50.4 |
2.1. Imaging Analysis
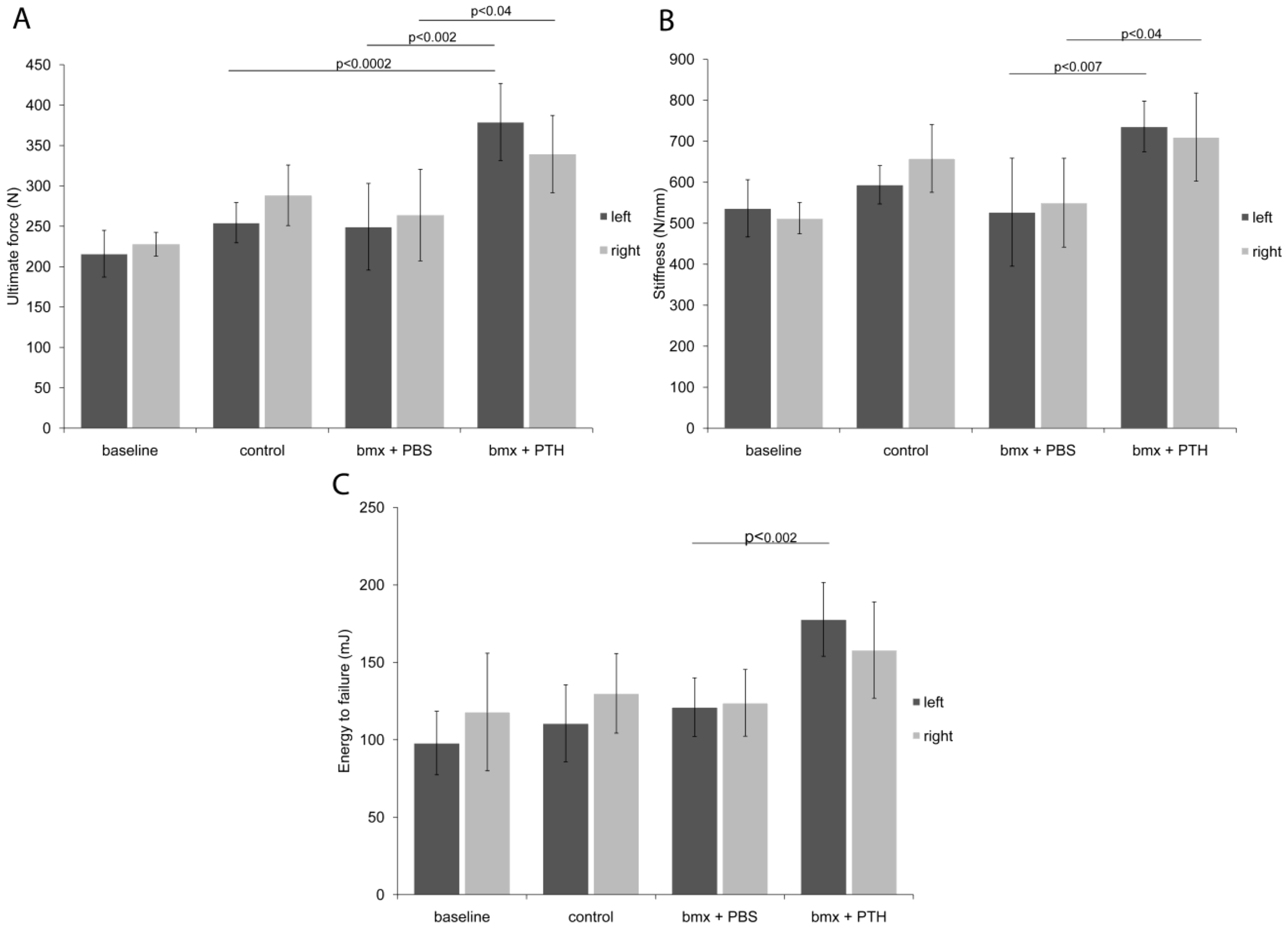
2.2. Biomechanical Testing
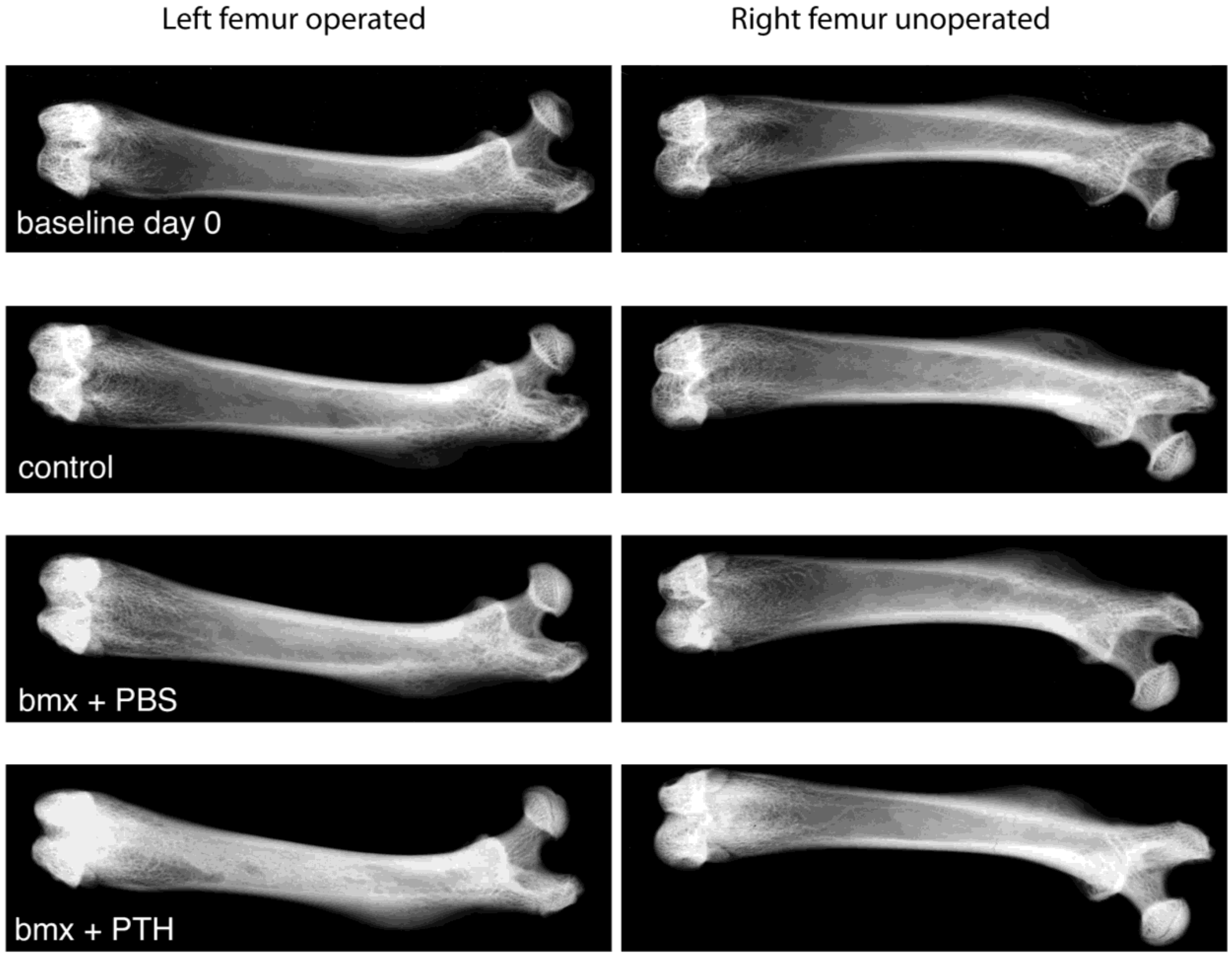
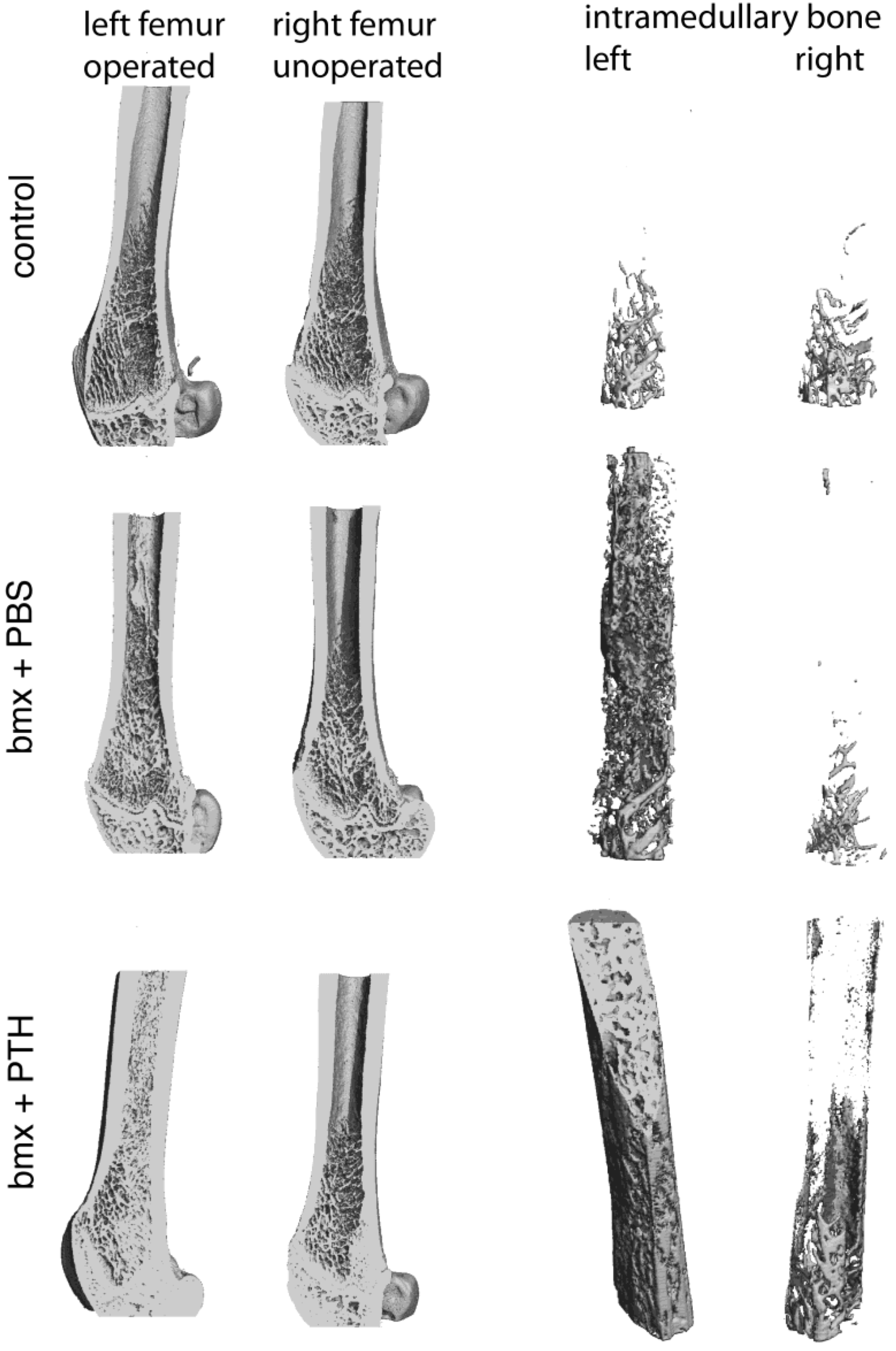
| Group | Total | |||
|---|---|---|---|---|
| Content (mg) | Density (mg/cm3) | Area (mm2) | ||
| Left femur | Baseline | 12.8 ± 1.3 | 934.5 ± 78.5 | 13.8 ± 2.0 |
| Control | 16.4 ± 1.9 a | 973.1 ± 42.1 a | 16.8 ± 1.9 a | |
| bmx + PBS | 15.2 ± 2.2 | 996.0 ± 62.0 | 15.2 ± 1.7 | |
| bmx + PTH | 18.9 ± 2.5 bcd | 1166.2 ± 68.5 bcd | 16.2 ± 1.8 | |
| Right femur | Baseline | 13.0 ± 1.3 | 936.1 ± 80.9 | 14.0 ± 2.1 |
| Control | 16.2 ± 1.9 | 964.3 ± 45.2 | 16.8 ± 1.8 | |
| bmx + PBS | 14.6 ± 1.9 | 973.5 ± 57.5 | 15.0 ± 1.6 | |
| bmx + PTH | 16.3 ± 2.4 | 1013.5 ± 75.1 | 16.0 ± 1.6 | |
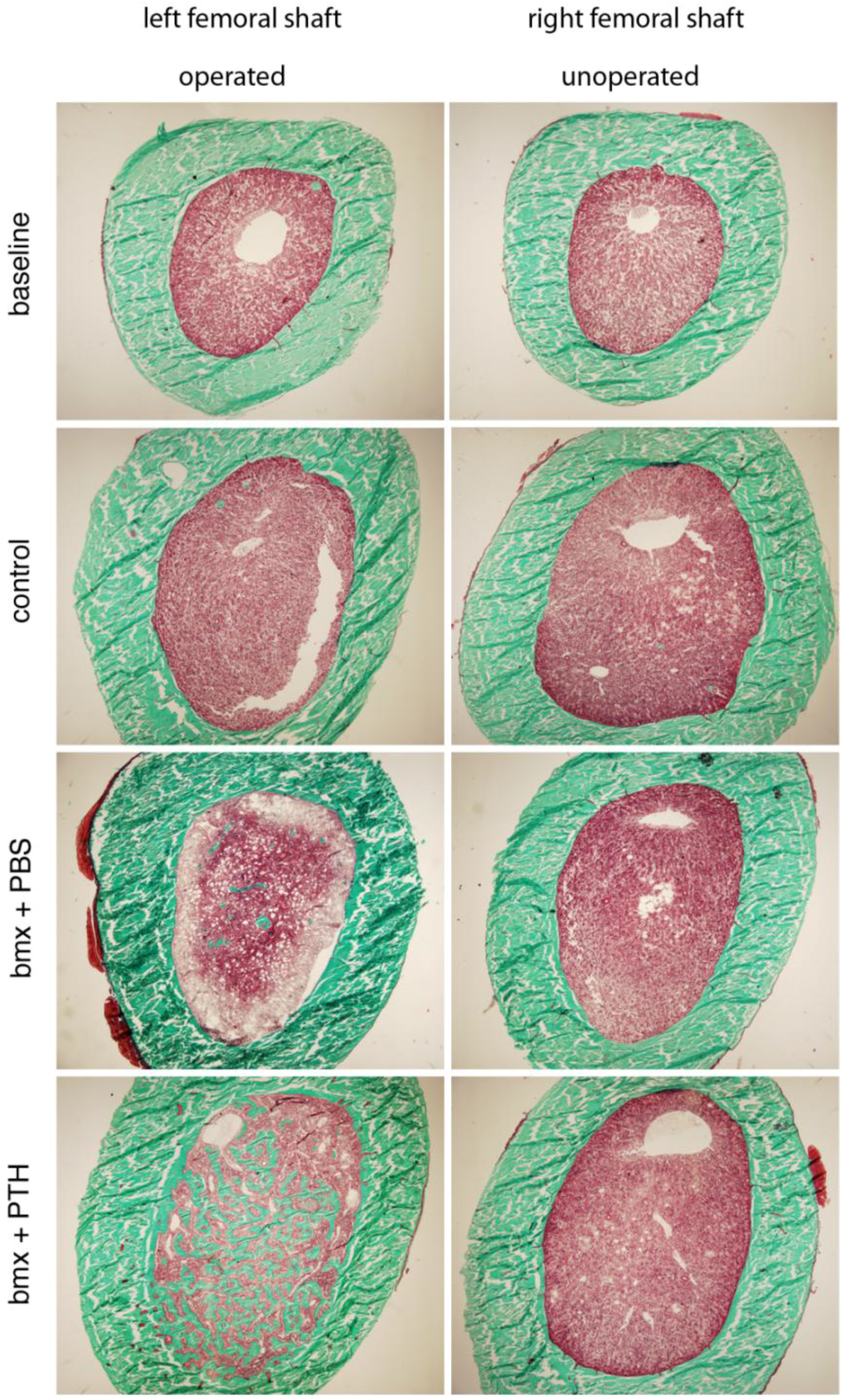
2.3. Histomorphometry

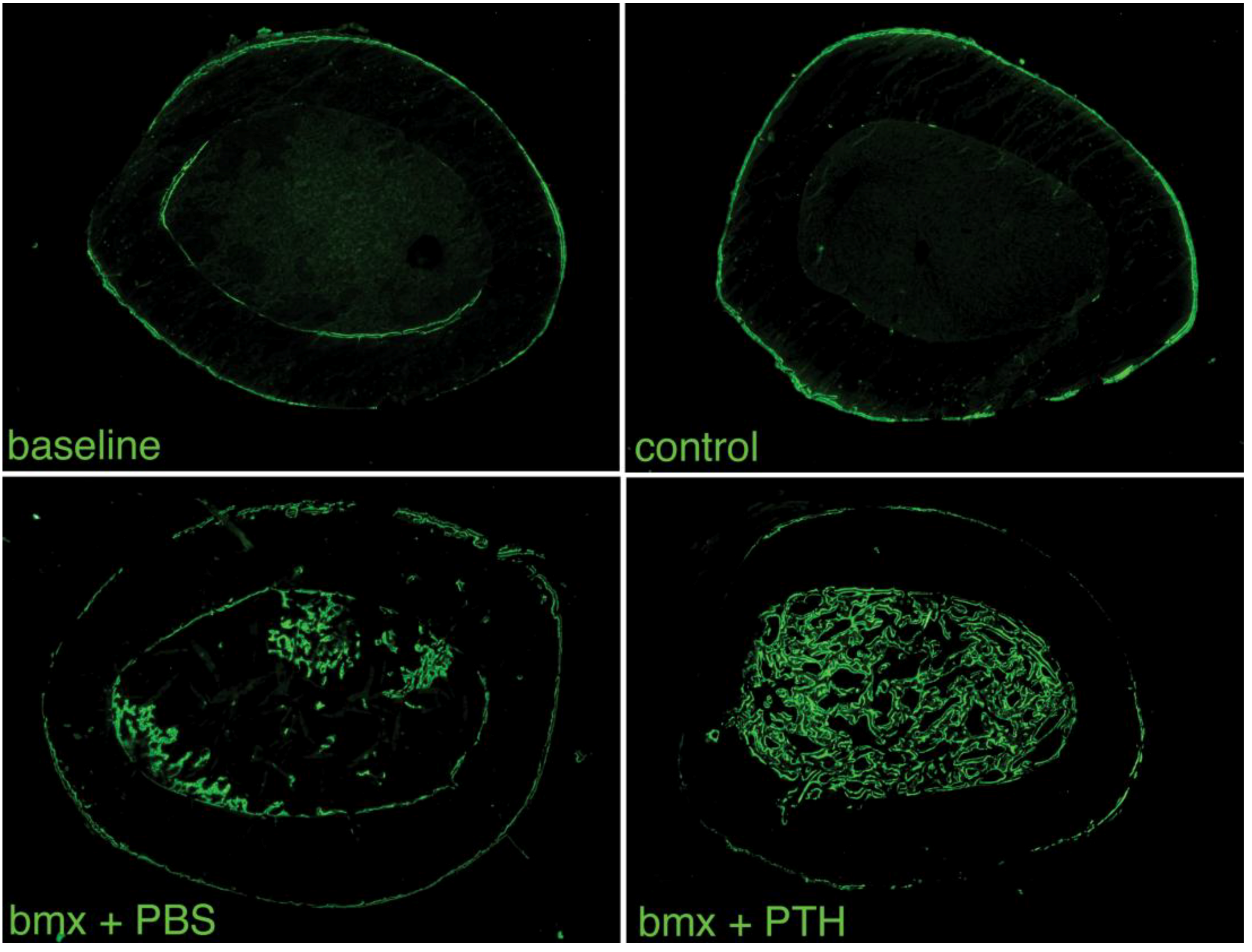
2.4. Serum Osteocalcin
| Group | Osteocalcin (mg/mL) | Level of Significance |
|---|---|---|
| Baseline | 58.4 ± 9.7 | p < 0.007 vs. Control |
| Control | 44.0 ± 10.9 | p < 0.0002 vs. bmx + PTH; p < 0.007 vs. Baseline |
| bmx + PBS | 45.9 ± 8.3 | p < 0.0001 vs. bmx + PTH |
| bmx + PTH | 61.8 ± 8.7 | p < 0.0001 vs. PTH; p < 0.0002 vs. Control |
3. Experimental
3.1. Animals
3.2. Experimental Design
3.3. Femoral Bone Marrow Ablation Procedure
3.4. PTH or PBS Administration
3.5. Bone Radiography
3.6. Bone Densitometry by Peripheral Quantitative Computed Tomography (pQCT)
3.7. Computed Tomography on a Microscale (microCT)
3.8. Biomechanical Testing of the Femoral Midshaft: Three-Point Bending Test
3.9. Histology
3.10. Microscopy
3.11. Biochemical Parameters
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Reeve, J.; Hesp, R.; Williams, D.; Hulme, P.; Klenerman, L.; Zanelli, J.M.; Darby, A.J.; Tregear, G.W.; Parsons, J.A. Anabolic effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. Lancet 1976, 1, 1035–1038. [Google Scholar]
- Zhang, Q.; Cuartas, E.; Mehta, N.; Gilligan, J.; Ke, H.Z.; Saltzman, W.M.; Kotas, M.; Ma, M.; Rajan, S.; Chalouni, C.; et al. Replacement of bone marrow by bone in rat femurs: The bone bioreactor. Tissue Eng. Part A 2008, 14, 237–246. [Google Scholar] [CrossRef]
- Zhang, Q.; Carlson, J.; Ke, H.Z.; Li, J.; Kim, M.; Murphy, K.; Mehta, N.; Gilligan, J.; Vignery, A. Dramatic increase in cortical thickness induced by femoral marrow ablation followed by a 3-month treatment with PTH in rats. J. Bone Miner. Res. 2010, 25, 1350–1359. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ozaki, K.; Suketa, Y. Alteration in bone metabolism with increasing age: Effects of zinc and vitamin D3 in aged rats. J. Pharmacobio-Dynam. 1989, 12, 67–73. [Google Scholar] [CrossRef]
- Riggs, B.L.; Melton, L.J., 3rd. The prevention and treatment of osteoporosis. N. Engl. J. Med. 1992, 327, 620–627. [Google Scholar] [CrossRef]
- Liang, C.T.; Barnes, J.; Seedor, J.G.; Quartuccio, H.A.; Bolander, M.; Jeffrey, J.J.; Rodan, G.A. Impaired bone activity in aged rats: Alterations at the cellular and molecular levels. Bone 1992, 13, 435–441. [Google Scholar] [CrossRef]
- Baumann, B.D.; Wronski, T.J. Response of cortical bone to antiresorptive agents and parathyroid hormone in aged ovariectomized rats. Bone 1995, 16, 247–253. [Google Scholar] [CrossRef]
- Friedl, G.; Turner, R.T.; Evans, G.L.; Dobnig, H. Intermittent parathyroid hormone (PTH) treatment and age-dependent effects on rat cancellous bone and mineral metabolism. J. Orthop. Res. 2007, 25, 1454–1464. [Google Scholar] [CrossRef]
- Qi, H.; Li, M.; Wronski, T.J. A comparison of the anabolic effects of parathyroid hormone at skeletal sites with moderate and severe osteopenia in aged ovariectomized rats. J. Bone Miner. Res. 1995, 10, 948–955. [Google Scholar]
- Tsuji, K.; Komori, T.; Noda, M. Aged mice require full transcription factor, Runx2/Cbfa1, gene dosage for cancellous bone regeneration after bone marrow ablation. J. Bone Miner. Res. 2004, 19, 1481–1489. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Gevers, G.; Raymaekers, G.; Devos, P.; Dequeker, J. Sex- and age-related changes in bone and serum osteocalcin. Calcified Tissue Int. 1990, 46, 179–182. [Google Scholar] [CrossRef]
- Fraher, L.J.; Avram, R.; Watson, P.H.; Hendy, G.N.; Henderson, J.E.; Chong, K.L.; Goltzman, D.; Morley, P.; Willick, G.E.; Whitfield, J.F.; et al. Comparison of the biochemical responses to human parathyroid hormone-(1–31)NH2 and hPTH-(1–34) in healthy humans. J. Clin. Endocrinol. Metab. 1999, 84, 2739–2743. [Google Scholar] [CrossRef]
- Ballica, R.; Valentijn, K.; Khachatryan, A.; Guerder, S.; Kapadia, S.; Gundberg, C.; Gilligan, J.; Flavell, R.A.; Vignery, A. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J. Bone Miner. Res. 1999, 14, 1067–1074. [Google Scholar] [CrossRef]
- Baron, R.; Tross, R.; Vignery, A. Evidence of sequential remodeling in rat trabecular bone: Morphology, dynamic histomorphometry, and changes during skeletal maturation. Anat. Rec. 1984, 208, 137–145. [Google Scholar] [CrossRef]
- Vignery, A.; Baron, R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat. Rec. 1980, 196, 191–200. [Google Scholar] [CrossRef]
- Gundberg, C.M.; Hauschka, P.V.; Lian, J.B.; Gallop, P.M. Osteocalcin: Isolation, characterization, and detection. Methods Enzymol. 1984, 107, 516–544. [Google Scholar]
- Carlson, J.; Zhang, Q.; Bennett, A.; Vignery, A. Deletion of mitogen-activated protein kinase phosphatase 1 modifies the response to mechanical bone marrow ablation in a mouse model. Comp. Med. 2009, 59, 221–226. [Google Scholar]
- Amsel, S.; Maniatis, A.; Tavassoli, M.; Crosby, W.H. The significance of intramedullary cancellous bone formation in the repair of bone marrow tissue. Anat. Rec. 1969, 164, 101–111. [Google Scholar] [CrossRef]
- Bab, I.A. Postablation bone marrow regeneration: an in vivo model to study differential regulation of bone formation and resorption. Bone 1995, 17, 437S–441S. [Google Scholar]
- Patt, H.M.; Maloney, M.A. Bone marrow regeneration after local injury: A review. Exp. Hematol. 1975, 3, 135–148. [Google Scholar]
- Dempster, D.W.; Cosman, F.; Parisien, M.; Shen, V.; Lindsay, R. Anabolic actions of parathyroid hormone on bone. Endocr. Rev. 1993, 14, 690–709. [Google Scholar]
- Recker, R.R.; Bare, S.P.; Smith, S.Y.; Varela, A.; Miller, M.A.; Morris, S.A.; Fox, J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone 2009, 44, 113–119. [Google Scholar]
- Wise, J.K.; Sena, K.; Vranizan, K.; Pollock, J.F.; Healy, K.E.; Hughes, W.F.; Sumner, D.R.; Virdi, A.S. Temporal gene expression profiling during rat femoral marrow ablation-induced intramembranous bone regeneration. PLoS One 2010. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Q.; Miller, C.; Bible, J.; Li, J.; Xu, X.; Mehta, N.; Gilligan, J.; Vignery, A.; Scholz, J.A.C. Additive Effects of Mechanical Marrow Ablation and PTH Treatment on de Novo Bone Formation in Mature Adult Rats. Cells 2012, 1, 1168-1181. https://doi.org/10.3390/cells1041168
Zhang Q, Miller C, Bible J, Li J, Xu X, Mehta N, Gilligan J, Vignery A, Scholz JAC. Additive Effects of Mechanical Marrow Ablation and PTH Treatment on de Novo Bone Formation in Mature Adult Rats. Cells. 2012; 1(4):1168-1181. https://doi.org/10.3390/cells1041168
Chicago/Turabian StyleZhang, Qing, Christopher Miller, Jesse Bible, Jiliang Li, Xiaoqing Xu, Nozer Mehta, James Gilligan, Agnès Vignery, and Jodi A. Carlson Scholz. 2012. "Additive Effects of Mechanical Marrow Ablation and PTH Treatment on de Novo Bone Formation in Mature Adult Rats" Cells 1, no. 4: 1168-1181. https://doi.org/10.3390/cells1041168




