Inflammaging, an Imbalanced Immune Response That Needs to Be Restored for Cancer Prevention and Treatment in the Elderly
Abstract
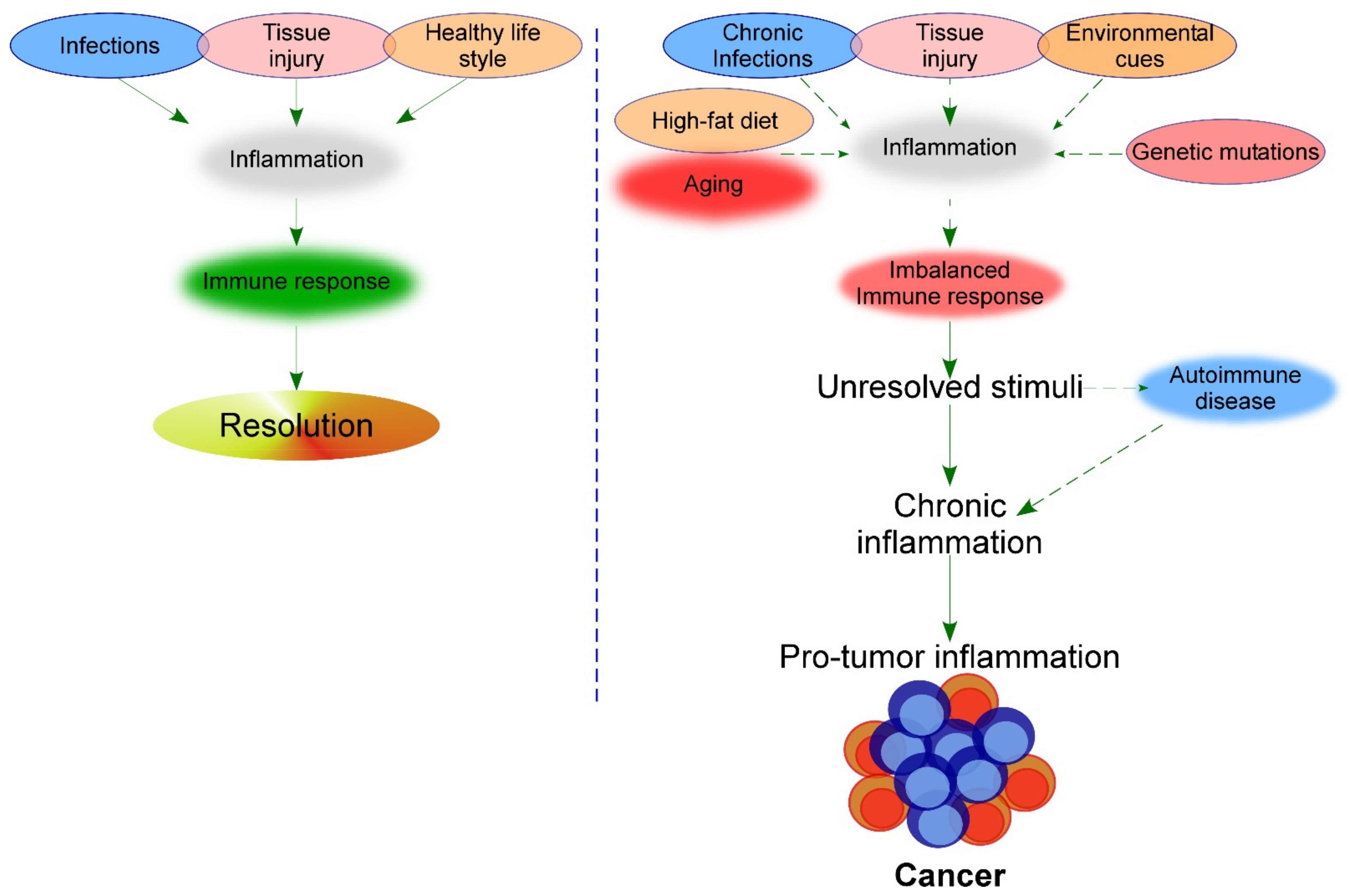
:1. Introduction: Immunosenescence and Inflammation during Aging, and its Consequences in Cancer and Other Age-Related Diseases
2. Variation of T-reg Cells and Th17 Cells during Aging and Their Impact on the Development of Inflammaging
2.1. Changes in the T-reg Cell Compartment during Aging and Impact in Inflammation and Cancer
2.2. Th17 Compartment and Its Delicate Balance with T-reg Cells
2.3. Changes in the Th17 Compartment during Aging and Implications for Autoimmunity and Cancer
3. Variation of NK Cells during Aging and Cancer and Their Impact on the Development of Inflammation
3.1. Immunoregulatory Activity of CD56bright NK Cells
3.2. Variation of NK Cells in Aging and Cancer
4. Impact of NLRP3 and Granzymes in Pyroptosis during the Immune Response and the Development of Inflammation
4.1. Impact of NLRP3 in Inflammatory Diseases
4.2. Impact of NLRP3 in Immunosenescence and Adoptive Cellular Immunotherapy in Cancer
4.3. Impact of Inflammatory Granzymes Released by Immune Cells on Inflammaging and the Immune Response
5. Association of Intrinsic and External Triggers of Inflammation with Cancer
5.1. Diet
5.2. Air Pollution
5.3. Genomic Instability
6. Preclinical Studies That Associate Inflammation with the Development of Different Typesof Cancer
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing Europe. Available online: https://ec.europa.eu/eurostat/web/products-statistical-books/-/ks-02-19-681 (accessed on 23 September 2021).
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, F. The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harb. Perspect. Med. 2016, 6, a025163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.-M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Drabkin, M.J.; Meyer, J.I.; Kanth, N.; Lobel, S.; Fogel, J.; Grossman, J.; Krumenacker, J.H. Age-Stratified Patterns of Thymic Involution on Multidetector CT. J. Thorac. Imaging 2018, 33, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Cho, W.C.; Ben-Yehuda, A. Cancer and Aging—The Inflammatory Connection. Aging Dis. 2017, 8, 611–627. [Google Scholar] [CrossRef] [Green Version]
- Coder, B.D.; Wang, H.; Ruan, L.; Su, D.-M. Thymic Involution Perturbs Negative Selection Leading to Autoreactive T Cells That Induce Chronic Inflammation. J. Immunol. 2015, 194, 5825–5837. [Google Scholar] [CrossRef] [Green Version]
- Fröbel, J.; Landspersky, T.; Percin, G.; Schreck, C.; Rahmig, S.; Ori, A.; Nowak, D.; Essers, M.; Waskow, C.; Oostendorp, R.A.J. The Hematopoietic Bone Marrow Niche Ecosystem. Front. Cell Dev. Biol. 2021, 9, 705410. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Teixeira, V.R.; Alencar-Silva, T.; Simonassi-Paiva, B.; Pereira, R.W.; Pogue, R.; Carvalho, J.L. Hallmarks of Aging and Immunosenescence: Connecting the Dots. Cytokine Growth Factor Rev. 2021, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Beerman, I.; Maloney, W.J.; Weissmann, I.L.; Rossi, D.J. Stem Cells and the Aging Hematopoietic System. Curr. Opin. Immunol. 2010, 22, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Wang, Q.; Xie, Z.; Li, J. The Elevated Level of IL-1α in the Bone Marrow of Aged Mice Leads to MSC Senescence Partly by down-Regulating Bmi-1. Exp. Gerontol. 2021, 148, 111313. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Zhang, B.; Jia, X. IL-6 Regulates the Bone Metabolism and Inflammatory Microenvironment in Aging Mice by Inhibiting Setd7. Acta Histochem. 2021, 123, 151718. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, T.; Takemura, Y.; Goukassian, D.; Walsh, K. Ageing Is Associated with Diminished Apoptotic Cell Clearance in Vivo. Clin. Exp. Immunol. 2008, 152, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Gasparoto, T.H.; Dalboni, T.M.; Amôr, N.G.; Abe, A.E.; Perri, G.; Lara, V.S.; Vieira, N.A.; Gasparoto, C.T.; Campanelli, A.P. Fcγ Receptors on Aging Neutrophils. J. Appl. Oral. Sci. 2021, 29, e20200770. [Google Scholar] [CrossRef]
- Van Beek, A.A.; Van den Bossche, J.; Mastroberardino, P.G.; de Winther, M.P.J.; Leenen, P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019, 40, 113–127. [Google Scholar] [CrossRef]
- Solana, R.; Campos, C.; Pera, A.; Tarazona, R. Shaping of NK Cell Subsets by Aging. Curr. Opin. Immunol. 2014, 29, 56–61. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Jeyapalan, J.C.; Ferreira, M.; Sedivy, J.M.; Herbig, U. Accumulation of Senescent Cells in Mitotic Tissue of Aging Primates. Mech. Ageing Dev. 2007, 128, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired Immune Surveillance Accelerates Accumulation of Senescent Cells and Aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and Tumour Clearance Is Triggered by P53 Restoration in Murine Liver Carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagiv, A.; Biran, A.; Yon, M.; Simon, J.; Lowe, S.W.; Krizhanovsky, V. Granule Exocytosis Mediates Immune Surveillance of Senescent Cells. Oncogene 2013, 32, 1971–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battram, A.M.; Bachiller, M.; Martín-Antonio, B. Senescence in the Development and Response to Cancer with Immunotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2020, 21, 4346. [Google Scholar] [CrossRef]
- Tsukishiro, T.; Donnenberg, A.D.; Whiteside, T.L. Rapid Turnover of the CD8(+)CD28(-) T-Cell Subset of Effector Cells in the Circulation of Patients with Head and Neck Cancer. Cancer Immunol. Immunother. 2003, 52, 599–607. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Hoare, M.; Narita, M. Transmitting Senescence to the Cell Neighbourhood. Nat. Cell Biol 2013, 15, 887–889. [Google Scholar] [CrossRef]
- Onyema, O.O.; Decoster, L.; Njemini, R.; Forti, L.N.; Bautmans, I.; De Waele, M.; Mets, T. Chemotherapy-Induced Changes and Immunosenescence of CD8+ T-Cells in Patients with Breast Cancer. Anticancer Res. 2015, 35, 1481–1489. [Google Scholar]
- Bruni, E.; Cazzetta, V.; Donadon, M.; Cimino, M.; Torzilli, G.; Spata, G.; Leonardi, G.; Dieli, F.; Mikulak, J.; Mavilio, D. Chemotherapy Accelerates Immune-Senescence and Functional Impairments of Vδ2pos T Cells in Elderly Patients Affected by Liver Metastatic Colorectal Cancer. J. Immunother. Cancer 2019, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Martín-Antonio, B.; Granell, M.; Urbano-Ispizua, A. Genomic Polymorphisms of the Innate Immune System and Allogeneic Stem Cell Transplantation. Expert Rev. Hematol. 2010, 3, 411–427. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A Proteomic Atlas of Senescence-Associated Secretomes for Aging Biomarker Development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [Green Version]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and Metaflammation: The Yin and Yang of Type 2 Diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aunan, J.R.; Cho, W.C.; Søreide, K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; An, Y.; Zoli, M.; Simonsick, E.M.; Guralnik, J.M.; Bandinelli, S.; Boyd, C.M.; Ferrucci, L. Aging and the Burden of Multimorbidity: Associations with Inflammatory and Anabolic Hormonal Biomarkers. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 63–70. [Google Scholar] [CrossRef]
- Masters, S.L.; Dunne, A.; Subramanian, S.L.; Hull, R.L.; Tannahill, G.M.; Sharp, F.A.; Becker, C.; Franchi, L.; Yoshihara, E.; Chen, Z.; et al. Activation of the NLRP3 Inflammasome by Islet Amyloid Polypeptide Provides a Mechanism for Enhanced IL-1β in Type 2 Diabetes. Nat. Immunol. 2010, 11, 897–904. [Google Scholar] [CrossRef]
- Stienstra, R.; Joosten, L.A.B.; Koenen, T.; van Tits, B.; van Diepen, J.A.; van den Berg, S.A.A.; Rensen, P.C.N.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The Inflammasome-Mediated Caspase-1 Activation Controls Adipocyte Differentiation and Insulin Sensitivity. Cell Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Van’t Klooster, C.C.; Ridker, P.M.; Hjortnaes, J.; van der Graaf, Y.; Asselbergs, F.W.; Westerink, J.; Aerts, J.G.J.V.; Visseren, F.L.J. On behalf of the UCC-SMART study group The Relation between Systemic Inflammation and Incident Cancer in Patients with Stable Cardiovascular Disease: A Cohort Study. Eur. Heart J. 2019, 40, 3901–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkilä, K.; Harris, R.; Lowe, G.; Rumley, A.; Yarnell, J.; Gallacher, J.; Ben-Shlomo, Y.; Ebrahim, S.; Lawlor, D.A. Associations of Circulating C-Reactive Protein and Interleukin-6 with Cancer Risk: Findings from Two Prospective Cohorts and a Meta-Analysis. Cancer Causes Control 2009, 20, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Föger-Samwald, U.; Ellinger, I. Immunology of Osteoporosis: A Mini-Review. Gerontology 2016, 62, 128–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldner, M.J.; Neurath, M.F. Colitis-Associated Cancer: The Role of T Cells in Tumor Development. Semin. Immunopathol. 2009, 31, 249–256. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, J.; Chen, K.; Ma, P.; Lei, Q.; Xing, S.; Cao, Z.; Sun, S.; Yu, Z.; Liu, Y.; et al. Perspectives of Tumor-Infiltrating Lymphocyte Treatment in Solid Tumors. BMC Med. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Martin-Antonio, B.; Yang, H.; Ku, S.; Lee, D.A.; Cooper, L.J.N.; Decker, W.K.; Li, S.; Robinson, S.N.; Sekine, T.; et al. Antigen Presenting Cell-Mediated Expansion of Human Umbilical Cord Blood Yields Log-Scale Expansion of Natural Killer Cells with Anti-Myeloma Activity. PLoS One 2013, 8, e76781. [Google Scholar] [CrossRef]
- Bachiller, M.; Battram, A.M.; Perez-Amill, L.; Martín-Antonio, B. Natural Killer Cells in Immunotherapy: Are We Nearly There? Cancers 2020, 12, 3139. [Google Scholar] [CrossRef]
- Martín-Antonio, B.; Suñe, G.; Najjar, A.; Perez-Amill, L.; Antoñana-Vildosola, A.; Castella, M.; León, S.; Velasco-de Andrés, M.; Lozano, F.; Lozano, E.; et al. Extracellular NK Histones Promote Immune Cell Anti-Tumor Activity by Inducing Cell Clusters through Binding to CD138 Receptor. J. Immunother. Cancer 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Chen, X.; Wang, Z.; Liang, X.; Zhang, T.; Liu, M.; Zhou, N.; Lv, J.; Tang, K.; et al. Gasdermin E-Mediated Target Cell Pyroptosis by CAR T Cells Triggers Cytokine Release Syndrome. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xuan, B.; Liu, Y.; Wang, L.; He, L.; Meng, X.; Zhou, T.; Wang, Y. Updating the NLRC4 Inflammasome: From Bacterial Infections to Autoimmunity and Cancer. Front. Immunol. 2021, 12, 702527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from Cytotoxic Lymphocytes Cleaves GSDMB to Trigger Pyroptosis in Target Cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef]
- Martín-Antonio, B.; Suñe, G.; Perez-Amill, L.; Castella, M.; Urbano-Ispizua, A. Natural Killer Cells: Angels and Devils for Immunotherapy. Int. J. Mol. Sci. 2017, 18, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Amill, L.; Marzal, B.; Urbano-Ispizua, A.; Juan, M.; Martín-Antonio, B. CAR-T Cell Therapy: A Door Is Open to Find Innumerable Possibilities of Treatments for Cancer Patients. Turk. J. Haematol. 2018, 35, 217–228. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Bucci, L.; Ostan, R.; Giampieri, E.; Cevenini, E.; Pini, E.; Scurti, M.; Vescovini, R.; Sansoni, P.; Caruso, C.; Mari, D.; et al. Immune Parameters Identify Italian Centenarians with a Longer Five-Year Survival Independent of Their Health and Functional Status. Exp. Gerontol. 2014, 54, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Santos-Lozano, A.; Valenzuela, P.L.; Llavero, F.; Lista, S.; Carrera-Bastos, P.; Hampel, H.; Pareja-Galeano, H.; Gálvez, B.G.; López, J.A.; Vázquez, J.; et al. Successful Aging: Insights from Proteome Analyses of Healthy Centenarians. Aging 2020, 12, 3502–3515. [Google Scholar] [CrossRef]
- Zelle-Rieser, C.; Thangavadivel, S.; Biedermann, R.; Brunner, A.; Stoitzner, P.; Willenbacher, E.; Greil, R.; Jöhrer, K. T Cells in Multiple Myeloma Display Features of Exhaustion and Senescence at the Tumor Site. J. Hematol. Oncol. 2016, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Zitvogel, L. CD4+ T Cells at the Center of Inflammaging. Cell Metab. 2020, 32, 4–5. [Google Scholar] [CrossRef]
- Thomas, R.; Oh, J.; Wang, W.; Su, D.-M. Thymic Atrophy Creates Holes in Treg-Mediated Immuno-Regulation via Impairment of an Antigen-Specific Clone. Immunology 2021, 163, 478–492. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When Worlds Collide: Th17 and Treg Cells in Cancer and Autoimmunity. Cell Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Fu, J.; Zhou, Y. Metabolism Controls the Balance of Th17/T-Regulatory Cells. Front. Immunol. 2017, 0. [Google Scholar] [CrossRef] [Green Version]
- Feuerer, M.; Hill, J.A.; Mathis, D.; Benoist, C. Foxp3 + Regulatory T Cells: Differentiation, Specification, Subphenotypes. Nat. Immunol. 2009, 10, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T Cells and the Immune Aging Process: A Mini-Review. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lages, C.S.; Suffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional Regulatory T Cells Accumulate in Aged Hosts and Promote Chronic Infectious Disease Reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef] [Green Version]
- Gregg, R.; Smith, C.M.; Clark, F.J.; Dunnion, D.; Khan, N.; Chakraverty, R.; Nayak, L.; Moss, P.A. The Number of Human Peripheral Blood CD4+ CD25high Regulatory T Cells Increases with Age. Clin. Exp. Immunol. 2005, 140, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Moriizumi, E. CD4+CD25- T Cells in Aged Mice Are Hyporesponsive and Exhibit Suppressive Activity. J. Immunol. 2003, 170, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.A. Regulatory T-Cells: Diverse Phenotypes Integral to Immune Homeostasis and Suppression. Toxicol. Pathol. 2012, 40, 186–204. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Dominguez, A.L.; Lustgarten, J. High Accumulation of T Regulatory Cells Prevents the Activation of Immune Responses in Aged Animals. J. Immunol. 2006, 177, 8348–8355. [Google Scholar] [CrossRef]
- Simone, R.; Zicca, A.; Saverino, D. The Frequency of Regulatory CD3+CD8+CD28- CD25+ T Lymphocytes in Human Peripheral Blood Increases with Age. J. Leukoc. Biol. 2008, 84, 1454–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression — Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wan, Z.; Gao, X.; Yang, G.; Liu, L. Reprogramming Immune Cells for Enhanced Cancer Immunotherapy: Targets and Strategies. Front. Immunol. 2021, 12, 609762. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Franco, F.; Tsui, Y.-C.; Xie, X.; Trefny, M.P.; Zappasodi, R.; Mohmood, S.R.; Fernández-García, J.; Tsai, C.-H.; Schulze, I.; et al. CD36-Mediated Metabolic Adaptation Supports Regulatory T Cell Survival and Function in Tumors. Nat. Immunol. 2020, 21, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.R.M.; Rihova, L.; Zahradova, L.; Klincova, M.; Penka, M.; Hajek, R. Increased T Regulatory Cells Are Associated with Adverse Clinical Features and Predict Progression in Multiple Myeloma. PLoS ONE 2012, 7, e47077. [Google Scholar] [CrossRef]
- Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Toubi, E.; Porat, B.-S.; Shoenfeld, Y. Autoimmunity in the Elderly: Insights from Basic Science and Clinics - A Mini-Review. Gerontology 2017, 63, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Barsheshet, Y.; Wildbaum, G.; Levy, E.; Vitenshtein, A.; Akinseye, C.; Griggs, J.; Lira, S.A.; Karin, N. CCR8+FOXp3+ Treg Cells as Master Drivers of Immune Regulation. Proc. Natl. Acad. Sci. USA 2017, 114, 6086–6091. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Hurez, V.J.; Thibodeaux, S.R.; Kious, M.J.; Liu, A.; Lin, P.; Murthy, K.; Pandeswara, S.; Shin, T.; Curiel, T.J. Aged Regulatory T Cells Protect from Autoimmune Inflammation despite Reduced STAT3 Activation and Decreased Constraint of IL-17 Producing T Cells. Aging Cell 2012, 11, 509–519. [Google Scholar] [CrossRef]
- Fan, Y.-G.; Zhai, J.-M.; Wang, W.; Feng, B.; Yao, G.-L.; An, Y.-H.; Zeng, C. IL-35 over-Expression Is Associated with Genesis of Gastric Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 2845–2849. [Google Scholar] [CrossRef] [Green Version]
- Van Herk, E.H.; Velde, A.A. Treg Subsets in Inflammatory Bowel Disease and Colorectal Carcinoma: Characteristics, Role, and Therapeutic Targets. J. Gastroenterol. Hepatol. 2016, 31, 1393–1404. [Google Scholar] [CrossRef]
- Cutler, C.S.; Koreth, J.; Ritz, J. Mechanistic Approaches for the Prevention and Treatment of Chronic GVHD. Blood 2017, 129, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Whangbo, J.S.; Antin, J.H.; Koreth, J. The Role of Regulatory T Cells in Graft-versus-Host Disease Management. Expert Rev. Hematol. 2020, 13, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Qayed, M.; Wang, T.; Hemmer, M.T.; Spellman, S.; Arora, M.; Couriel, D.; Alousi, A.; Pidala, J.; Abdel-Azim, H.; Aljurf, M.; et al. Influence of Age on Acute and Chronic GVHD in Children Undergoing HLA-Identical Sibling Bone Marrow Transplantation for Acute Leukemia: Implications for Prophylaxis. Biol. Blood Marrow Transplant. 2018, 24, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-López, J.; Fernández, J.L.; Lumbreras, E.; Serrano, J.; Martínez-Losada, C.; Martín, C.; Hernández-Rivas, J.M.; Sánchez-García, J. Machine Learning Applied to Gene Expression Analysis of T-Lymphocytes in Patients with CGVHD. Bone Marrow Transplant. 2020, 55, 1668–1670. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Blume, J.; Roy, U.; Teh, P.P.; Vasanthakumar, A.; Beller, A.; Liao, Y.; Heinrich, F.; Arenzana, T.L.; Hackney, J.A.; et al. C-Maf-Dependent Treg Cell Control of Intestinal TH17 Cells and IgA Establishes Host-Microbiota Homeostasis. Nat. Immunol. 2019, 20, 471–481. [Google Scholar] [CrossRef]
- Othy, S.; Jairaman, A.; Dynes, J.L.; Dong, T.X.; Tune, C.; Yeromin, A.V.; Zavala, A.; Akunwafo, C.; Chen, F.; Parker, I.; et al. Regulatory T Cells Suppress Th17 Cell Ca2+ Signaling in the Spinal Cord during Murine Autoimmune Neuroinflammation. PNAS 2020, 117, 20088–20099. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Qin, Y.; Tang, G.; Cai, G.; Liu, Y.; Zhang, J.; Zhang, P.; Shen, Q.; Shen, L.; et al. Th17, Synchronically Increased with Tregs and Bregs, Promoted by Tumour Cells via Cell-Contact in Primary Hepatic Carcinoma. Clin. Exp. Immunol. 2018, 192, 181–192. [Google Scholar] [CrossRef]
- Schmitt, V.; Rink, L.; Uciechowski, P. The Th17/Treg Balance Is Disturbed during Aging. Exp. Gerontol. 2013, 48, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e6. [Google Scholar] [CrossRef]
- Gaur, P.; Qadir, G.A.; Upadhyay, S.; Singh, A.K.; Shukla, N.K.; Das, S.N. Skewed Immunological Balance between Th17 (CD4(+)IL17A (+)) and Treg (CD4 (+)CD25 (+)FOXP3 (+)) Cells in Human Oral Squamous Cell Carcinoma. Cell Oncol. 2012, 35, 335–343. [Google Scholar] [CrossRef]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.-H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.-A.; Lee, J.; Park, J.-S.; Jhun, J.-Y.; Moon, Y.-M.; Cho, M.-L.; Kim, H.-Y. Increased Th17 Differentiation in Aged Mice Is Significantly Associated with High IL-1β Level and Low IL-2 Expression. Exp. Gerontol. 2014, 49, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Prabhala, R.H.; Pelluru, D.; Fulciniti, M.; Prabhala, H.K.; Nanjappa, P.; Song, W.; Pai, C.; Amin, S.; Tai, Y.-T.; Richardson, P.G.; et al. Elevated IL-17 Produced by TH17 Cells Promotes Myeloma Cell Growth and Inhibits Immune Function in Multiple Myeloma. Blood 2010, 115, 5385–5392. [Google Scholar] [CrossRef]
- Dhodapkar, K.M.; Barbuto, S.; Matthews, P.; Kukreja, A.; Mazumder, A.; Vesole, D.; Jagannath, S.; Dhodapkar, M.V. Dendritic Cells Mediate the Induction of Polyfunctional Human IL17-Producing Cells (Th17-1 Cells) Enriched in the Bone Marrow of Patients with Myeloma. Blood 2008, 112, 2878–2885. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2019, 0. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Altomare, E.; Botta, C.; Gallo Cantafio, M.E.; Sarvide, S.; Caracciolo, D.; Riillo, C.; Gaspari, M.; Taverna, D.; Conforti, F.; et al. MiR-21 Antagonism Abrogates Th17 Tumor Promoting Functions in Multiple Myeloma. Leukemia 2021, 35, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Sun, J.; Han, J.; Jiang, X.; Wang, Z.; Chen, L. Interleukin-17 Induces Pyroptosis in Osteoblasts through the NLRP3 Inflammasome Pathway in Vitro. Int. Immunopharmacol. 2021, 96, 107781. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Zhou, X.; Xie, P.; Li, J. A Unique Role of T Helper 17 Cells in Different Treatment Stages of Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Garcia Perez, L.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-Driven Interleukin-17-Producing Cells and Eosinophils Synergize to Accelerate Multiple Myeloma Progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Prabhala, R.H.; Neri, P.; Bae, J.E.; Tassone, P.; Shammas, M.A.; Allam, C.K.; Daley, J.F.; Chauhan, D.; Blanchard, E.; Thatte, H.S.; et al. Dysfunctional T Regulatory Cells in Multiple Myeloma. Blood 2006, 107, 301–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, K.; Marchionni, L.; Anderson, J.; Pardoll, D.; Roodman, G.D.; Borrello, I. A Novel Role of IL-17–Producing Lymphocytes in Mediating Lytic Bone Disease in Multiple Myeloma. Blood 2010, 116, 3554–3563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, D.; Piantanida, E.; Gallazzi, M.; Bartalena, L.; Tanda, M.L.; Bruno, A.; Mortara, L. Immunological Drivers in Graves’ Disease: NK Cells as a Master Switcher. Front. Endocrinol. 2020, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK Cells in Autoimmune Diseases: Linking Innate and Adaptive Immune Responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef]
- Laroni, A.; Armentani, E.; Kerlero de Rosbo, N.; Ivaldi, F.; Marcenaro, E.; Sivori, S.; Gandhi, R.; Weiner, H.L.; Moretta, A.; Mancardi, G.L.; et al. Dysregulation of Regulatory CD56(Bright) NK Cells/T Cells Interactions in Multiple Sclerosis. J. Autoimmun. 2016, 72, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Waggoner, S.N.; Cornberg, M.; Selin, L.K.; Welsh, R.M. Natural Killer Cells Act as Rheostats Modulating Antiviral T Cells. Nature 2011, 481, 394–398. [Google Scholar] [CrossRef]
- Airas, L.; Saraste, M.; Rinta, S.; Elovaara, I.; Huang, Y.-H.; Wiendl, H. Finnish Multiple Sclerosis and Pregnancy Study Group Immunoregulatory Factors in Multiple Sclerosis Patients during and after Pregnancy: Relevance of Natural Killer Cells. Clin. Exp. Immunol. 2008, 151, 235–243. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef]
- Morandi, F.; Horenstein, A.L.; Chillemi, A.; Quarona, V.; Chiesa, S.; Imperatori, A.; Zanellato, S.; Mortara, L.; Gattorno, M.; Pistoia, V.; et al. CD56brightCD16- NK Cells Produce Adenosine through a CD38-Mediated Pathway and Act as Regulatory Cells Inhibiting Autologous CD4+ T Cell Proliferation. J. Immunol. 2015, 195, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, M.; Papewalis, C.; Stenzel, W.; Jacobs, B.; Meyer, K.L.; Deenen, R.; Willenberg, H.S.; Schinner, S.; Thiel, A.; Scherbaum, W.A.; et al. Immunoregulatory Natural Killer Cells Suppress Autoimmunity by Down-Regulating Antigen-Specific CD8+ T Cells in Mice. Endocrinology 2012, 153, 4367–4379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Correa, B.; Campos, C.; Pera, A.; Bergua, J.M.; Arcos, M.J.; Bañas, H.; Casado, J.G.; Morgado, S.; Duran, E.; Solana, R.; et al. Natural Killer Cell Immunosenescence in Acute Myeloid Leukaemia Patients: New Targets for Immunotherapeutic Strategies? Cancer Immunol. Immunother. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Kaszubowska, L.; Foerster, J.; Kaczor, J.J.; Schetz, D.; Ślebioda, T.J.; Kmieć, Z. NK Cells of the Oldest Seniors Represent Constant and Resistant to Stimulation High Expression of Cellular Protective Proteins SIRT1 and HSP70. Immun. Ageing 2018, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barberi, C.; De Pasquale, C.; Allegra, A.; Sidoti Migliore, G.; Oliveri, D.; Loiacono, F.; Innao, V.; Musolino, C.; Pende, D.; Cantoni, C.; et al. Myeloma Cells Induce the Accumulation of Activated CD94low NK Cells by Cell-to-Cell Contacts Involving CD56 Molecules. Blood Adv. 2020, 4, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Chretien, A.-S.; Fauriat, C.; Orlanducci, F.; Galseran, C.; Rey, J.; Bouvier Borg, G.; Gautherot, E.; Granjeaud, S.; Hamel-Broza, J.-F.; Demerle, C.; et al. Natural Killer Defective Maturation Is Associated with Adverse Clinical Outcome in Patients with Acute Myeloid Leukemia. Front. Immunol. 2017, 8, 573. [Google Scholar] [CrossRef]
- Fauriat, C.; Just-Landi, S.; Mallet, F.; Arnoulet, C.; Sainty, D.; Olive, D.; Costello, R.T. Deficient Expression of NCR in NK Cells from Acute Myeloid Leukemia: Evolution during Leukemia Treatment and Impact of Leukemia Cells in NCRdull Phenotype Induction. Blood 2007, 109, 323–330. [Google Scholar] [CrossRef]
- Stringaris, K.; Sekine, T.; Khoder, A.; Alsuliman, A.; Razzaghi, B.; Sargeant, R.; Pavlu, J.; Brisley, G.; de Lavallade, H.; Sarvaria, A.; et al. Leukemia-Induced Phenotypic and Functional Defects in Natural Killer Cells Predict Failure to Achieve Remission in Acute Myeloid Leukemia. Haematologica 2014, 99, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Crinier, A.; Dumas, P.-Y.; Escalière, B.; Piperoglou, C.; Gil, L.; Villacreces, A.; Vély, F.; Ivanovic, Z.; Milpied, P.; Narni-Mancinelli, É.; et al. Single-Cell Profiling Reveals the Trajectories of Natural Killer Cell Differentiation in Bone Marrow and a Stress Signature Induced by Acute Myeloid Leukemia. Cell Mol. Immunol. 2021, 18, 1290–1304. [Google Scholar] [CrossRef]
- Chretien, A.-S.; Devillier, R.; Granjeaud, S.; Cordier, C.; Demerle, C.; Salem, N.; Wlosik, J.; Orlanducci, F.; Gorvel, L.; Fattori, S.; et al. High-Dimensional Mass Cytometry Analysis of NK Cell Alterations in AML Identifies a Subgroup with Adverse Clinical Outcome. Proc. Natl. Acad. Sci. USA 2021, 118, e2020459118. [Google Scholar] [CrossRef]
- Mukherjee, N.; Ji, N.; Hurez, V.; Curiel, T.J.; Montgomery, M.O.; Braun, A.J.; Nicolas, M.; Aguilera, M.; Kaushik, D.; Liu, Q.; et al. Intratumoral CD56bright Natural Killer Cells Are Associated with Improved Survival in Bladder Cancer. Oncotarget 2018, 9, 36492–36502. [Google Scholar] [CrossRef] [Green Version]
- De Jonge, K.; Ebering, A.; Nassiri, S.; Maby-El Hajjami, H.; Ouertatani-Sakouhi, H.; Baumgaertner, P.; Speiser, D.E. Circulating CD56 Bright NK Cells Inversely Correlate with Survival of Melanoma Patients. Sci. Rep. 2019, 9, 4487. [Google Scholar] [CrossRef] [Green Version]
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Zhou, Y.; Ni, X.; Tong, X.; Xu, X.; Dong, Z.; Sun, R.; Tian, Z.; Wei, H. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 2017, 47, 1100–1113.e6. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK Cells Regulate Key Developmental Processes at the Human Fetal-Maternal Interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Stambler, I.; Caruso, C. Innate and Adaptive Immunity in Aging and Longevity: The Foundation of Resilience. Aging Dis. 2020, 11, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.K.; Zhu, X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging Insights into Molecular Mechanisms Underlying Pyroptosis and Functions of Inflammasomes in Diseases. J. Cell Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 Priming in Inflammaging and Age Related Diseases. Cytokine Growth Factor Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 Inflammasomes Are Required for Atherogenesis and Activated by Cholesterol Crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, F.; Xing, S.; Gong, Z.; Mu, W.; Xing, Q. Silence of NLRP3 Suppresses Atherosclerosis and Stabilizes Plaques in Apolipoprotein E-Deficient Mice. Mediators Inflamm. 2014, 2014, 507208. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, T.; Kritikou, E.; Venema, W.; van Duijn, J.; van Santbrink, P.J.; Slütter, B.; Foks, A.C.; Bot, I.; Kuiper, J. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Sun, S.; Ling, H.; Ma, J.; Zhang, X.; Xie, Z.; Zhan, N.; Zheng, W.; Li, M.; Qin, Y.; et al. Protectin DX Restores Treg/Th17 Cell Balance in Rheumatoid Arthritis by Inhibiting NLRP3 Inflammasome via MiR-20a. Cell Death Dis. 2021, 12, 280. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, S.; Zhang, X.; Wang, L.; Zhao, L.; Wan, W.; Xue, Y.; Lv, L. Leptin Facilitates the Differentiation of Th17 Cells from MRL/Mp-Fas Lpr Lupus Mice by Activating NLRP3 Inflammasome. Innate Immun. 2020, 26, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-H.; Ham, S.; Lee, A.; Möller, A.; Kim, T.S. NLRP3 Negatively Regulates Treg Differentiation through Kpna2-Mediated Nuclear Translocation. J. Biol. Chem. 2019, 294, 17951–17961. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Kanneganti, T.-D.; Vandanmagsar, B.; Zhu, X.; Ravussin, A.; Adijiang, A.; Owen, J.S.; Thomas, M.J.; Francis, J.; Parks, J.S.; et al. The Nlrp3 Inflammasome Promotes Age-Related Thymic Demise and Immunosenescence. Cell Rep. 2012, 1, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Rood, J.; Kumar, K.G.; Butler, A.A.; Dixit, V.D. Obesity Accelerates Thymic Aging. Blood 2009, 114, 3803–3812. [Google Scholar] [CrossRef] [Green Version]
- Camell, C.D.; Günther, P.; Lee, A.; Goldberg, E.L.; Spadaro, O.; Youm, Y.-H.; Bartke, A.; Hubbard, G.B.; Ikeno, Y.; Ruddle, N.H.; et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019, 30, 1024–1039.e6. [Google Scholar] [CrossRef]
- Martin-Antonio, B.; Najjar, A.; Robinson, S.N.; Chew, C.; Li, S.; Yvon, E.; Thomas, M.W.; Mc Niece, I.; Orlowski, R.; Muñoz-Pinedo, C.; et al. Transmissible Cytotoxicity of Multiple Myeloma Cells by Cord Blood-Derived NK Cells Is Mediated by Vesicle Trafficking. Cell Death Differ. 2015, 22, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Chen, S.; Cao, M.; Fan, X.; Yang, T.; Huang, Y.; Song, X.; Li, Y.; Ye, L.; Shen, N.; et al. Antigen-Specific CD8+ T Cell Feedback Activates NLRP3 Inflammasome in Antigen-Presenting Cells through Perforin. Nat. Commun. 2017, 8, 15402. [Google Scholar] [CrossRef] [Green Version]
- Castella, M.; Caballero-Baños, M.; Ortiz-Maldonado, V.; González-Navarro, E.A.; Suñé, G.; Antoñana-Vidósola, A.; Boronat, A.; Marzal, B.; Millán, L.; Martín-Antonio, B.; et al. Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-Automatic Bioreactor: Experience From an Academic Phase I Clinical Trial. Front. Immunol. 2020, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Perez-Amill, L.; Suñe, G.; Antoñana-Vildosola, A.; Castella, M.; Najjar, A.; Bonet, J.; Fernández-Fuentes, N.; Inogés, S.; López, A.; Bueno, C.; et al. Preclinical Development of a Humanized Chimeric Antigen Receptor against B Cell Maturation Antigen for Multiple Myeloma. Haematologica 2021, 106, 173–184. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to CAR T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.M.; Sakemura, R.; Cox, M.J.; Yang, N.; Khadka, R.H.; Forsman, C.L.; Hansen, M.J.; Jin, F.; Ayasoufi, K.; Hefazi, M.; et al. GM-CSF Inhibition Reduces Cytokine Release Syndrome and Neuroinflammation but Enhances CAR-T Cell Function in Xenografts. Blood 2019, 133, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogilenko, D.A.; Shpynov, O.; Andhey, P.S.; Arthur, L.; Swain, A.; Esaulova, E.; Brioschi, S.; Shchukina, I.; Kerndl, M.; Bambouskova, M.; et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK+ CD8+ T Cells as Conserved Hallmark of Inflammaging. Immunity 2021, 54, 99-115.e12. [Google Scholar] [CrossRef]
- Turner, C.T.; Zeglinski, M.R.; Richardson, K.C.; Zhao, H.; Shen, Y.; Papp, A.; Bird, P.I.; Granville, D.J. Granzyme K Expressed by Classically Activated Macrophages Contributes to Inflammation and Impaired Remodeling. J. Invest. Dermatol. 2019, 139, 930–939. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.A.; Franks, Z.; Bhatnagar, A.; Rowley, J.W.; Manne, B.K.; Supiano, M.A.; Schwertz, H.; Weyrich, A.S.; Rondina, M.T. Granzyme A in Human Platelets Regulates the Synthesis of Proinflammatory Cytokines by Monocytes in Aging. J. Immunol. 2018, 200, 295–304. [Google Scholar] [CrossRef]
- Garzón-Tituaña, M.; Sierra-Monzón, J.L.; Comas, L.; Santiago, L.; Khaliulina-Ushakova, T.; Uranga-Murillo, I.; Ramirez-Labrada, A.; Tapia, E.; Morte-Romea, E.; Algarate, S.; et al. Granzyme A Inhibition Reduces Inflammation and Increases Survival during Abdominal Sepsis. Theranostics 2021, 11, 3781–3795. [Google Scholar] [CrossRef]
- Santiago, L.; Castro, M.; Sanz-Pamplona, R.; Garzón, M.; Ramirez-Labrada, A.; Tapia, E.; Moreno, V.; Layunta, E.; Gil-Gómez, G.; Garrido, M.; et al. Extracellular Granzyme A Promotes Colorectal Cancer Development by Enhancing Gut Inflammation. Cell Rep. 2020, 32, 107847. [Google Scholar] [CrossRef]
- Bade, B.; Boettcher, H.E.; Lohrmann, J.; Hink-Schauer, C.; Bratke, K.; Jenne, D.E.; Virchow, J.C.; Luttmann, W. Differential Expression of the Granzymes A, K and M and Perforin in Human Peripheral Blood Lymphocytes. Int. Immunol. 2005, 17, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- De Poot, S.A.H.; Bovenschen, N. Granzyme M: Behind Enemy Lines. Cell Death Differ. 2014, 21, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, J.J.; Grant, M.D. The Immune Response Against Human Cytomegalovirus Links Cellular to Systemic Senescence. Cells 2020, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Anthony, D.A.; Andrews, D.M.; Chow, M.; Watt, S.V.; House, C.; Akira, S.; Bird, P.I.; Trapani, J.A.; Smyth, M.J. A Role for Granzyme M in TLR4-Driven Inflammation and Endotoxicosis. J. Immunol. 2010, 185, 1794–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wensink, A.C.; Wiewel, M.A.; Jongeneel, L.H.; Boes, M.; van der Poll, T.; Hack, C.E.; Bovenschen, N. Granzyme M and K Release in Human Experimental Endotoxemia. Immunobiology 2016, 221, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Souza-Fonseca-Guimaraes, F.; Krasnova, Y.; Putoczki, T.; Miles, K.; MacDonald, K.P.; Town, L.; Shi, W.; Gobe, G.C.; McDade, L.; Mielke, L.A.; et al. Granzyme M Has a Critical Role in Providing Innate Immune Protection in Ulcerative Colitis. Cell Death Dis. 2016, 7, e2302. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; van Aart, C.; Morisse, J.; Mullee, A.; Huybrechts, I. Chronic Inflammation towards Cancer Incidence: A Systematic Review and Meta-Analysis of Epidemiological Studies. Crit. Rev. Oncol. Hematol. 2021, 157, 103177. [Google Scholar] [CrossRef]
- Dobloug, G.C.; Garen, T.; Brunborg, C.; Gran, J.T.; Molberg, Ø. Survival and Cancer Risk in an Unselected and Complete Norwegian Idiopathic Inflammatory Myopathy Cohort. Semin. Arthritis. Rheum. 2015, 45, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Oldroyd, A.G.S.; Allard, A.B.; Callen, J.P.; Chinoy, H.; Chung, L.; Fiorentino, D.; George, M.D.; Gordon, P.; Kolstad, K.; Kurtzman, D.J.B.; et al. A Systematic Review and Meta-Analysis to Inform Cancer Screening Guidelines in Idiopathic Inflammatory Myopathies. Rheumatology 2021, 60, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Momohara, M.; Muro, Y.; Mitsuma, T.; Katayama, M.; Yanaba, K.; Nara, M.; Kakeda, M.; Kono, M.; Akiyama, M. Strong Correlation between Cancer Progression and Anti-Transcription Intermediary Factor 1γ Antibodies in Dermatomyositis Patients. Clin. Exp. Rheumatol. 2018, 36, 990–995. [Google Scholar] [PubMed]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J. Am. Coll. Nutr. 2015, 34 (Suppl. 1), 10–13. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Zhou, C.; Zhuang, J.; Tang, S.; Yu, J.; Tian, J.; Feng, F.; Liu, L.; Zhang, T.; et al. Meta-Analysis of the Association between the Dietary Inflammatory Index (DII) and Breast Cancer Risk. Eur. J. Clin. Nutr. 2019, 73, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-L.; Ren, Z.-J.; Zhang, Q.; Ren, P.-W.; Yang, B.; Liu, L.-R.; Dong, Q. Meta-Analysis of the Association between the Inflammatory Potential of Diet and Urologic Cancer Risk. PLoS ONE 2018, 13, e0204845. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Veranth, J.M.; Moss, T.A.; Chow, J.C.; Labban, R.; Nichols, W.K.; Walton, J.C.; Watson, J.G.; Yost, G.S. Correlation of in Vitro Cytokine Responses with the Chemical Composition of Soil-Derived Particulate Matter. Environ. Health Perspect 2006, 114, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-B.; Shim, J.-Y.; Park, B.; Lee, Y.-J. Long-Term Exposure to Air Pollutants and Cancer Mortality: A Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2018, 15, 2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollyea, D.A.; Harris, C.; Rabe, J.L.; Hedin, B.R.; De Arras, L.; Katz, S.; Wheeler, E.; Bejar, R.; Walter, M.J.; Jordan, C.T.; et al. Myelodysplastic Syndrome-Associated Spliceosome Gene Mutations Enhance Innate Immune Signaling. Haematologica 2019, 104, e388–e392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.C.-W.; North, K.; Kim, E.; Jang, E.; Obeng, E.; Lu, S.X.; Liu, B.; Inoue, D.; Yoshimi, A.; Ki, M.; et al. Synthetic Lethal and Convergent Biological Effects of Cancer-Associated Spliceosomal Gene Mutations. Cancer Cell 2018, 34, 225–241.e8. [Google Scholar] [CrossRef] [Green Version]
- Basiorka, A.A.; McGraw, K.L.; Eksioglu, E.A.; Chen, X.; Johnson, J.; Zhang, L.; Zhang, Q.; Irvine, B.A.; Cluzeau, T.; Sallman, D.A.; et al. The NLRP3 Inflammasome Functions as a Driver of the Myelodysplastic Syndrome Phenotype. Blood 2016, 128, 2960–2975. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rosenfeld, N.; et al. Mutant P53 Prolongs NF-ΚB Activation and Promotes Chronic Inflammation and Inflammation-Associated Colorectal Cancer. Cancer Cell 2013, 23, 634–646. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Chan, R.L.Y.; Luo, X.M.; Wu, W.K.K.; Shin, V.Y.; Cho, C.H. Animal Models of Gastrointestinal Inflammation and Cancer. Life Sci. 2014, 108, 1–6. [Google Scholar] [CrossRef]
- Takahashi, H.; Ogata, H.; Nishigaki, R.; Broide, D.H.; Karin, M. Tobacco Smoke Promotes Lung Tumorigenesis by Triggering IKKbeta- and JNK1-Dependent Inflammation. Cancer Cell 2010, 17, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkinson, A.M. Role of Inflammation in Mouse Lung Tumorigenesis: A Review. Exp. Lung Res. 2005, 31, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Enzler, T.; Gillessen, S.; Manis, J.P.; Ferguson, D.; Fleming, J.; Alt, F.W.; Mihm, M.; Dranoff, G. Deficiencies of GM-CSF and Interferon Gamma Link Inflammation and Cancer. J. Exp. Med. 2003, 197, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Dougan, M.; Li, D.; Neuberg, D.; Mihm, M.; Googe, P.; Wong, K.-K.; Dranoff, G. A Dual Role for the Immune Response in a Mouse Model of Inflammation-Associated Lung Cancer. J. Clin. Investig. 2011, 121, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.S.L.; Ye, Y.-N.; Shin, V.Y.; Yuen, S.-T.; Leung, S.-Y.; Wong, B.C.Y.; Cho, C.-H. Cigarette Smoke Exposure Increases Ulcerative Colitis-Associated Colonic Adenoma Formation in Mice. Carcinogenesis 2003, 24, 1407–1413. [Google Scholar] [CrossRef] [Green Version]
- Erdman, S.E.; Rao, V.P.; Poutahidis, T.; Rogers, A.B.; Taylor, C.L.; Jackson, E.A.; Ge, Z.; Lee, C.W.; Schauer, D.B.; Wogan, G.N.; et al. Nitric Oxide and TNF-Alpha Trigger Colonic Inflammation and Carcinogenesis in Helicobacter Hepaticus-Infected, Rag2-Deficient Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1027–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Xiao, J.; Zhou, X.; Xu, M.; Hu, C.; Xu, X.; Lu, Y.; Liu, C.; Xue, S.; Nie, L.; et al. STK4 Regulates TLR Pathways and Protects against Chronic Inflammation-Related Hepatocellular Carcinoma. J. Clin. Investig. 2015, 125, 4239–4254. [Google Scholar] [CrossRef] [Green Version]
| Age-Related Diseases | Mediators | References |
|---|---|---|
| Atherosclerosis | Secretion of IL1β, IL18 and IL6 among others | [7,40] |
| Cardiovascular diseases | CRP and IL6 in blood | [7] |
| Frailty, Sarcopenia | Inflammatory markers in blood, IL6 | [41] |
| Decline of innate and adaptive immune system | Immunosenescence | [8,9] |
| Type 2 diabetes | Secretion of IL1β among others | [34,42,43] |
| Cancer | CRP, IL6, immunosenescence | [7,25,26,35,36,44,45] |
| Osteoporosis, bone remodeling | IL1, IL6, TNFα | [46] |
| Neurodegenerative disease | Immune cells infiltration | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-López, J.; Martín-Antonio, B. Inflammaging, an Imbalanced Immune Response That Needs to Be Restored for Cancer Prevention and Treatment in the Elderly. Cells 2021, 10, 2562. https://doi.org/10.3390/cells10102562
Serrano-López J, Martín-Antonio B. Inflammaging, an Imbalanced Immune Response That Needs to Be Restored for Cancer Prevention and Treatment in the Elderly. Cells. 2021; 10(10):2562. https://doi.org/10.3390/cells10102562
Chicago/Turabian StyleSerrano-López, Juana, and Beatriz Martín-Antonio. 2021. "Inflammaging, an Imbalanced Immune Response That Needs to Be Restored for Cancer Prevention and Treatment in the Elderly" Cells 10, no. 10: 2562. https://doi.org/10.3390/cells10102562






