Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings
Abstract
:1. Introduction
2. Materials and Methods
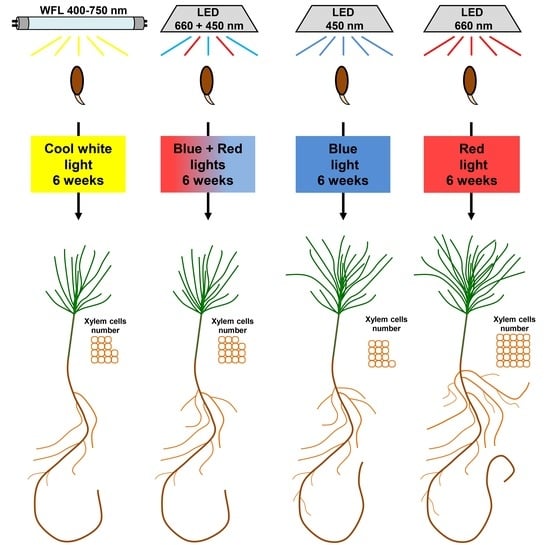
2.1. Plant Materials and Experimental Design
2.2. Pigment Contents
2.3. Measurements of CO2 Gas Exchange
2.4. Determination of Photochemical Activity
2.5. RNA Extraction and qRT-PCR
2.6. Histochemical Studies of the Hypocotyls
2.7. Statistics
3. Results
3.1. Growth and Morphological Parameters
3.2. Contents of Photosynthetic Pigments
3.3. Fluorescence Parameters and CO2 Gas Exchange
3.4. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casal, J.J. Shade avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef] [Green Version]
- Taulavuori, K.; Sarala, M.; Karhu, J.; Taulavuori, E.; Kubin, E.; Laine, K.; Poikolainen, J.; Pesonen, E. Elongation of Scots pine seedlings under blue light depletion. Silva Fenn. 2005, 39, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Franklin, K.A. Photomorphogenesis: Plants feel blue in the shade. Curr. Biol. 2016, 26, R1275–R1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Alakärppä, E.; Taulavuori, E.; Valledor, L.; Marttila, T.; Jokipii-Lukkari, S.; Karppinen, K.; Nguyen, N.; Taulavuori, K.; Häggman, H. Early growth of Scots pine seedlings is affected by seed origin and light quality. J. Plant Physiol. 2019, 237, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M. Photocycle and signaling mechanisms of plant cryptochromes. Curr. Opin. Plant Biol. 2016, 33, 108–115. [Google Scholar] [CrossRef]
- Mathews, S. Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell. 2010, 22, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapham, D.H.; Kolukisaoglu, H.Ü.; Larsson, C.T.; Qamaruddin, M.; Ekberg, I.; Wiegmann-Eirund, C.; Schneider-Poetsch, H.A.W.; von Arnold, S. Phytochrome types in Picea and Pinus. Expression patterns of PHYA-related types. Plant Mol. Biol. 1999, 40, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.H.; Ekberg, I.; Eriksson, G.; Norell, L.; Vince-Prue, D. Requirement for far-red light to maintain secondary needle extension growth in northern but not southern populations of Pinus sylvestris (Scots pine). Physiol. Plantarum. 2002, 114, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zdarska, M.; Dobisová, T.; Gelová, Z.; Pernisová, M.; Dabravolski, S.; Hejátko, J. Illuminating light, cytokinin, and ethylene signalling crosstalk in plant development. J. Exp. Bot. 2015, 66, 4913–4931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Rougemont, J.; Ljung, K.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S.; et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, O.S.; Deng, X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Emery, R.N.; Pharis, R.P.; Reid, D.M. The interaction of light quality and irradiance with gibberellins, cytokinins and auxin in regulating growth of Helianthus annuus hypocotyls. Plant Cell Environ. 2007, 30, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; Keuskamp, D.H.; Sasidharan, R.; Djakovic-Petrovic, T.; de Wit, M.; Voesenek, L.A. Light quality controls shoot elongation through regulation of multiple hormones. Plant Signal. Behav. 2009, 4, 755–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurepin, L.V.; Walton, L.J.; Hayward, A.; Emery, R.N.; Pharis, R.P.; Reid, D.M. Interactions between plant hormones and light quality signaling in regulating the shoot growth of Arabidopsis thaliana seedlings. Botany 2012, 90, 237–246. [Google Scholar] [CrossRef]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.K.; Finlayson, S.A. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol. 2014, 164, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ji, R.; Li, H.; Zhao, T.; Liu, J.; Lin, C.; Liu, B. CONSTANS-LIKE 7 (COL7) is involved in Phytochrome B (phyB)-mediated light-quality regulation of auxin homeostasis. Mol. Plant 2014, 7, 1429–1440. [Google Scholar] [CrossRef] [Green Version]
- OuYang, F.; Mao, J.F.; Wang, J.; Zhang, S.; Li, Y. Transcriptome analysis reveals that red and blue light regulate growth and phytohormone metabolism in Norway spruce [Picea abies (L.) Karst.]. PLoS ONE 2015, 10, e0127896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of zinc on Scots pine (Pinus sylvestris L.) seedlings grown in hydroculture. Plant Physiol. Bioch. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Packer, L., Douce, R., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Lankin, A.V.; Kreslavski, V.D.; Khudyakova, A.Y.; Zharmukhamedov, S.K.; Allakhverdiev, S.I. Effect of naphthalene on photosystem 2 photochemical activity of pea plants. Biochemistry 2014, 79, 1216–1225. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Kolosova, N.; Miller, B.; Ralph, S.; Ellis, B.E.; Douglas, C.; Ritland, K.; Bohlmann, J. Isolation of high-quality RNA from gymnosperm and angiosperm trees. Biotechniques 2004, 36, 821–824. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Vankova, R.; Zlobin, I.E.; Dobrev, P.; Ivanov, Y.V.; Kartashov, A.V.; Kuznetsov, V.V. Comparative analysis of abscisic acid levels and expression of abscisic acid-related genes in Scots pine and Norway spruce seedlings under water deficit. Plant Physiol. Bioch. 2019, 140, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.P.; Loqué, D. Histochemical staining of Arabidopsis thaliana secondary cell wall elements. J. Vis. Exp. 2014, 87, 51381. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.W.; Liu, S.Q.; Wang, Y.; Liu, J.K.; Feng, L. Effects of different LED light qualities on growth, photosynthetic characteristics and nutritional quality of savoy. Ying Yong Sheng Tai Xue Bao 2014, 25, 1955–1962. [Google Scholar]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranade, S.S.; Delhomme, N.; García-Gil, M.R. Global gene expression analysis in etiolated and de-etiolated seedlings in conifers. PLoS ONE 2019, 14, e0219272. [Google Scholar] [CrossRef] [PubMed]
- Gyula, P.; Schäfer, E.; Nagy, F. Light perception and signalling in higher plants. Curr. Opin. Plant Biol. 2003, 6, 446–452. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, M.J.; Guo, Y.P. Regulation of photosynthesis by light quality and its mechanism in plants. Ying Yong Sheng Tai Xue Bao 2008, 19, 1619–1624. [Google Scholar] [PubMed]
- Kreslavski, V.D.; Strokina, V.V.; Pashkovskiy, P.P.; Balakhnina, T.I.; Voloshin, R.A.; Alwasel, S.; Kosobryukhov, A.A.; Allakhverdiev, S.I. Deficiencies in phytochromes A and B and cryptochrome 1 affect the resistance of the photosynthetic apparatus to high-intensity light in Solanum lycopersicum. J. Photoch. Photobio. B 2020, 210, 111976. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Strokina, V.V.; Khudyakova, A.Y.; Shirshikova, G.N.; Kosobryukhov, A.A.; Pashkovskiy, P.P.; Alwasel, S.; Allakhverdiev, S.I. Effect of high-intensity light and UV-B on photosynthetic activity and the expression of certain light-responsive genes in A. thaliana phyA and phyB mutants. BBA-Bioenergetics 2021, 1862, 148445. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, J.; Qu, L.; Hager, J.; Chen, Z.; Zhao, H.; Deng, X.W. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 2001, 13, 2589–2607. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Ruttink, T.; Hostyn, V.; Sterck, L.; Van Driessche, K.; Boerjan, W. Gene expression during the induction, maintenance, and release of dormancy in apical buds of poplar. J. Exp. Bot. 2007, 58, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Sweere, U.; Eichenberg, K.; Lohrmann, J.; Mira-Rodado, V.; Bäurle, I.; Kudla, J.; Nagy, F.; Schäfer, E.; Harter, K. Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 2001, 294, 1108–1111. [Google Scholar] [CrossRef] [Green Version]
- Fankhauser, C. Light perception in plants: Cytokinins and red light join forces to keep phytochrome B active. Trends Plant Sci. 2002, 7, 143–145. [Google Scholar] [CrossRef]
- Al-Sady, B.; Ni, W.; Kircher, S.; Schäfer, E.; Quail, P.H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 2006, 23, 439–446. [Google Scholar] [CrossRef]
- Thomas, T.H.; Hare, P.D.; van Staden, J. Phytochrome and cytokinin responses. Plant Growth Regul. 1997, 23, 105–122. [Google Scholar] [CrossRef]
- Mira-Rodado, V.; Sweere, U.; Grefen, C.; Kunkel, T.; Fejes, E.; Nagy, F.; Schäfer, E.; Harter, K. Functional cross-talk between two-component and phytochrome B signal transduction in Arabidopsis. J. Exp. Bot. 2007, 58, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Durante, L.; Ferri, M. Combined elicitation of methyl-jasmonate and red light on stilbene and anthocyanin biosynthesis. J. Plant Physiol. 2012, 169, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Kim, S.A.; Choi, S.J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]






| Gene Bank ID | Gene Description | Plant | Gene | Primer 5′-3′ | ||
|---|---|---|---|---|---|---|
| Forward | Reverse | |||||
| 1 | ALN42232.1 (uniprot.org) | Histidine-containing phosphotransfer 1 | Pinus pinaster | HPT1 | GCTCAAGTATAGGAGCGCGG | CCAGCTTGTTTTTCACGAGGT |
| 2 | FJ717710.1 (ncbi.nlm.nih.gov) | Type-A Response Regulators | Pinus pinea | RR-A | CAGAAGGCGCTCAAGAGTTT | TTGTTGGTCCCTGGATCTTC |
| 3 | AY289600.1 (ncbi.nlm.nih.gov) | Auxin-induced protein 1 (IAA1) | Pinus taeda | Aux/IAA | GCCACCTGTCAAAGATTTCAG | TGAGGTCCACCTTTCTGAGA |
| 4 | ABO77179.1 (uniprot.org) | Photosystem II protein D1 | Pinus sylvestris | psbA | TGAAGGTTACAGATTCGGTCA | TGAATATGCAACAGCAATCCA |
| 5 | K7R334 (uniprot.org) | Cryptochrome 1 | Pinus sylvestris | Cry1 | TATGGTGCACAGGGCAGATG | AAGCTGCAGAAGCTGTTCCT |
| 6 | T2FFB6 (uniprot.org) | Cryptochrome 2 | Picea abies | Cry2 | TTCCCTGGCTGCAACAGAAA | CCCAACATTGCTAGGCAGGA |
| 7 | AIY54822.1 (uniprot.org) | Phytochrome P | Pinus sylvestris | phyP | GGCATGTCCCTTGTTCAGGA | CTTCTGTGGGCCAAAGGTCT |
| 8 | AFV79519.1 (uniprot.org) | Phytochrome N | Pinus sylvestris | phyN | GGCTCAGAGGAGGACAAAGG | TTCTGCCCGGTCACATCTTG |
| 9 | A7Y6Q6 (uniprot.org) | Phytochrome O | Pinus sylvestris | phyO | AGATGTGACGTGGCAAAGGA | TGCGGGATTCCACTCAGAAC |
| 10 | D5ABG4 (uniprot.org) | Phytochrome-interacting factor 3 | Picea sitchensis | PIF3 | ATCAGCACTTCCTGGTTCCG | CAGGCTGAGTTGTTCCAGGT |
| 11 | AF543757.1 (ncbi.nlm.nih.gov) | Chalcone synthase | Pinus uliginosa | CHS | ATGGCTGCAGGAATGATGAAGG | AGTGCCAATAGCGAGGATG |
| 12 | S50350.1 (ncbi.nlm.nih.gov) | Stilbene synthase | Pinus sylvestris | STS | TCCGACTGGAACAAGTTGTTC | GCTTGGCCTCCACCCGATCAAG |
| 13 | CBB44933.1 (uniprot.org) | Actin 1 | Pinus sylvestris | Act1 | TTAGCAACTGGGATGACATGGA | CCTGAATGGCAACATACATAGCA |
| Parameter | WFL | BRL | BL | RL |
|---|---|---|---|---|
| Pn, µmol CO2 m−2 s−1 | 14.4 ± 2.1 ab | 16.3 ± 0.2 a | 13.7 ± 0.3 b | 12.1 ± 0.3 c |
| R, µmol CO2 m−2 s−1 | 8.7 ± 0.8 a | 8.8 ± 0.1 a | 5.1 ± 0.2 b | 2.1 ± 0.1 c |
| R/Pn | 0.62 ± 0.12 a | 0.54 ± 0.06 a | 0.37 ± 0.02 b | 0.17 ± 0.01c |
| (Pn – R), µmol CO2 m−2 s−1 | 5.7 ± 0.5 d | 7.5 ± 0.2 c | 8.6 ± 0.1b | 10.0 ± 0.2 a |
| Fv/Fm | 0.814 ± 0.005 a | 0.803 ± 0.001 a | 0.815 ± 0.007 a | 0.812 ± 0.006 a |
| PIABS | 7.73 ± 0.39 b | 7.85 ± 0.64 b | 6.45 ± 0.82 b | 10.21 ± 0.59 a |
| YII | 0.52 ± 0.01 b | 0.44 ± 0.03 c | 0.50 ± 0.01 b | 0.57 ± 0.01 a |
| NPQ | 0.86 ± 0.10 b | 1.67 ± 0.21 a | 0.95 ± 0.12 ab | 0.66 ± 0.17 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells 2021, 10, 3284. https://doi.org/10.3390/cells10123284
Pashkovskiy P, Kreslavski VD, Ivanov Y, Ivanova A, Kartashov A, Shmarev A, Strokina V, Kuznetsov VV, Allakhverdiev SI. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells. 2021; 10(12):3284. https://doi.org/10.3390/cells10123284
Chicago/Turabian StylePashkovskiy, Pavel, Vladimir D. Kreslavski, Yury Ivanov, Alexandra Ivanova, Alexander Kartashov, Alexander Shmarev, Valeriya Strokina, Vladimir V. Kuznetsov, and Suleyman I. Allakhverdiev. 2021. "Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings" Cells 10, no. 12: 3284. https://doi.org/10.3390/cells10123284