1. Introduction
Cyclophilin A (Cyp A) is a universally distributed protein that exists in both intracellular and extracellular forms [
1,
2]. Several cells, such as endothelial cells, vascular smooth muscle cells and platelets, secrete Cyp A in response to increased oxidative stress [
3]. Cyp A is known to mediate the progression of atherosclerosis by increasing adhesion, transmigration and differentiation of monocytes, as well as the formation of foam cells [
2]. In high-glucose conditions, Cyp A can induce apoptosis of macrophages [
4]. It is possible that the increased incidence of apoptotic cells is due to inadequate clearance of apoptotic cells in the vascular lumen, a process called efferocytosis. Efferocytosis involves several sequential events, including finding of apoptotic cells by phagocytes, recognition of apoptotic cells (ACs) by phagocytic receptors, activation of intracellular signaling cascades with cytoskeletal rearrangements for AC engulfment and post-engulfment processes for phagosome maturation and AC degradation [
5]. These mechanisms are regulated by several signal molecules, such as ‘
find-me’, ‘
eat-me’ and ‘
don’t-eat-me’ signals. Cells undergoing apoptosis express molecules such as ‘find-me’ signals to attract phagocytes, whereas, dying cells also express ‘
eat-me’ signals on the cell surface to indicate they should be engulfed by macrophages. The best-characterized ‘
eat-me’ signal is phosphatidylserine on the cell surface of ACs. Healthy cells display ‘
don’t-eat-me’ signals, such as CD 47 and CD 31, on the cell surface to avoid efferocytosis. The prompt and efficient clearance of apoptotic cells by phagocytosis is essential to maintenance of tissue homeostasis and prevention of secondary necrosis [
6]. The necrotic core is the hallmark of the vulnerable atherosclerotic plaque. Although apoptotic cells are cleared quickly in almost all other tissue beds, their removal appears to be significantly impaired in diseased blood vessels [
7]. Macrophages containing abundant apoptotic material are detected in plaques, supporting that efferocytosis occurs in atherosclerosis [
8].
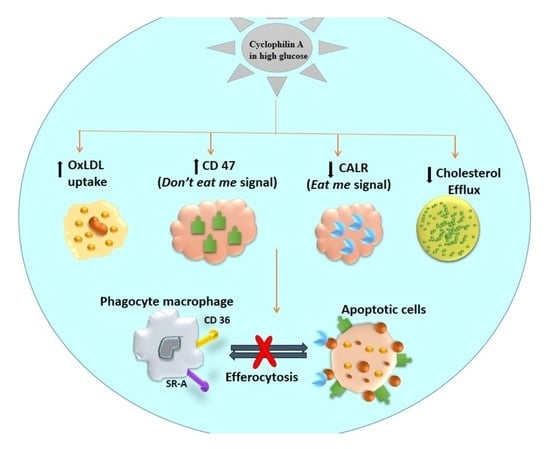
We report here that Cyp A impairs efferocytosis in apoptotic macrophages associated with atherosclerotic lesions. Impaired efferocytosis, together with an increase in macrophage apoptosis, can modulate the severity of atherosclerotic lesion. This mechanism could be important for rapid progression of atherosclerotic lesions in type 2 diabetes mellitus, where circulating levels of Cyp A are elevated [
9].
2. Materials and Methods
2.1. In Vitro Culture Model
RAW 264.7 macrophages, obtained from American Type Culture Collection (ATCC® TIB-71™ USA, were maintained in a high-glucose conditions (20 mM = 360 mg/dL) and cultured in Dulbecco’s Modified Eagle medium supplemented with 10% FBS and antibiotics (penicillin 0.1 μg/μL and streptomycin 0.1 μg/μL). To analyze the efficiency of in vitro efferocytosis, macrophages were primed with 300 ng/mL of Cyp A in high-glucose conditions for 24 h.
2.2. In Vitro Efferocytosis Assays
Efferocytosis is a specialized process in which macrophages clear apoptotic cells to maintain homeostasis. In order to determine whether cyclophilin A influences efferocytosis, an in vitro efferocytosis assay was carried out in the presence and absence of Cyp A using labelled macrophages by both flow cytometry and confocal microscopy assay.
2.3. Flow Cytometry Assay
An in vitro efferocytosis assay was performed, as previously described [
9]. Primary human coronary aortic SMCs (HCASMCs) were labeled with 20 μM orange CMTMR CellTracker fluorescent probes (C2927; Life Technologies, Waltham, MA, USA) for 1 h, then cultured overnight in serum-free media. Simultaneously, RAW macrophages were labeled with 20 μM green CMFDA CellTracker probe (C7025; Life Technologies, Waltham, MA, USA) for 1 h, then cultured overnight in standard media with serum supplementation. In the morning, apoptotic HCASMCs were induced to undergo apoptosis by treatment with 1 µM Staurosporin for 3 h. Later, they were harvested and manually counted. Apoptotic cells (1 × 10
5) were then added to the cultured macrophages, and co-culturing was performed for an additional 1.5 h. At that point, all adherent cells were trypsinized and subjected to FACS using a BD FACS caliber (530 nm [FL1] and >575 nm [FL4]), as described in previously published protocols [
10]. Cells that were dual-positive for green (phagocyte) and orange (SMC) represented phagocytosed cells. The efferocytosis rate was then defined as the ratio of dual-positive cells (phagocytosed ABs) to orange-positive/green-negative cells (uneaten apoptotic cells).
2.4. Confocal Microscopy Assay
Non-adherent, non-phagocytosed cells, post coculture of macrophages with SMCs, were washed off. The remaining cells were fixed and stained with Hoechst 33258 (Sigma-Aldrich Chemicals Private Limited, Bangalore, India) and analyzed under an inverted fluorescence microscope using NIS-Elements software. The efferocytosis rate was analyzed by counting the number of efferocytosed cells per field.
2.5. Cholesterol Efflux Culture Assays Using Cholesterol Efflux Assay Kit (Cell-Based)
Cholesterol efflux (reverse cholesterol transport) is a process whereby intracellular cholesterol is transported from macrophages to extracellular acceptors, such as apoprotein A. Reverse cholesterol transport plays an important role in preventing development of atherosclerosis by reducing accumulation of cholesterol in the wall of arteries. To analyze whether cyclophilin A impairs cholesterol efflux in macrophages, we used a high-throughput cell-based cholesterol efflux assay kit (ab196985) with fluorescently labeled cholesterol, according to the manufacturer’s instructions. Briefly, cultured macrophages were labelled with premixed labeling reagent containing fluorescently labeled cholesterol for 16 h and incubated at 37 °C in a humidified incubator with 5% CO2. After overnight incubation, the labeling reagent was removed, and cells were treated with human serum as cholesterol acceptors in DMEM media. Then, the cells were washed and incubated for 5 h in a 37 °C incubator containing 5% CO2. After incubation, fluorescence was measured (Ex/Em = 482/515 nm) both calorimetrically and by IVIS spectrum in vivo imaging assay. The cell monolayer was solubilized by adding 100 µL of cell lysis buffer for 30 min at room temperature, and fluorescence was measured again. Cholesterol efflux was calculated by dividing the fluorescence intensity of the media by total fluorescence intensity of the cell lysate after the same treatment and media. The value obtained was multiplied by 100 to obtain % cholesterol efflux. The final % cholesterol efflux was determined by subtracting the % cholesterol efflux obtained for the control. High-density lipoprotein (HDL) was used as cholesterol acceptor for a positive control. For negative control, serum containing DMEM media was used.
2.6. In Vitro Silencing of Cyclophilin A Gene
Macrophage cells were transfected with Cyp A-siRNA using mission siRNA transfection reagent (Sigma-Aldrich Chemicals Private Limited, Bangalore, India) for 48 h at 37 °C. Primers used for Cyp A mRNA target sequence were 5′- TGGTGTTTGGCAAAGTGAAAGAAGGCATGAATATTGTGGAGGCCATGGAGCGCTTTG-3′. The efficiency of silencing was determined by measuring relative mRNA expression in quantitative real-time PCR (ABI Prism 7900HT sequence detection system) [
2].
2.7. Gene-Expression Analysis of ABCA1 mRNA by RT-PCR
ABCA1 can inhibit lipid-laden-foam cell formation by increasing the reverse cholesterol transport of excessive cholesterol from lipid-loaded macrophages and conserve lipid homeostasis in cells. To examine the effects of cyclophilin A on macrophage ABCA1 expression and ABCA1 mediated cholesterol efflux, we next analyzed the gene expression of ABCA1 in cyclophilin A primed macrophages by quantitative real-time PCR using ABI Prism 7900HT sequence detection system. Briefly, RNA isolated from macrophages of different treatments was reverse-transcribed, and real-time PCR was performed using specific primers of ABCA1 and beta 2 microglobulin. The reactions were performed in triplicate in 384-well plates at 50 °C, 2 min; 95 °C, 10 min; 95 °C, 15 s; 58 °C, 1 min; and 72 °C, 0.30 min, for 40 cycles. Ct values calculated from the expression levels of ABCA1 gene was normalized to endogenous cellular beta 2 microglobulin. The primer sequences of ABCA1 and beta 2 microglobulin were:
ABCA1:
Forward primer 5′-TCCACAAGGTATTTTTGCAAGGC-3′
Reverse primer 5′-ACTATGCAGCAATGTTTTTGTGGC-3′
beta-2-microglobulin:
Forward primer 5′-CCAGCGTACTCCAAAGATTCAG-3′
Reverse primer 5′-GTAAGTCAACTTCAATGTCGGATG-3′
2.8. Protein Sample Preparation for LC/MS/MS Analysis In Vitro
To investigate the molecular link between Cyp A and known ligand receptors involved in efferocytosis, we sorted dual-positive cells after co culturing using flow cytometry; the dual-positive cells were obtained from the in vitro efferocytosis assay. The phagocytosed dual-positive cells for green (phagocyte) and orange (SMC) were used for LC/MS/MS analysis. Protein lysates were prepared in 0.5% RapiGest TM SF surfactant in 50 mM NH4HCO3 buffer (Waters Corporation, Milford, MA, USA). Total protein content was estimated by Bradford assay. Peptide was produced for each sample (100 µg of protein) using trypsin digestion, followed by centrifugation at 14,000 rpm for 12 min, and the supernatants were collected and stored at −20 °C until LC/MS/MS analysis.
2.9. Liquid Chromatography and Mass Spectrometry
Peptide samples were analyzed by using a nano ACQUITY UPLC® system (Waters Corporation, Milford, MA, USA) coupled with a quadrupole-time-of-flight (Q/TOF) mass spectrometer (SYNAPT-G2, Waters Corporation, Milford, MA, USA) controlled by MassLynx4.1 SCN781 software (Waters Corporation, Milford, MA, USA). Peptides eluted from the nano LC were monitored by the SYNAPT® G2 High Definitions MS™ System (HDMSE System Waters Corporation, Milford, MA, USA). Three technical replicate runs were performed for each sample.
2.10. Data Analysis and Bioinformatics
LC/MSE data were analyzed by Protein Lynx Global SERVER™ v2.5.3 (PLGS, Waters Corporation, Milford, MA, USA), which helps both with protein identification and relative quantification. Homo sapiens database from NCBI was used for database search. Protein identification was carried out by setting the parameters as at least one fragment-ion match for each peptide, at least three fragment-ion matches for protein or a minimum of two peptide matches for identification. Precursor and fragment ions were defined by setting the mass tolerance at 10 and 20 ppm, respectively. Oxidation of methionine was chosen as variable modification, and carbamidomethylation of cysteine was chosen as fixed modification. Dataset was normalized by auto-normalization of PLGS. Label-free quantitative analyses were performed. Samples were compared with respect to normalized peak area/intensity of identified peptides. Number of peptides, score, and sequence coverage parameters were identified for each protein. The reference sequence identifications (RefSeq) obtained after PLGS analysis were converted into gene symbols using Biological Database Network (BioDBnet). Database for Annotation, Visualization and Integrated Discovery (DAVID) was used for categorizing gene symbols into different biological functions. Statistical analysis and graphical representations were done using MS-Excel 2013. The molecular link between Cyp A and ligand receptors involved in efferocytosis was explored using protein docking. To understand the topological relationship between Cyp A and ligand receptors, we selected 28 known genes reported in the efferocytosis process. These genes include CALR, MFGE8, CX3CL1, ABCA6, ICAM3, GAS6, APOH, PROS1, C1QB, ANXA1, CD 47, LRP1, MBL2, SIRPA, NR1H3, PPARG, LRPAP1, TGFB1, BAI1, TIMD4, CD14, MERTK, CD36, ELMO1, DOCK1, AKT1, PANX1 and GULP1. The similarity of amino-acid sequences between proteins was analyzed using the Schrodinger platform.
2.11. Quantification of CD 47 by Confocal Microscopy
For immunostaining of CD 47, RAW macrophages were incubated with primary antibody of mouse anti-CD 47 antibody (1:100) (Novus Biologicals, CO, USA) at 4 °C overnight, followed by incubation with secondary antibody of Alexa flour 488-conjugated anti-mouse antibody (1:200) (Abcam, Cambridge, UK) for 1 h at room temperature. Cells were counterstained with 10 μg/mL of Hoechst 33258 (Sigma) for 5 min and quantified using NIS-Elements Viewer microscope-imaging software with a 63 × 1.3 numerical aperture oil-immersion lens using dual excitation (488 nm for FITC) and emission (515–540 nm for FITC) filter sets.
2.12. Western Blot Analysis
After treatment, cells were lysed in cell lysis buffer containing protease inhibitor cocktail (Sigma-Aldrich). The total cell lysates were loaded on SDS-PAGE and electro-transferred into nitrocellulose membrane, followed by incubation with the appropriate primary antibody at 4 °C overnight. The primary antibodies used were mouse anti-cyclophilin A (ab-58144) (1:1000), mouse anti-β Actin (sc-47778) (1:1000), mouse anti-CD 47 antibody (1:1000) (B6H12.2, Novus Biologicals) and rabbit anti-calreticulin (1:1000) (ab-2907). The membranes were later incubated with specific secondary antibodies: anti-mouse IgG-HRP (Abcam) at a dilution of 1:5000. The proteins were visualized with Clarity Western ECL substrate. The bands were analyzed by Quantity One 1D image-analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
2.13. In Vivo Study Model
Further, we used New Zealand white rabbits (NZW) (n = 12) to demonstrate the effect of Cyp A on impaired efferocytosis. All animal experiments were performed according to experimental protocols that were approved by the Institute Animal Ethics Committee, RGCB (IAEC/803/SURYA/2018). The animals were categorized into two groups. Group 1 animals (n = 6) were fed with a normal diet (ND) containing 16.6% fiber, 14.5% protein and 8.3% mineral ash. Animals of group II (n = 6) were fed a high-cholesterol diet (HFD), which consisted of 0.5% (w/w) cholesterol/kg (5 g cholesterol, 150 g fat/kg rabbit chow), 2.6% sugar and 3% saturated fatty acids. To induce fatty streak formation, the animals were fed with HFD for 12 weeks. All animals had unobstructed admittance to water and were conserved on a 12-hlight–dark cycle in a pathogen-free environment. All animals were observed daily. At the end of the 12 weeks, blood samples were collected, and animals were euthanized by intramuscular injection of a combination of xylazine and thiopental. Aortae were isolated for histopathological analysis. Blood samples were collected from the marginal ear vein, and plasma was prepared and stored at −80 °C. Low-density lipoprotein (LDL), triglycerides and glucose levels in serum were measured using the Biosystems kit (LDL-code no: 11585 and Triglycerides-code no: 11528; Biosystems S.A., Barcelona, Spain), following the manufacturer’s instructions. Plasma Cyp A levels were determined by enzyme-linked immunosorbent assay (ELISA; R&D systems, USCN Life Science Inc., TX, USA), as per the manufacturer’s instructions.
2.14. ORO Staining
Enface staining of rabbit aortae was performed using Oil Red O Staining, as mentioned earlier [
4]. Briefly, whole rabbit aortae were collected and fixed on a petri dish with black wax with PBS and cut open vertically to expose the inner area of the aorta, washed and rinsed with 60% isopropanol. Rabbit aortae were stained with ORO stain for 30 min, followed by rinsing with 60% isopropanol for 2 s to remove excess lipids. Lipid-laden plaque area was analyzed by measuring the ratio of ORO-stained plaque areas to plaque area from total aorta within the aortic surface using ImageJ software.
2.15. Histological Analysis
Whole aortae were isolated for morphological analysis, fixed for 24 h with 10% phosphate-buffered formalin and embedded in paraffin. Cross-sections of seven microns thickness were prepared, and sections were stained with haematoxylin and eosin, Masson’s trichrome stain or used for immunostaining. Statistical analyses were performed using Image-Pro Plus software. Lesion area distinguished from acellular region on staining was analyzed by microscopy using NIS elements software.
2.16. Immunohistochemistry
For histopathological analysis of various proteins, the formaldehyde-fixed paraffin sections of tissue were incubated with primary antibodies, anti α smooth muscle actin (α-SMA) (ab-7817; 1:200; Abcam), cyclophilin A (ab41684; 1:200; Abcam), calreticulin (ab-2907; 1:100; Abcam) and CD 47 (B6H12.2; 1:100; Novus Biologicals) overnight at 4 °C. Species- and isotype-matched IgG was used as negative control. Anti-rabbit IgG-HRP (ab-97051) and anti-mouse IgG HRP (ab-6789) were used as secondary antibodies at a dilution of 1: 400 and 1:200, respectively. Quantitative analysis was performed manually by analyzing diaminobenzidine-tetrahydrochloride- (DAB) positive areas using ImageJ software.
2.17. Statistical Analysis
All experiments were performed in triplicate. Variable comparison between two groups was done by Fisher’s exact t test. Analysis of variance (ANOVA) using R package, Geisser Greenhouse’s epsilon, was used to analyze the differences among various cell treatments. Continuous variables with normal distribution were expressed as mean ± SD. Data were analyzed using a linear mixed effects model, with the ‘treatment’ (introduction of Cyp A and siRNA) and ‘control’ groups treated as fixed-effect variables. The repetitions were considered as random effects nested within the fixed effect. In vivo data analysis was performed using ImageJ (version 1.45s, Bethesda, MD, USA), and in vitro data were analyzed using Graph Pad Prism (Graph Pad Software, San Diego, CA, USA). p < 0.05 was considered statistically significant.
4. Discussion
Our studies provide a mechanism for impaired efferocytosis in apoptotic macrophages, a known determinant of the severity of atherosclerosis and vulnerability of plaques to rupture. We discovered that cyclophilin A (Cyp A), well-recognized to participate in several mechanisms in the progression of atherosclerosis, can disturb efferocytosis in apoptotic macrophages. Cyp A increases the expression of the don’t-eat-me signal, CD 47, and decreases expression of the phagocyte receptor ligand calreticulin in macrophages. Cyp A also reduces ABCA1-mediated cholesterol efflux in foam cells derived from macrophages. Our findings are significant, as Cyp A effects observed in this study could synergize with other mechanisms of Cyp A in acceleration of progression of atherosclerosis.
Efferocytosis involves several sequential events. These include (i) recognition of apoptotic cells by phagocytic receptors, (ii) activation of intracellular signaling cascades and cytoskeletal rearrangements for engulfment of apoptotic cells and (iii) post-engulfment process for phagosome maturation and degradation of apoptotic cells [
5]. These mechanisms are regulated by
find-me,
eat-me and
don’t-eat-me signals. Cells that undergo apoptosis express
find-me signals to attract phagocytes. Dying cells express
eat-me signals on the cell surface to indicate their readiness to be engulfed by macrophages. The best-characterized
eat-me signal is phosphatidylserine on the surface of apoptotic cells. Healthy cells display
don’t-eat-me signals, such as CD 47 and CD31, to avoid efferocytosis. Prompt and efficient clearance of apoptotic cells by phagocytosis is essential to maintenance of tissue homeostasis and prevention of secondary necrosis [
6].
Apoptotic cells are cleared quickly in almost all tissue beds. However, their removal appears to be significantly impaired in atherosclerotic blood vessels [
7]. Macrophages containing abundant apoptotic material are detected in atherosclerotic plaques. Their accumulation is seen in the necrotic core of atherosclerotic lesions, which is a hallmark of atherosclerotic plaques vulnerable to rupture. There is also evidence that efferocytosis occurs in atherosclerosis [
8]. In normal tissue, efficient cross talk between apoptotic and phagocytic mechanisms regulates efferocytosis. Cyclophilin A reportedly increases macrophage apoptosis through mitochondria-mediated death-signaling pathways. An 8-fold increase in the number of apoptotic cells in the lesion area of HFD-fed rabbits was observed [
4]. Efferocytosis is impaired in atherosclerotic lesions [
11].
We observed that CD 47 is overexpressed in apoptotic macrophages [
12] in the presence of Cyp A and that this could impede clearance of apoptotic debris by neutralizing the action of calreticulin, a ligand for phagocyte receptors. Clearance of apoptotic cells by macrophages could be increased by mission-siRNA-mediated silencing of the Cyp A gene.
Cyp A also reduces cholesterol efflux from lesional macrophages, thus promoting foam-cell transformation. Rapid breakdown of accumulated apoptotic cell membranes and release of plaque-destabilizing proteases [
11] and pro-inflammatory cytokines [
13] can further accelerate atherosclerosis and promote lesion vulnerability [
14]. Cyp A levels were found to be high in advanced atherosclerotic lesions of aorta in high-fat diet-fed animals. Thus, our studies indicate that Cyp A, by impairing efferocytosis and increasing macrophage apoptosis, can contribute to the progression of atherosclerotic lesions.
Cyclophilin A is an immunophilin with PPIase enzymatic activity and plays an active role in protein folding [
15]. Cyclophilin A is also secreted from several cell types, such as monocyte [
2], endothelial cells [
16] and vascular smooth muscle cells [
17] in both extracellular and intracellular forms in response to stimuli such as high glucose, presence of modified LDL and oxidative stress. Plasma cyclophilin A levels contribute to a pro-inflammatory milieu and trigger vessel-wall inflammation. Overexpression of cyclophilin A in macrophages induces the expression of scavenger receptors, resulting in the formation of lipid-laden foam cells and subsequent lesion formation. Cyclophilin A therefore functions as a cytokine and causes atherosclerotic lesion formation [
2]. Plasma Cyp A levels are increased in patients with type 2 diabetes and clinically manifested vascular disease [
9]. Recently, we demonstrated that the mechanisms by which Cyp A can contribute to rapid progression of vascular lesions in high glucose conditions involve the induction of foam-cell formation through an increase in oxidative stress [
2], increasing the levels of other pro-inflammatory cytokines [
2] and increasing macrophage apoptosis [
4]. The present finding suggests an additional mechanism.
Macrophage foam cells are abundant in atherosclerotic plaques vulnerable to rupture [
18]. Normally, macrophages unload extra cholesterol to apolipoprotein A1 and high-density lipoproteins via an efflux pathway. This is mediated by ATP-binding cassette transporters ABCA1 and ABCG1. Cholesterol efflux modulated by high-density lipoprotein is an atheroprotective mechanism [
19,
20,
21,
22,
23,
24,
25,
26]. In atherosclerotic lesions, transfer of cholesterol to intimal smooth muscle cells increases cholesterol levels in these cells, converting them into macrophage-like cells [
27,
28]. They secrete pro-inflammatory cytokines and make atherosclerotic plaques unstable [
29]. A series of cell-based cholesterol-efflux experiments in our study indicate that the reverse cholesterol-efflux ability of macrophages is impaired in the presence of Cyp A.
An important cause of advancement of atherosclerosis is increased apoptosis of cells in lesions. A secondary reason is defective efferocytosis when apoptotic cells are poor substrates for phagocytes [
14]. Macrophages are the key effector cells that identify
eat-me, find-me and
don’t-eat-me signals. Healthy cells express CD 47, a
don’t-eat-me signal that protects them from macrophage engulfment [
30,
31]. CD 47 is consistently upregulated in atherosclerotic plaques in humans, as well as mice [
12]. In mice, lesional efferocytosis was improved, and necrotic areas were reduced after administration of CD 47-blocking antibodies [
14]. Our results support the view that upregulation of CD 47 in the presence of Cyp A could impair macrophage efferocytosis in atherosclerotic lesions. An increase in apoptotic material in lesions also suggests in adequate efferocytosis [
32].
Calreticulin, a calcium-binding chaperon, is a well-known
eat-me-signal or pro-apoptotic molecule expressed on the surface of apoptotic cells. It stimulates phagocytosis and immunogenicity of apoptotic cells, possibly by interfering with the PS–C1q interaction [
33]. During efferocytosis, macrophages display several receptors that bind either directly or indirectly, via bridging molecules, to calreticulin. Several studies suggest that apoptotic cells in atherosclerotic lesions express lower amounts of calreticulin [
14].
Calreticulin and Cyp A are multifunctional proteins, and their interactions play an important role in several cellular processes [
34]. The Ca
2+ binding site of the P-domain of calreticulin is proline-rich [
35]. Cyp A can bind to proline and thus potentially affect Ca
2+-binding activity of calreticulin by forming a complex [
34]. In string analysis of the proteomics data, we observed an association of calreticulin and C1q proteins with Cyp A. We also found that Cyp A can repress the apoptotic effect of calreticulin by upregulating CD 47. We have not confirmed these findings in Cyp A knockout animals.