Hair-Follicle-Associated Pluripotent (HAP) Stem Cells Can Extensively Differentiate to Tyrosine-Hydroxylase-Expressing Dopamine-Secreting Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. C57BL/6 Mice
2.2. Isolation of Vibrissa Hair Follicles and Induction of Dopaminergic Neurons from HAP Stem Cells In Vitro
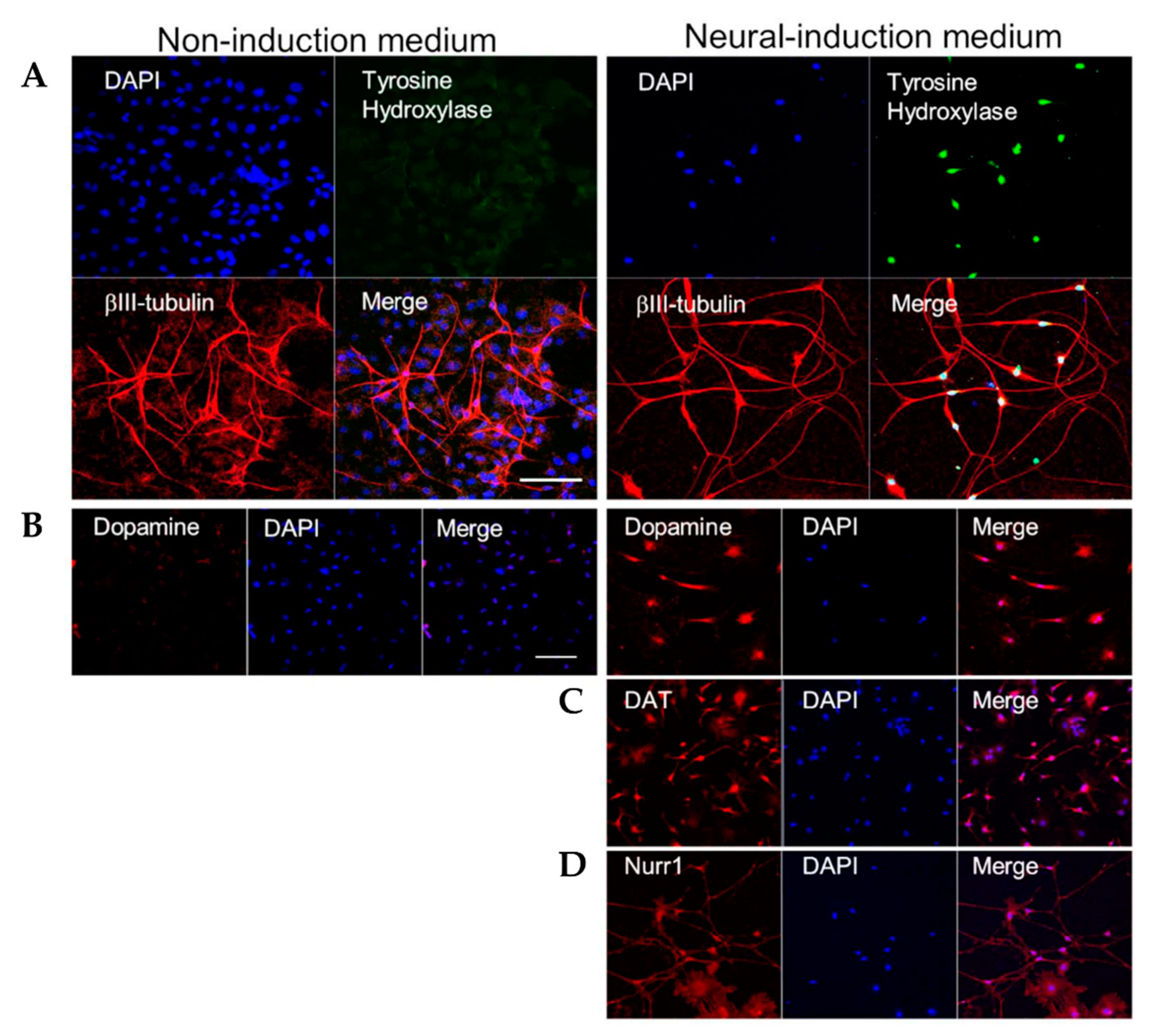
2.3. Immunofluorescence Staining
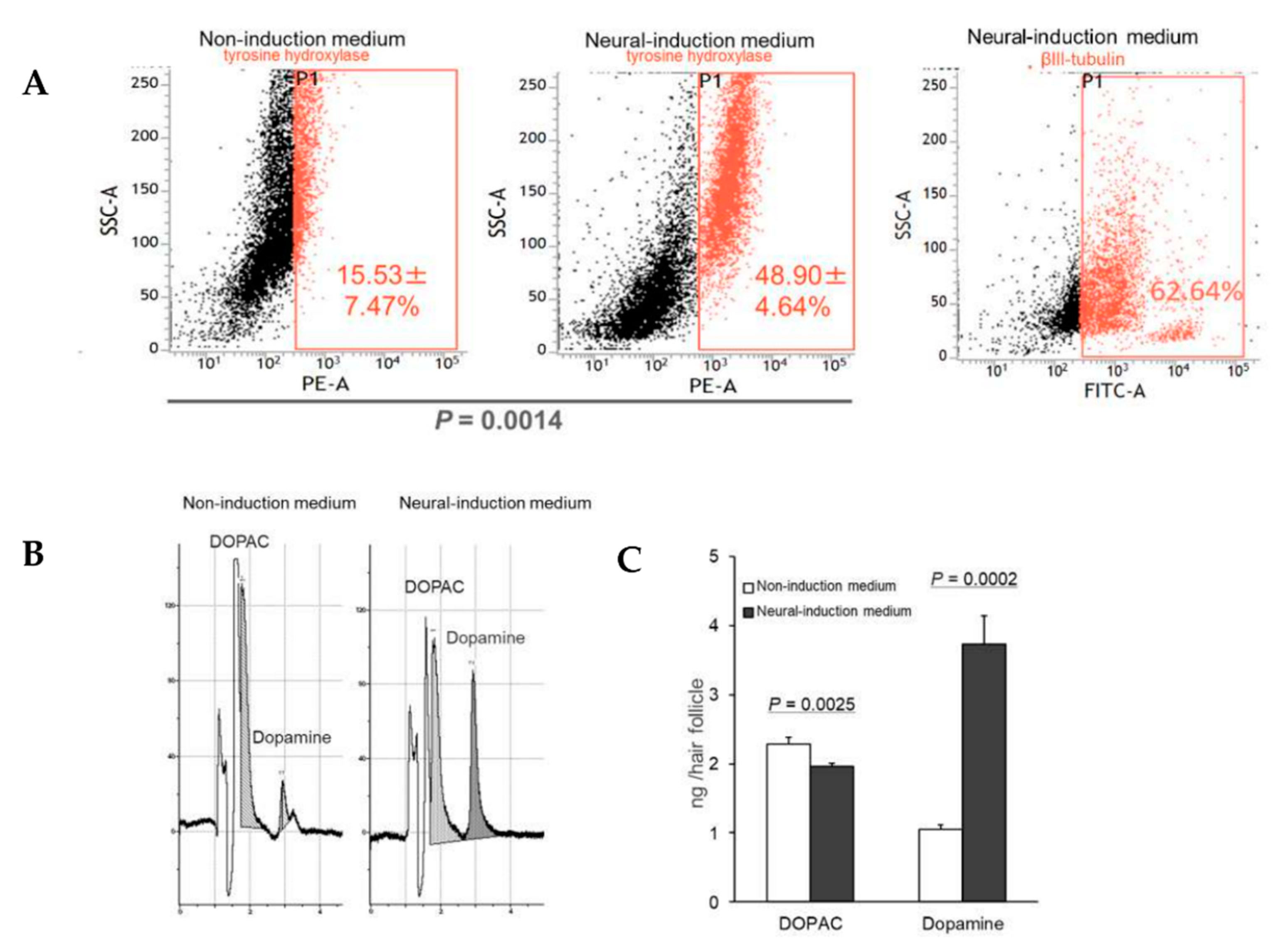
2.4. Fluorescence-Activated Cell Sorting (FACS)
2.5. High Performance Liquid Chromatography (HPLC)
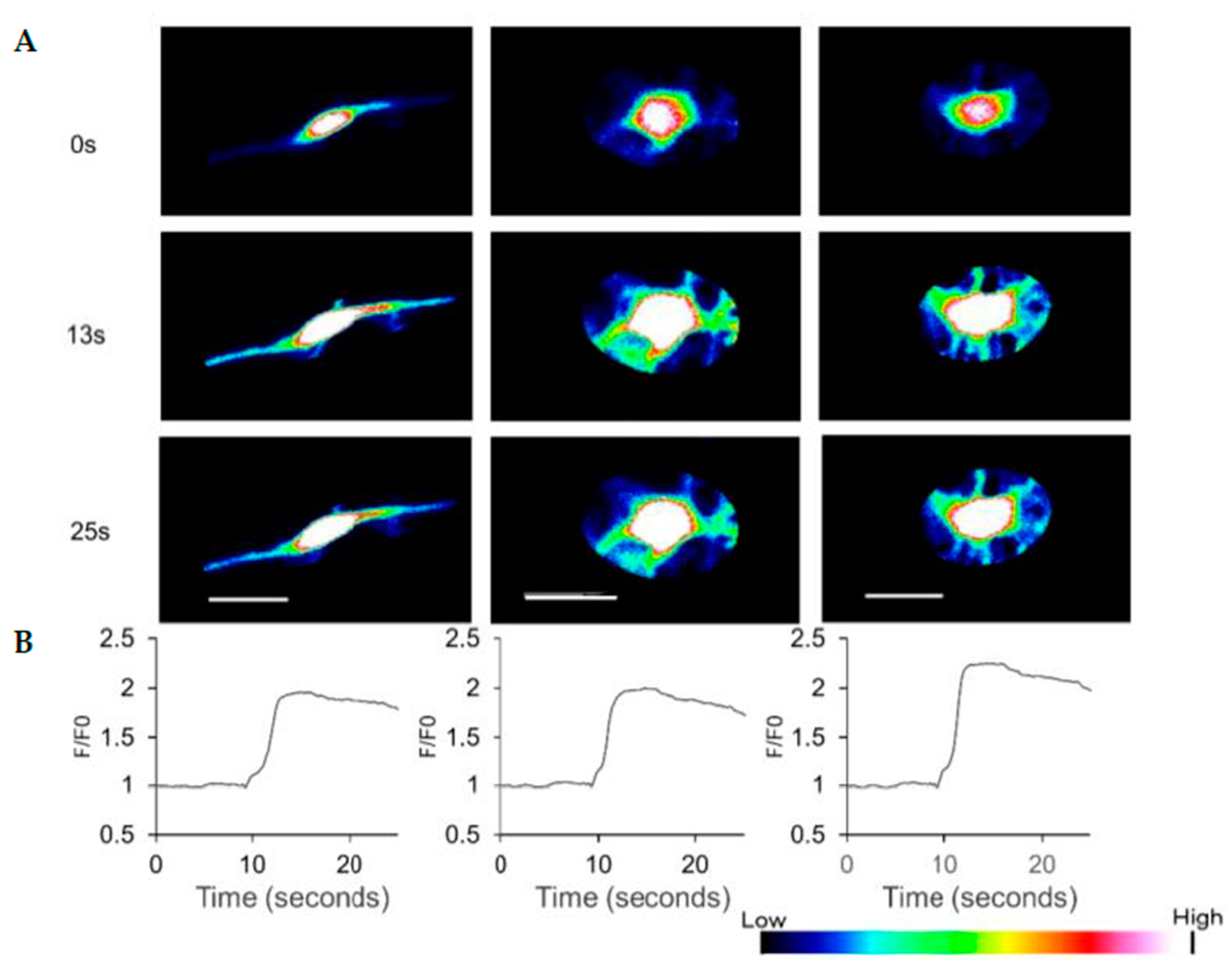
2.6. Ca2+ Imaging
2.7. Statistical Analysis
3. Results
3.1. HAP Stem Cells Differentiate Efficiently to Dopaminergic Neurons
3.2. HAP Stem Cells Differentiated to Dopaminergic Neurons That Extensively Proliferate
3.3. HAP Stem Cells Differentiated to Dopaminergic Neurons Secreted Dopamine at High Levels
3.4. HAP Stem Cells Differentiated to Dopaminergic Neurons Have Increased Ca2+ Levels When Treated with ATP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yashiro, M.; Mii, S.; Aki, R.; Hamada, Y.; Arakawa, N.; Kawahara, K.; Hoffman, R.M.; Amoh, Y. From hair to heart: Nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 2015, 14, 2362–2366. [Google Scholar] [CrossRef]
- Yamazaki, A.; Yashiro, M.; Mii, S.; Aki, R.; Hamada, Y.; Arakawa, N.; Kawahara, K.; Hoffman, R.M.; Amoh, Y. Isoproterenol directs hair follicle-associated pluripotent (HAP) stem cells to differentiate in vitro to cardiac muscle cells which can be induced to form beating heart-muscle tissue sheets. Cell Cycle 2016, 15, 760–765. [Google Scholar] [CrossRef]
- Hoffman, R.M. The pluripotency of hair follicle stem cells. Cell Cycle 2006, 5, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Amoh, Y.; Kanoh, M.; Niiyama, S.; Kawahara, K.; Satoh, Y.; Katsuoka, K.; Hoffman, R.M. Human and mouse hair follicles contain both multipotent and monopotent stem cells. Cell Cycle 2009, 8, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Tohgi, N.; Mii, S.; Hamada, Y.; Arakawa, N.; Aki, R.; Singh, S.R.; Hoffman, R.M.; Amoh, Y. Hair-follicle-associated pluripotent stem cells derived from cryopreserved intact human hair follicles sustain multilineage differentiation potential. Sci. Rep. 2019, 9, 9326. [Google Scholar] [CrossRef]
- Yu, H.; Fang, D.; Kumar, S.M.; Li, L.; Nguyen, T.K.; Acs, G.; Herlyn, M.; Xu, X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am. J. Pathol. 2006, 168, 1879–1888. [Google Scholar] [CrossRef]
- Amoh, Y.; Li, L.; Campillo, R.; Kawahara, K.; Katsuoka, K.; Penman, S.; Hoffman, R.M. Implanted hair follicle stem cells form Schwann cells which support repair of severed peripheral nerves. Proc. Natl. Acad. Sci. USA 2005, 102, 17734–17738. [Google Scholar] [CrossRef]
- Amoh, Y.; Li, L.; Katsuoka, K.; Hoffman, R.M. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008, 7, 1865–1869. [Google Scholar] [CrossRef]
- Amoh, Y.; Hamada, Y.; Aki, R.; Kawahara, K.; Hoffman, R.M.; Katsuoka, K. Direct transplantation of uncultured hair-follicle pluripotent stem (hfPS) cells promotes the recovery of peripheral nerve injury. J. Cell Biochem. 2010, 110, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Amoh, Y.; Aki, R.; Hamada, Y.; Niiyama, S.; Eshima, K.; Kawahara, K.; Sato, Y.; Tani, Y.; Hoffman, R.M.; Katsuoka, K. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J. Dermatol. 2012, 39, 33–38. [Google Scholar] [CrossRef]
- Amoh, Y.; Katsuoka, K.; Hoffman, R.M. Peripheral-nerve and spinal-cord regeneration in mice using hair-follicle-associated pluripotent (HAP) stem cells. Methods Mol. Biol. 2016, 1453, 21–32. [Google Scholar] [CrossRef]
- Yamazaki, A.; Obara, K.; Tohgi, N.; Shirai, K.; Mii, S.; Hamada, Y.; Arakawa, N.; Aki, R.; Hoffman, R.M.; Amoh, Y. Implanted hair-follicle-associated pluripotent (HAP) stem cells encapsulated in polyvinylidene fluoride membrane cylinders promote effective recovery of peripheral nerve injury. Cell Cycle 2017, 16, 1927–1932. [Google Scholar] [CrossRef]
- Tohgi, N.; Obara, K.; Yashiro, M.; Hamada, Y.; Arakawa, N.; Mii, S.; Aki, R.; Hoffman, R.M.; Amoh, Y. Human hair-follicle associated pluripotent (hHAP) stem cells differentiate to cardiac-muscle cells. Cell Cycle 2017, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Uchugonova, A.; Kimura, H.; Zhang, C.; Zhao, M.; Zhang, L.; Koenig, K.; Duong, J.; Aki, R.; Saito, N.; et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 2011, 10, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, N.; Nobakht, M.; Pourheydar, B.; Golmohammadi, M.G. Rat hair follicle stem cells differentiate and promote recovery following spinal cord injury. Neural Regen Res. 2013, 8, 3365–3372. [Google Scholar] [CrossRef]
- Kajiura, S.; Mii, S.; Aki, R.; Hamada, Y.; Arakawa, N.; Kawahara, K.; Li, L.; Katsuoka, K.; Hoffman, R.M.; Amoh, Y. Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair Fol-licle-associated pluripotent stem cells. Tissue Eng. Part C 2015, 21, 825–831. [Google Scholar] [CrossRef]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Yamamori, S.; Suzuki, E.; Watanabe, S.; Sato, T.; Miyaoka, H.; Azuma, S.; Ikegami, S.; Kuwahara, R.; Suzuki-Migishima, R.; et al. A single amino acid mutation in SNAP-25 induces anxiety-related behavior in mouse. PLoS ONE 2011, 6, e25158. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Song, B.; Cha, Y.; Ko, S.; Jeon, J.; Lee, N.; Seo, H.; Park, K.J.; Lee, I.H.; Lopes, C.; Feitosa, M.; et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Investig. 2020, 130, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Doi, D.; Samata, B.; Katsukawa, M.; Kikuchi, T.; Morizane, A.; Ono, Y.; Sekiguchi, K.; Nakagawa, M.; Parmar, M.; Takahashi, J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014, 2, 337–350. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Narytnyk, A.; Verdon, B.; Loughney, A.; Sweeney, M.; Clewes, O.; Taggart, M.J.; Sieber-Blum, M. Differentiation of human epidermal neural crest stem cells (hEPI-NCSC) into virtually homogenous populations of dopaminergic neurons. Stem Cell Rev. 2014, 10, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Hassanzadeh, G.; Joghataei, M.T.; Soleimani, M.; Moradi, F.; Mohammadpour, S.; Ghorbani, J.; Safavi, A.; Sarbishegi, M.; Mahabadi, V.P.; et al. In vitro differentiation of neural stem cells derived from human olfactory bulb into dopaminergic-like neurons. Eur. J. Neurosci. 2017, 45, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 3594. [Google Scholar] [CrossRef]
- Hartfield, E.M.; Yamasaki-Mann, M.; Fernandes, H.J.R.; Vowles, J.; James, W.S.; Cowley, S.A.; Wade-Martin, R. Physiological characterisation of human iPS-derived dopaminergic neurons. PLoS ONE 2014, 9, e87388. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Koo, S.Y.; Studer, L. Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities. Front. Cell Dev. Biol. 2020, 8, 729. [Google Scholar] [CrossRef]
- Li, L.; Mignone, J.; Yang, M.; Matic, M.; Penman, S.; Enikolopov, G.; Hoffman, R.M. Nestin expression in hair follicle sheath progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9958–9961. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamane, M.; Takaoka, N.; Obara, K.; Shirai, K.; Aki, R.; Hamada, Y.; Arakawa, N.; Hoffman, R.M.; Amoh, Y. Hair-Follicle-Associated Pluripotent (HAP) Stem Cells Can Extensively Differentiate to Tyrosine-Hydroxylase-Expressing Dopamine-Secreting Neurons. Cells 2021, 10, 864. https://doi.org/10.3390/cells10040864
Yamane M, Takaoka N, Obara K, Shirai K, Aki R, Hamada Y, Arakawa N, Hoffman RM, Amoh Y. Hair-Follicle-Associated Pluripotent (HAP) Stem Cells Can Extensively Differentiate to Tyrosine-Hydroxylase-Expressing Dopamine-Secreting Neurons. Cells. 2021; 10(4):864. https://doi.org/10.3390/cells10040864
Chicago/Turabian StyleYamane, Michiko, Nanako Takaoka, Koya Obara, Kyoumi Shirai, Ryoichi Aki, Yuko Hamada, Nobuko Arakawa, Robert M. Hoffman, and Yasuyuki Amoh. 2021. "Hair-Follicle-Associated Pluripotent (HAP) Stem Cells Can Extensively Differentiate to Tyrosine-Hydroxylase-Expressing Dopamine-Secreting Neurons" Cells 10, no. 4: 864. https://doi.org/10.3390/cells10040864
APA StyleYamane, M., Takaoka, N., Obara, K., Shirai, K., Aki, R., Hamada, Y., Arakawa, N., Hoffman, R. M., & Amoh, Y. (2021). Hair-Follicle-Associated Pluripotent (HAP) Stem Cells Can Extensively Differentiate to Tyrosine-Hydroxylase-Expressing Dopamine-Secreting Neurons. Cells, 10(4), 864. https://doi.org/10.3390/cells10040864




