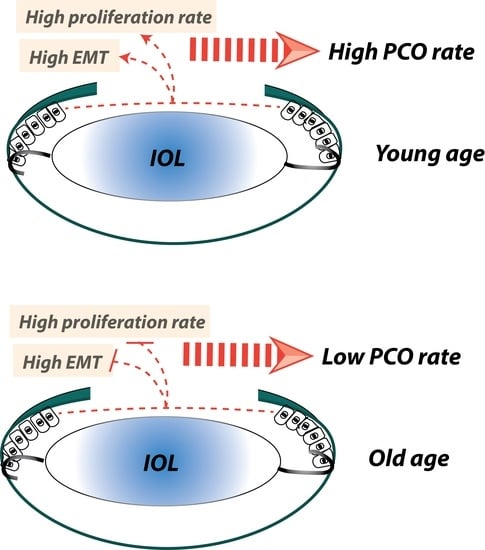
Aged Lens Epithelial Cells Suppress Proliferation and Epithelial–Mesenchymal Transition-Relevance for Posterior Capsule Opacification
Abstract
:1. Introduction
2. Methods
2.1. Reagents
2.2. Cell Culture and Lens Epithelial Cell Aging Model
2.3. Animals
2.4. Cell Proliferation Assay
2.5. RNAseq and Data Analysis
2.6. Gene Set Enrichment Analysis (GSEA)
2.7. TGFβ2 Treatment
2.8. Cell Immunofluorescence Staining
2.9. Mouse Cataract Surgery
2.10. Immunofluorescence Staining of Paraffin Sections
2.11. Confocal Microscopy
2.12. Immunoblot Assay
2.13. Statistics
3. Results
3.1. FHL124 Is a Suitable Human Lens Epithelial Cell Line for Aging Study
3.2. Aging Induces Substantial Gene Expression Changes in Lens Epithelial Cells
3.3. Aging Decelerates Proliferation in Lens Epithelial Cells
3.4. Aged Lens Epithelial Cells Suppress Epithelial–Mesenchymal Transition (EMT)
3.5. Lens Epithelial Cells Gradually Decrease αSMA and Fibronectin Expression in Aging
3.6. High Passage Lens Epithelial Cells Are Less Sensitive to TGFβ2-Mediated EMT
3.7. Aged Mice Alleviate Lens Epithelial Cell EMT Signaling after Cataract Surgery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, A.; Gilbert, C.; Rahi, J. Epidemiology of cataract in childhood: A global perspective. J. Cataract. Refract. Surg. 1997, 23 (Suppl. S1), 601–604. [Google Scholar] [CrossRef]
- Vella, M.; Wickremasinghe, S.; Gupta, N.; Andreou, P.; Sinha, A. YAG laser capsulotomy, an unusual complication. Eye 2004, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Basti, S.; Ravishankar, U.; Gupta, S. Results of a prospective evaluation of three methods of management of pediatric cataracts. Ophthalmology 1996, 103, 713–720. [Google Scholar] [CrossRef]
- Knight-Nanan, D.; O’Keefe, M.; Bowell, R. Outcome and complications of intraocular lenses in children with cataract. J. Cataract. Refract. Surg. 1996, 22, 730–736. [Google Scholar] [CrossRef]
- Koch, D.D.; Kohnen, T. Retrospective comparison of techniques to prevent secondary cataract formation after posterior chamber intraocular lens implantation in infants and children. J. Cataract. Refract. Surg. 1997, 23 (Suppl. S1), 657–663. [Google Scholar] [CrossRef]
- Vasavada, A.; Chauhan, H. Intraocular lens implantation in infants with congenital cataracts. J. Cataract. Refract. Surg. 1994, 20, 592–598. [Google Scholar] [CrossRef]
- O’Keefe, M.; Fenton, S.; Lanigan, B. Visual outcomes and complications of posterior chamber intraocular lens implantation in the first year of life. J. Cataract. Refract. Surg. 2001, 27, 2006–2011. [Google Scholar] [CrossRef]
- Vasavada, A.R.; Praveen, M.R.; Tassignon, M.J.; Shah, S.K.; Vasavada, V.A.; Vasavada, V.A.; Van Looveren, J.; De Veuster, I.; Trivedi, R.H. Posterior capsule management in congenital cataract surgery. J. Cataract. Refract. Surg. 2011, 37, 173–193. [Google Scholar] [CrossRef]
- Ursell, P.G.; Dhariwal, M.; Majirska, K.; Ender, F.; Kalson-Ray, S.; Venerus, A.; Miglio, C.; Bouchet, C. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: A UK Real World Evidence study. Eye 2018, 32, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Ursell, P.G.; Dhariwal, M.; O’Boyle, D.; Khan, J.; Venerus, A. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: A real-world evidence study of 20,763 eyes. Eye 2020, 34, 960–968. [Google Scholar] [CrossRef]
- Lindholm, J.M.; Laine, I.; Tuuminen, R. Five-Year Cumulative Incidence and Risk Factors of Nd:YAG Capsulotomy in 10 044 Hydrophobic Acrylic 1-Piece and 3-Piece Intraocular Lenses. Am. J. Ophthalmol. 2019, 200, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wormstone, I.M. Posterior capsule opacification: A cell biological perspective. Exp. Eye Res. 2002, 74, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Wormstone, I.M.; Wormstone, Y.M.; Smith, A.J.O.; Eldred, J.A. Posterior capsule opacification: What’s in the bag? Prog. Retin. Eye Res. 2021, 82, 100905. [Google Scholar] [CrossRef]
- Walker, J.L.; Wolff, I.M.; Zhang, L.; Menko, A.S. Activation of SRC kinases signals induction of posterior capsule opacification. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Taiyab, A.; Holms, J.; West-Mays, J.A. beta-Catenin/Smad3 Interaction Regulates Transforming Growth Factor-beta-Induced Epithelial to Mesenchymal Transition in the Lens. Int. J. Mol. Sci. 2019, 20, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, D.Y.; Lovicu, F.J. Enhanced EGF receptor-signaling potentiates TGFbeta-induced lens epithelial-mesenchymal transition. Exp. Eye Res. 2019, 185, 107693. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Kanwar, M.; Wang, Y.; Jackson, E.E.; Faranda, A.P.; Duncan, M.K. Fibronectin has multifunctional roles in posterior capsular opacification (PCO). Matrix Biol. 2020, 90, 79–108. [Google Scholar] [CrossRef]
- Tomasetti, C.; Poling, J.; Roberts, N.J.; London, N.R., Jr.; Pittman, M.E.; Haffner, M.C.; Rizzo, A.; Baras, A.; Karim, B.; Kim, A.; et al. Cell division rates decrease with age, providing a potential explanation for the age-dependent deceleration in cancer incidence. Proc. Natl. Acad. Sci. USA 2019, 116, 20482–20488. [Google Scholar] [CrossRef] [Green Version]
- Wormstone, I.M.; Liu, C.S.; Rakic, J.M.; Marcantonio, J.M.; Vrensen, G.F.; Duncan, G. Human lens epithelial cell proliferation in a protein-free medium. Investig. Ophthalmol. Vis. Sci. 1997, 38, 396–404. [Google Scholar]
- Reddan, J.R.; Lindemann, C.B.; Hitt, A.L.; Bagchi, M.; Raphtis, E.M.; Pena, J.T.; Dziedzic, D.C. Generation of two non-transfected human lens cell lines. Investig. Ophthalmol. Vis. Sci. 1999, 40, S970. [Google Scholar]
- Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X.; Fan, X. Aging lens epithelium is susceptible to ferroptosis. Free Radic. Biol. Med. 2021, 167, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Whitson, J.A.; Zhang, X.; Medvedovic, M.; Chen, J.; Wei, Z.; Monnier, V.M.; Fan, X. Transcriptome of the GSH-Depleted Lens Reveals Changes in Detoxification and EMT Signaling Genes, Transport Systems, and Lipid Homeostasis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2666–2684. [Google Scholar] [CrossRef]
- Hoang, T.V.; Kumar, P.K.; Sutharzan, S.; Tsonis, P.A.; Liang, C.; Robinson, M.L. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol. Vis. 2014, 20, 1491–1517. [Google Scholar] [PubMed]
- Zhao, Y.; Zheng, D.; Cvekl, A. A comprehensive spatial-temporal transcriptomic analysis of differentiating nascent mouse lens epithelial and fiber cells. Exp. Eye Res. 2018, 175, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Faranda, A.P.; Shihan, M.H.; Wang, Y.; Duncan, M.K. The aging mouse lens transcriptome. Exp. Eye Res. 2021, 209, 108663. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Caty, J.; Whitson, J.; Zhang, A.D.; Srinivasagan, R.; Kavanagh, T.J.; Yan, H.; Fan, X. Reduced Glutathione Level Promotes Epithelial-Mesenchymal Transition in Lens Epithelial Cells via a Wnt/beta-Catenin-Mediated Pathway: Relevance for Cataract Therapy. Am. J. Pathol. 2017, 187, 2399–2412. [Google Scholar] [CrossRef] [Green Version]
- Eldred, J.A.; Hodgkinson, L.M.; Dawes, L.J.; Reddan, J.R.; Edwards, D.R.; Wormstone, I.M. MMP2 activity is critical for TGFbeta2-induced matrix contraction--implications for fibrosis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4085–4098. [Google Scholar] [CrossRef]
- Wormstone, I.M.; Tamiya, S.; Eldred, J.A.; Lazaridis, K.; Chantry, A.; Reddan, J.R.; Anderson, I.; Duncan, G. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp. Eye Res. 2004, 78, 705–714. [Google Scholar] [CrossRef]
- Lachke, S.A.; Ho, J.W.; Kryukov, G.V.; O’Connell, D.J.; Aboukhalil, A.; Bulyk, M.L.; Park, P.J.; Maas, R.L. iSyTE: Integrated Systems Tool for Eye gene discovery. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1617–1627. [Google Scholar] [CrossRef] [Green Version]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Das, S.J.; Wishart, T.F.L.; Jandeleit-Dahm, K.; Lovicu, F.J. Nox4-mediated ROS production is involved, but not essential for TGFbeta-induced lens EMT leading to cataract. Exp. Eye Res. 2020, 192, 107918. [Google Scholar] [CrossRef]
- Terrell, A.M.; Anand, D.; Smith, S.F.; Dang, C.A.; Waters, S.M.; Pathania, M.; Beebe, D.C.; Lachke, S.A. Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genes. Exp. Eye Res. 2015, 131, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Bigagli, E.; Luceri, C.; Scartabelli, T.; Dolara, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Giovannelli, L. Long-term Neuroglial Cocultures as a Brain Aging Model: Hallmarks of Senescence, MicroRNA Expression Profiles, and Comparison with In Vivo Models. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. From cells to organisms: Can we learn about aging from cells in culture? Exp. Gerontol. 2001, 36, 607–618. [Google Scholar] [CrossRef]
- Marko Bunc, S.M.H.; Jose, M.P.; Julijana, K.; Orlu, M. Characterisation of an In Vitro Aged Cell Culture Model. Farmacia 2019, 67, 240–247. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Li, L.; Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014, 157, 935–949. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yan, Q.; Wolf, N.S. Long-term caloric restriction delays age-related decline in proliferation capacity of murine lens epithelial cells in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 1997, 38, 100–107. [Google Scholar]
- Dawes, L.J.; Duncan, G.; Wormstone, I.M. Age-related differences in signaling efficiency of human lens cells underpin differential wound healing response rates following cataract surgery. Investig. Ophthalmol. Vis. Sci. 2013, 54, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Imran, S.A.M.; Yazid, M.D.; Idrus, R.B.H.; Maarof, M.; Nordin, A.; Razali, R.A.; Lokanathan, Y. Is There an Interconnection between Epithelial-Mesenchymal Transition (EMT) and Telomere Shortening in Aging? Int. J. Mol. Sci. 2021, 22, 3888. [Google Scholar] [CrossRef]
- Santos, F.; Moreira, C.; Nobrega-Pereira, S.; Bernardes de Jesus, B. New Insights into the Role of Epithelial(-)Mesenchymal Transition during Aging. Int. J. Mol. Sci. 2019, 20, 891. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arter. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef] [Green Version]
- Chilosi, M.; Poletti, V.; Zamo, A.; Lestani, M.; Montagna, L.; Piccoli, P.; Pedron, S.; Bertaso, M.; Scarpa, A.; Murer, B.; et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 2003, 162, 1495–1502. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, H.; Yu, S.; Lu, Y.; Wu, T. Research progress in the role and mechanism of Cadherin-11 in different diseases. J. Cancer 2021, 12, 1190–1199. [Google Scholar] [CrossRef]
- Kaur, H.; Phillips-Mason, P.J.; Burden-Gulley, S.M.; Kerstetter-Fogle, A.E.; Basilion, J.P.; Sloan, A.E.; Brady-Kalnay, S.M. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol. Cancer Res. 2012, 10, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, E.S.; Topham, J.T.; Karasinska, J.M.; Lee, M.K.C.; Williamson, L.M.; Mendis, S.; Denroche, R.E.; Jang, G.H.; Kalloger, S.E.; Moore, R.A.; et al. Delving into Early-onset Pancreatic Ductal Adenocarcinoma: How Does Age Fit In? Clin. Cancer Res. 2021, 27, 246–254. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Gordon, P.; Hao, C.; Huangfu, J.; Fan, E.; Zhang, X.; Yan, H.; Fan, X. Aged Lens Epithelial Cells Suppress Proliferation and Epithelial–Mesenchymal Transition-Relevance for Posterior Capsule Opacification. Cells 2022, 11, 2001. https://doi.org/10.3390/cells11132001
Wei Z, Gordon P, Hao C, Huangfu J, Fan E, Zhang X, Yan H, Fan X. Aged Lens Epithelial Cells Suppress Proliferation and Epithelial–Mesenchymal Transition-Relevance for Posterior Capsule Opacification. Cells. 2022; 11(13):2001. https://doi.org/10.3390/cells11132001
Chicago/Turabian StyleWei, Zongbo, Pasley Gordon, Caili Hao, Jingru Huangfu, Emily Fan, Xiang Zhang, Hong Yan, and Xingjun Fan. 2022. "Aged Lens Epithelial Cells Suppress Proliferation and Epithelial–Mesenchymal Transition-Relevance for Posterior Capsule Opacification" Cells 11, no. 13: 2001. https://doi.org/10.3390/cells11132001