Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Live Sensor Dyes
2.3. Immunostaining
2.4. Scratch Assay
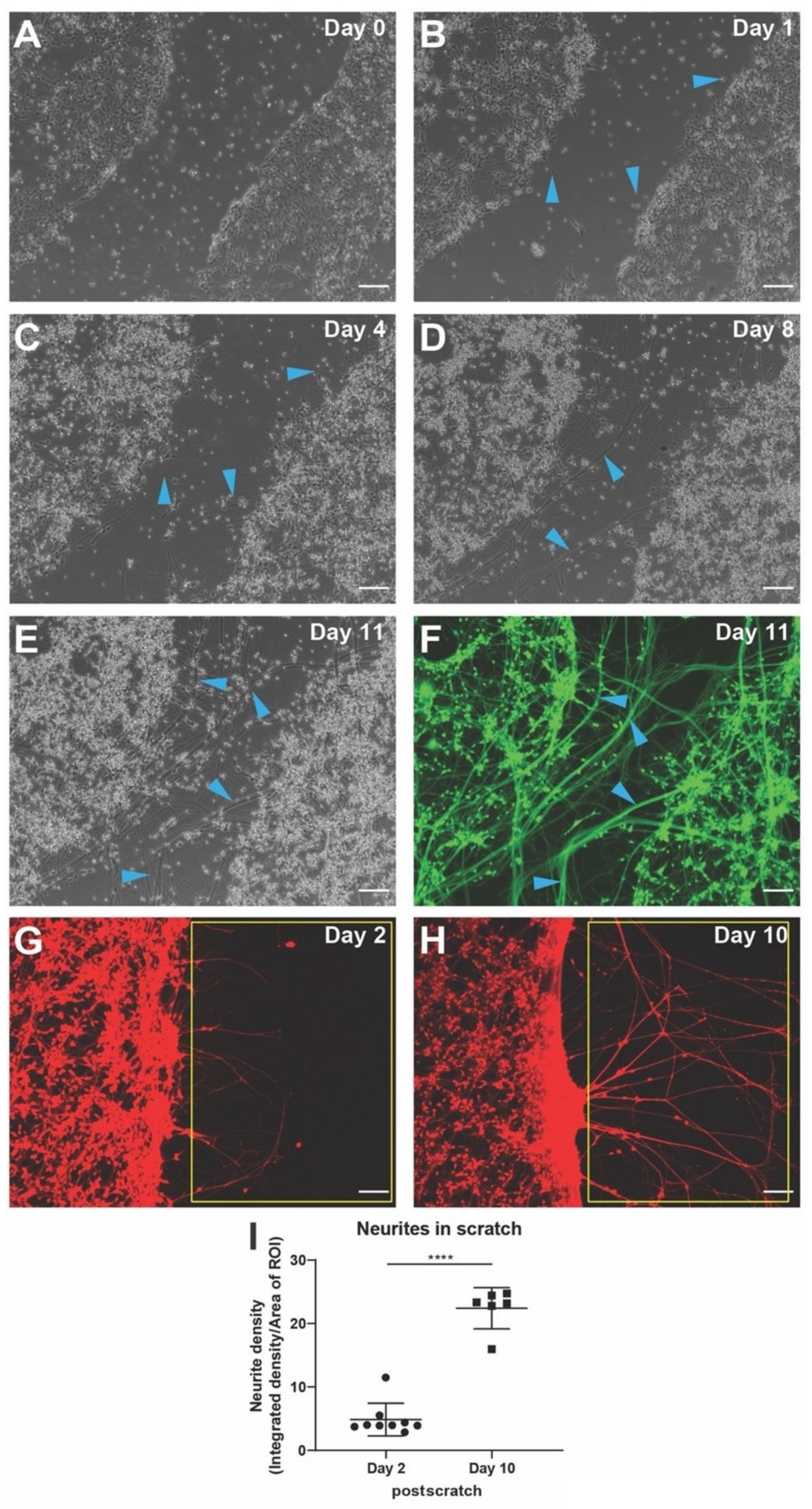
2.5. Neurite Density Quantification
2.6. Statistics
3. Results and Discussion
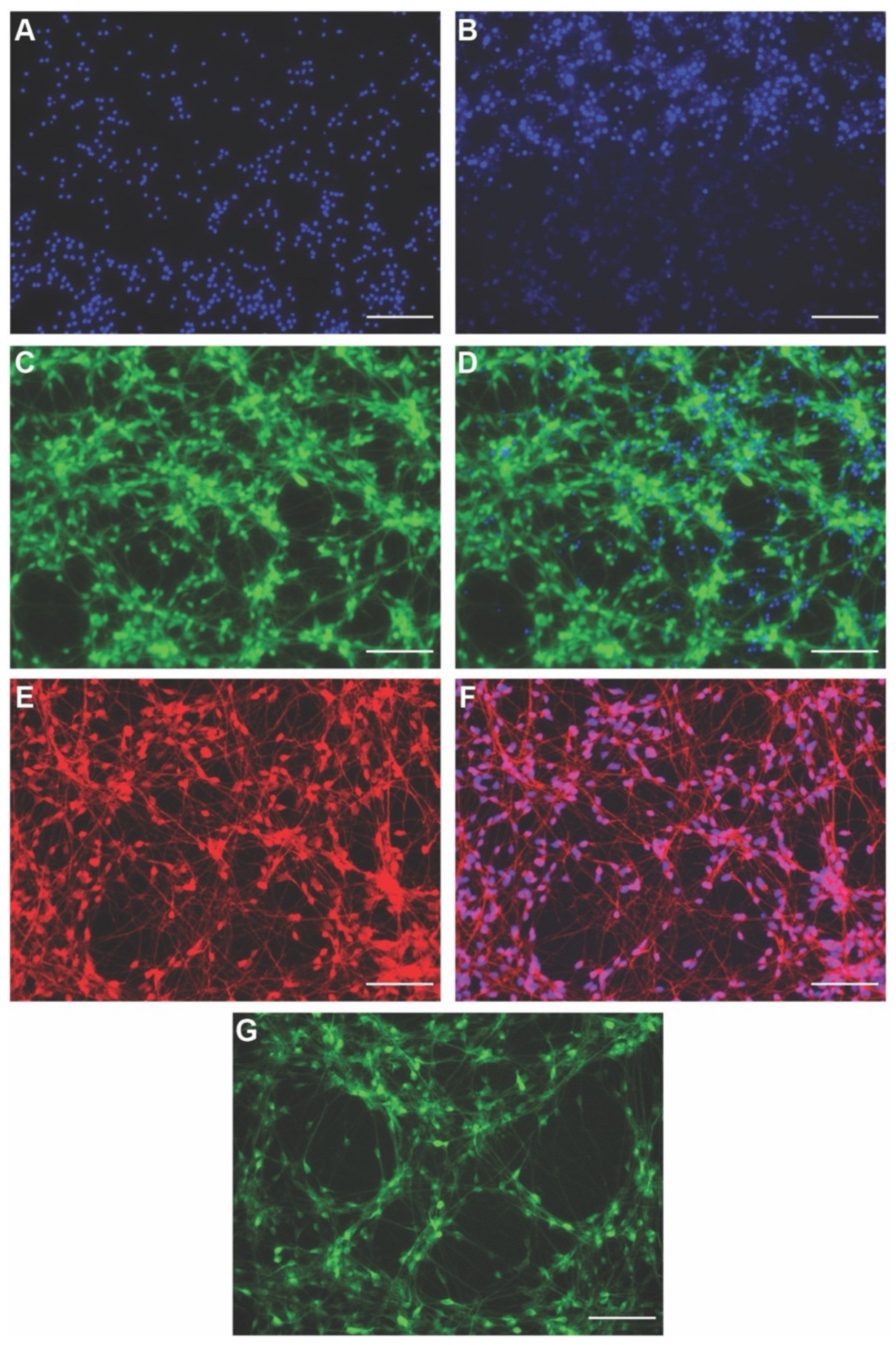
3.1. Establishing Human Induced Neural Stem Cell (hiNSC)-Derived Neuron Cultures
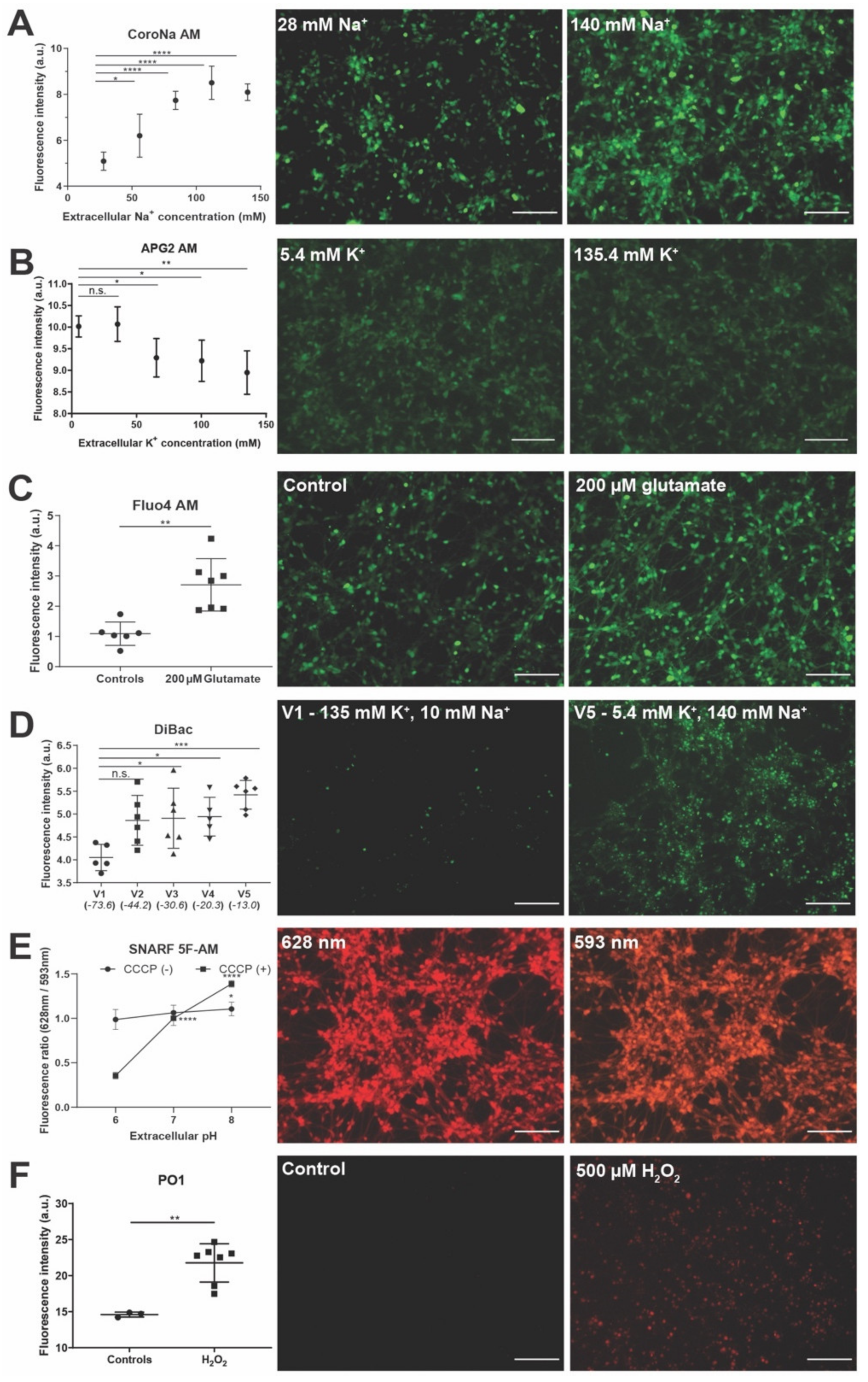
3.2. Live Sensors for Detecting Healthy Neurons and Their Morphology in hiNSC-Derived Neurons
3.3. Live Sensors for Detecting Changes in Intracellular Ion Concentrations and Resting Membrane Potential in hiNSC-Derived Neurons
3.4. Live Sensors for Detecting Changes in Cellular State Such as pH and Metabolism in hiNSC-Derived Neurons
3.5. Establishing Scratch Assay for Neuronal Regeneration and Quantitative Determination of Neurite Outgrowth from Injured Neurons Using hiNSC-Derived Neurons
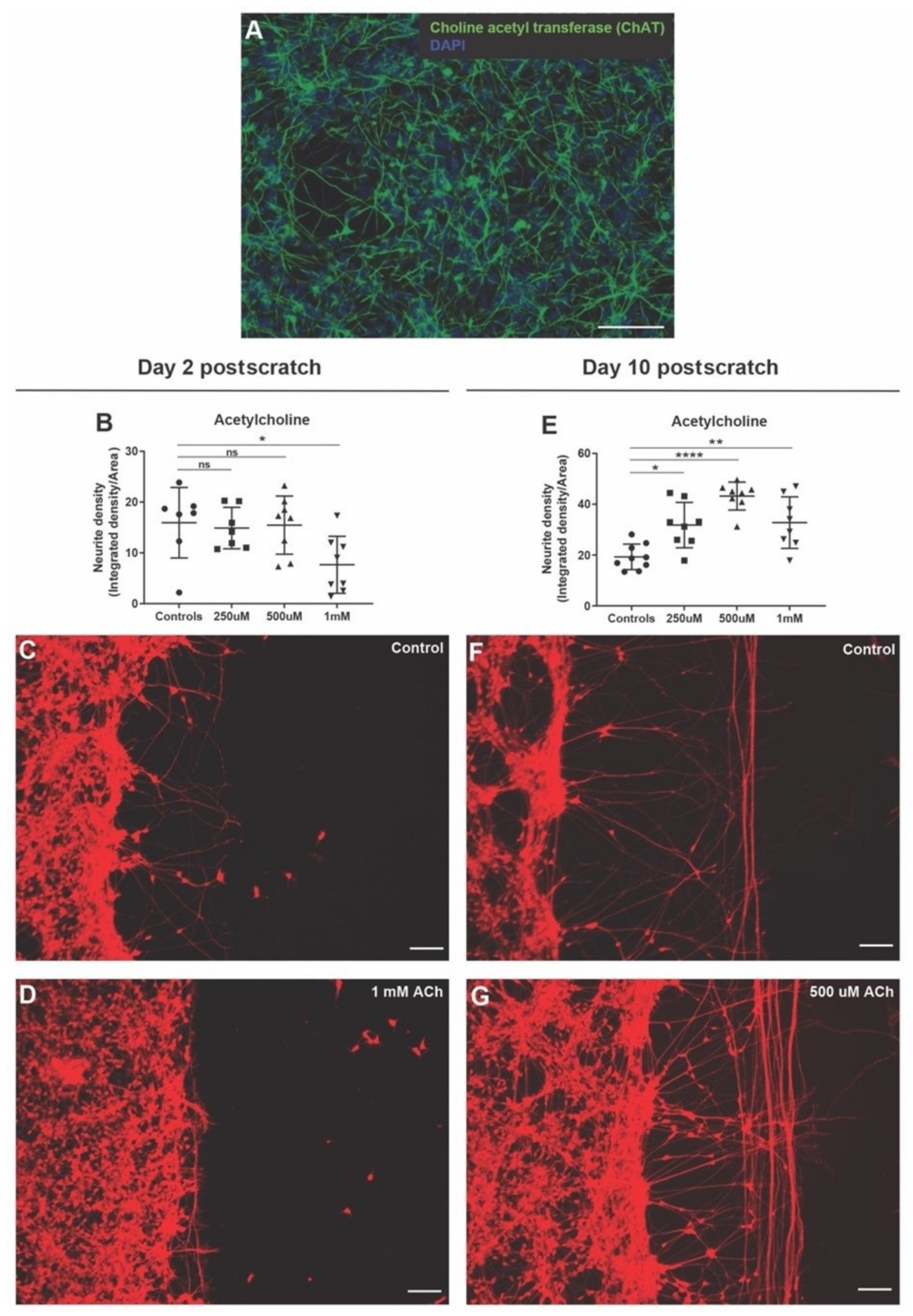
3.6. Acetylcholine Has Biphasic Effect on Neurite Outgrowth after Scratch Injury
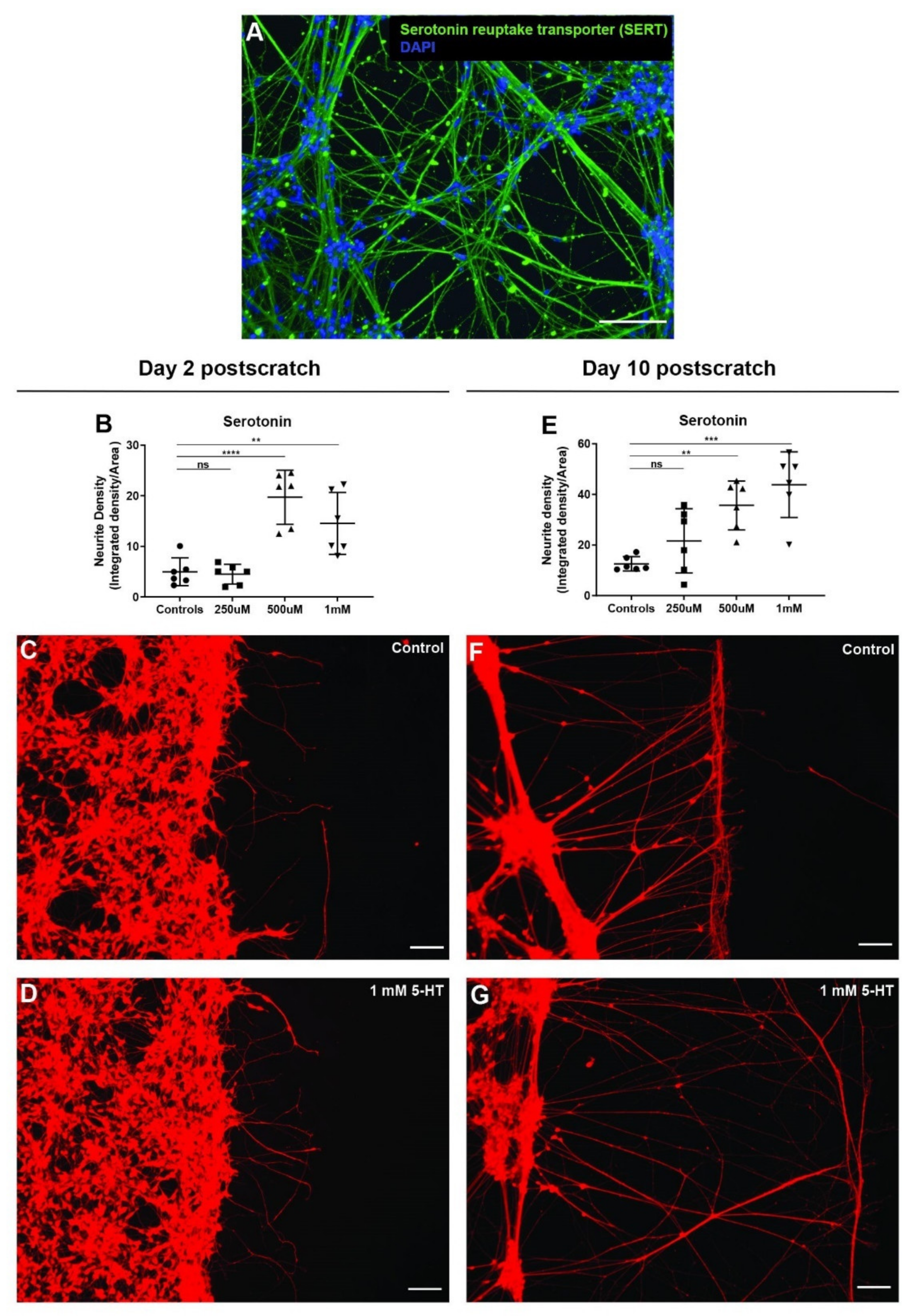
3.7. Serotonin Significantly Enhances Neurite Outgrowth after Scratch Injury
3.8. GABA Has No Effect on Neurite Outgrowth after Scratch
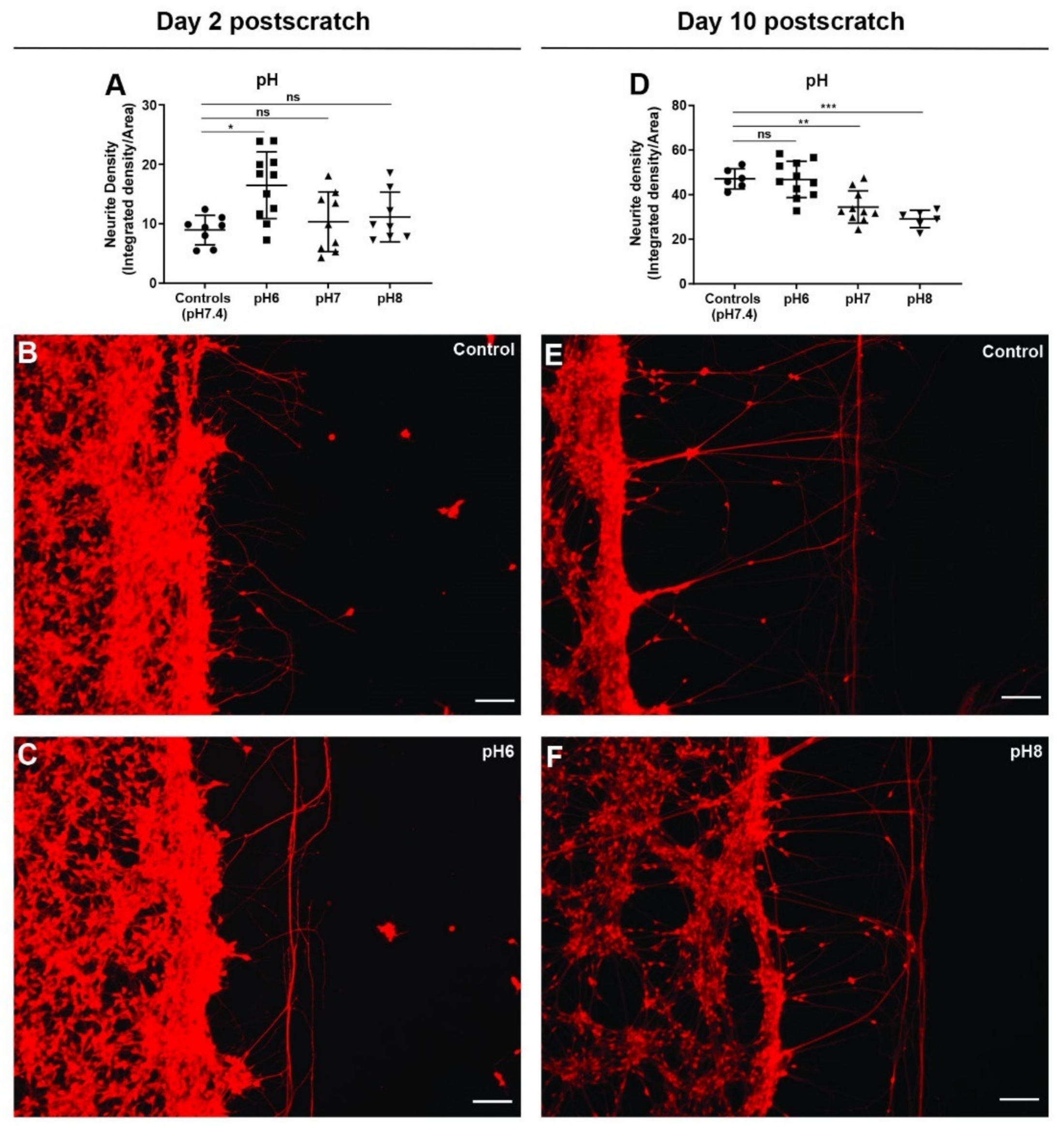
3.9. Extracellular pH Change Has Biphasic Effect on Neurite Outgrowth after Scratch Injury
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef]
- Nuccitelli, R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat. Prot. Dosim. 2003, 106, 375–383. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, J.; Xiong, L.; Liu, J.; Prindle, A.; Yuan, F.; Arjes, H.A.; Tsimring, L.; Suel, G.M. Species-Independent Attraction to Biofilms through Electrical Signaling. Cell 2017, 168, 200–209.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, M.; Martyniuk, C.J. The bioelectric code: An ancient computational medium for dynamic control of growth and form. Biosystems 2018, 164, 76–93. [Google Scholar] [CrossRef]
- Bates, E. Ion channels in development and cancer. Annu. Rev. Cell Dev. Biol. 2015, 31, 231–247. [Google Scholar] [CrossRef]
- Harris, M.P. Bioelectric signaling as a unique regulator of development and regeneration. Development 2021, 148, dev180794. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E. Plant biology: Electric defence. Nature 2013, 500, 404–405. [Google Scholar] [CrossRef]
- Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef]
- Stanger, B.Z. Organ size determination and the limits of regulation. Cell Cycle 2008, 7, 318–324. [Google Scholar] [CrossRef]
- Thompson, D. On Growith and Form; Cambridge University Press: Cambridge, UK, 1942. [Google Scholar]
- Harvey, K.F.; Hariharan, I.K. The hippo pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011288. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Pai, V.P.; Lemire, J.M.; Pare, J.F.; Lin, G.; Chen, Y.; Levin, M. Endogenous Gradients of Resting Potential Instructively Pattern Embryonic Neural Tissue via Notch Signaling and Regulation of Proliferation. J. Neurosci. 2015, 35, 4366–4385. [Google Scholar] [CrossRef] [Green Version]
- Pai, V.P.; Aw, S.; Shomrat, T.; Lemire, J.M.; Levin, M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 2012, 139, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.W.; Masak, G.; Yang, J.; Davidson, L.A. From biomechanics to mechanobiology: Xenopus provides direct access to the physical principles that shape the embryo. Curr. Opin. Genet. Dev. 2020, 63, 71–77. [Google Scholar] [CrossRef]
- Davidson, L.A. Mechanical design in embryos: Mechanical signalling, robustness and developmental defects. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150516. [Google Scholar] [CrossRef] [Green Version]
- Boulan, L.; Leopold, P. What determines organ size during development and regeneration? Development 2021, 148, dev196063. [Google Scholar] [CrossRef]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [Green Version]
- Pai, V.P.; Levin, M. HCN2 Channel-induced Rescue of Brain, Eye, Heart, and Gut Teratogenesis Caused by Nicotine, Ethanol, and Aberrant Notch Signaling. Wound Repair Regen. 2022. [CrossRef]
- Pai, V.P.; Pietak, A.; Willocq, V.; Ye, B.; Shi, N.Q.; Levin, M. HCN2 Rescues brain defects by enforcing endogenous voltage pre-patterns. Nat. Commun. 2018, 9, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, V.P.; Cervera, J.; Mafe, S.; Willocq, V.; Lederer, E.K.; Levin, M. HCN2 Channel-Induced Rescue of Brain Teratogenesis via Local and Long-Range Bioelectric Repair. Front. Cell. Neurosci. 2020, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Churchill, C.D.M.; Winter, P.; Tuszynski, J.A.; Levin, M. EDEn-Electroceutical Design Environment: Ion Channel Tissue Expression Database with Small Molecule Modulators. iScience 2019, 11, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Durant, F.; Whited, J.L. Finding Solutions for Fibrosis: Understanding the Innate Mechanisms Used by Super-Regenerator Vertebrates to Combat Scarring. Adv. Sci. 2021, 8, e2100407. [Google Scholar] [CrossRef]
- Slater, P.G.; Palacios, M.; Larrain, J. Xenopus, a Model to Study Wound Healing and Regeneration: Experimental Approaches. Cold Spring Harb. Protoc. 2021, 2021, pdb-top100966. [Google Scholar] [CrossRef]
- Kaliya-Perumal, A.K.; Ingham, P.W. Musculoskeletal regeneration: A zebrafish perspective. Biochimie 2022, 196, 171–181. [Google Scholar] [CrossRef]
- Chifflet, S.; Hernandez, J.A. The Epithelial Sodium Channel and the Processes of Wound Healing. BioMed Res. Int. 2016, 2016, 5675047. [Google Scholar] [CrossRef] [Green Version]
- Franklin, B.M.; Voss, S.R.; Osborn, J.L. Ion channel signaling influences cellular proliferation and phagocyte activity during axolotl tail regeneration. Mech. Dev. 2017, 146, 42–54. [Google Scholar] [CrossRef]
- Zhang, W.; Das, P.; Kelangi, S.; Bei, M. Potassium channels as potential drug targets for limb wound repair and regeneration. Precis. Clin. Med. 2020, 3, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Reid, B.; Zhao, M. The Electrical Response to Injury: Molecular Mechanisms and Wound Healing. Adv. Wound Care 2014, 3, 184–201. [Google Scholar] [CrossRef] [Green Version]
- Chifflet, S.; Hernandez, J.A.; Grasso, S. A possible role for membrane depolarization in epithelial wound healing. Am. J. Physiol. Physiol. 2005, 288, C1420–C1430. [Google Scholar] [CrossRef] [Green Version]
- Messerli, M.A.; Graham, D.M. Extracellular electrical fields direct wound healing and regeneration. Biol. Bull. 2011, 221, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Li, C.; Choi, Y.J.; Levin, M.; Kaplan, D.L. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials 2013, 34, 6695–6705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bei, M. Kcnh2 and Kcnj8 interactively regulate skin wound healing and regeneration. Wound Repair Regen. 2015, 23, 797–806. [Google Scholar] [CrossRef]
- Borgens, R.B.; Robinson, K.R.; Vanable, J.W., Jr.; McGinnis, M.E.; McCaig, C.D. Electric Fields in Vertebrate Repair: Natural and Applied Voltages in Vertebrate Regeneration and Healing; Wiley-Liss: New York, NY, USA, 1989. [Google Scholar]
- Pai, V.P.; Levin, M. Bioelectric Control of Stem Cell Function. In Stem Cells: From Basic Research to Therapy; Calegari, F., Waskov, C., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 106–148. [Google Scholar]
- Adams, D.S.; Masi, A.; Levin, M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 2007, 134, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Tseng, A.S.; Levin, M. Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration. Anat. Rec. 2012, 295, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- Perathoner, S.; Daane, J.M.; Henrion, U.; Seebohm, G.; Higdon, C.W.; Johnson, S.L.; Nusslein-Volhard, C.; Harris, M.P. Bioelectric signaling regulates size in zebrafish fins. PLoS Genet. 2014, 10, e1004080. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R. A role for endogenous electric fields in wound healing. Curr. Top. Dev. Biol. 2003, 58, 1–26. [Google Scholar]
- Singer, M. The influence of the nerve in regeneration of the amphibian extremity. Q. Rev. Biol. 1952, 27, 169–200. [Google Scholar] [CrossRef]
- Mitogawa, K.; Makanae, A.; Satoh, A. Hyperinnervation improves Xenopus laevis limb regeneration. Dev. Biol. 2018, 433, 276–286. [Google Scholar] [CrossRef]
- Nieto-Diaz, M.; Pita-Thomas, D.W.; Munoz-Galdeano, T.; Martinez-Maza, C.; Navarro-Ruiz, R.; Reigada, D.; Yunta, M.; Caballero-Lopez, M.J.; Nieto-Sampedro, M.; Martinez-Maza, R. Deer antler innervation and regeneration. Front. Biosci. 2012, 17, 1389–1401. [Google Scholar] [CrossRef] [Green Version]
- Filoni, S.; Velloso, C.P.; Bernardini, S.; Cannata, S.M. Acquisition of nerve dependence for the formation of a regeneration blastema in amputated hindlimbs of larval Xenopus laevis: The role of limb innervation and that of limb differentiation. J. Exp. Zool. 1995, 273, 327–341. [Google Scholar] [CrossRef]
- Wislocki, G.B.; Singer, M. The occurrence and function of nerves in the growing antlers of deer. J. Comp. Neurol. 1946, 85, 1–19. [Google Scholar] [CrossRef]
- Kumar, A.; Brockes, J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef]
- Ge, S.; Khachemoune, A. The Importance of Cutaneous Innervation in Wound Healing: From Animal Studies to Clinical Applications. Int. J. Low. Extrem Wounds 2021, 15347346211045022. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, M.; Baguneid, M.; Bayat, A. The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm. Venereol. 2016, 96, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, E. Efficient Healing Takes Some Nerve: Electrical Stimulation Enhances Innervation in Cutaneous Human Wounds. J. Investig. Dermatol. 2017, 137, 543–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, B.M. Some principles of regeneration in mammalian systems. Anat. Rec. Part B New Anat. 2005, 287, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Chou, Y.H.; Chang, Y.J.; Teng, N.Y.; Hsu, H.L.; Chen, L. Interplay between cell migration and neurite outgrowth determines SH2B1beta-enhanced neurite regeneration of differentiated PC12 cells. PLoS ONE 2012, 7, e34999. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Wei, D.; Reid, B.; Zhao, S.; Pu, J.; Pan, T.; Yamoah, E.; Zhao, M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 2013, 14, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, L.F.; Poo, M.M. Neurites grow faster towards the cathode than the anode in a steady field. J. Exp. Zool. 1979, 209, 115–128. [Google Scholar] [CrossRef]
- Song, B.; Zhao, M.; Forrester, J.; McCaig, C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 2004, 117, 4681–4690. [Google Scholar] [CrossRef] [Green Version]
- Breier, J.M.; Radio, N.M.; Mundy, W.R.; Shafer, T.J. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol. Sci. 2008, 105, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Radio, N.M.; Breier, J.M.; Shafer, T.J.; Mundy, W.R. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicol. Sci. 2008, 105, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Young, A.; Machacek, D.W.; Dhara, S.K.; Macleish, P.R.; Benveniste, M.; Dodla, M.C.; Sturkie, C.D.; Stice, S.L. Ion channels and ionotropic receptors in human embryonic stem cell derived neural progenitors. Neuroscience 2011, 192, 793–805. [Google Scholar] [CrossRef] [Green Version]
- Dragunow, M. High-content analysis in neuroscience. Nat. Rev. Neurosci. 2008, 9, 779–788. [Google Scholar] [CrossRef]
- Harrill, J.A.; Robinette, B.L.; Freudenrich, T.; Mundy, W.R. Use of high content image analyses to detect chemical-mediated effects on neurite sub-populations in primary rat cortical neurons. Neurotoxicology 2013, 34, 61–73. [Google Scholar] [CrossRef]
- Cairns, D.M.; Chwalek, K.; Moore, Y.E.; Kelley, M.R.; Abbott, R.D.; Moss, S.; Kaplan, D.L. Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem Cell Rep. 2016, 7, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Cairns, D.M.; Giordano, J.E.; Conte, S.; Levin, M.; Kaplan, D.L. Ivermectin Promotes Peripheral Nerve Regeneration during Wound Healing. ACS Omega 2018, 3, 12392–12402. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Efthymiou, A.; Shaltouki, A.; Steiner, J.P.; Jha, B.; Heman-Ackah, S.M.; Swistowski, A.; Zeng, X.; Rao, M.S.; Malik, N. Functional screening assays with neurons generated from pluripotent stem cell-derived neural stem cells. J. Biomol. Screen. 2014, 19, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Rosati, J.; Ferrari, D.; Altieri, F.; Tardivo, S.; Ricciolini, C.; Fusilli, C.; Zalfa, C.; Profico, D.C.; Pinos, F.; Bernardini, L.; et al. Establishment of stable iPS-derived human neural stem cell lines suitable for cell therapies. Cell Death Dis. 2018, 9, 937. [Google Scholar] [CrossRef]
- Sirenko, O.; Hesley, J.; Rusyn, I.; Cromwell, E.F. High-content high-throughput assays for characterizing the viability and morphology of human iPSC-derived neuronal cultures. Assay Drug Dev. Technol. 2014, 12, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Kiechle, F.L. Hoechst 33342-induced apoptosis in BC3H-1 myocytes. Ann. Clin. Lab. Sci. 1997, 27, 260–275. [Google Scholar]
- Gilbert, D.F.; Erdmann, G.; Zhang, X.; Fritzsche, A.; Demir, K.; Jaedicke, A.; Muehlenberg, K.; Wanker, E.E.; Boutros, M. A novel multiplex cell viability assay for high-throughput RNAi screening. PLoS ONE 2011, 6, e28338. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; Belgacem, Y.H. Crosstalk among electrical activity, trophic factors and morphogenetic proteins in the regulation of neurotransmitter phenotype specification. J. Chem. Neuroanat. 2016, 73, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Belgacem, Y.H.; Borodinsky, L.N. Inversion of Sonic hedgehog action on its canonical pathway by electrical activity. Proc. Natl. Acad. Sci. USA 2015, 112, 4140–4145. [Google Scholar] [CrossRef] [Green Version]
- Beane, W.S.; Morokuma, J.; Lemire, J.M.; Levin, M. Bioelectric signaling regulates head and organ size during planarian regeneration. Development 2013, 140, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Pai, V.P.; Vandenberg, L.N.; Blackiston, D.; Levin, M. Neurally Derived Tissues in Xenopus laevis Embryos Exhibit a Consistent Bioelectrical Left-Right Asymmetry. Stem Cells Int. 2012, 2012, 353491. [Google Scholar] [CrossRef] [Green Version]
- Pai, V.P.; Lemire, J.M.; Chen, Y.; Lin, G.; Levin, M. Local and long-range endogenous resting potential gradients antagonistically regulate apoptosis and proliferation in the embryonic CNS. Int. J. Dev. Biol. 2015, 59, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.S.; Kenny, C.J.; Ganesh, V.; Jang, A.; Borges-Monroy, R.; Partlow, J.N.; Hill, R.S.; Shin, T.; Chen, A.Y.; Doan, R.N.; et al. Sodium Channel SCN3A (NaV1.3) Regulation of Human Cerebral Cortical Folding and Oral Motor Development. Neuron 2018, 99, 905–913.e907. [Google Scholar] [CrossRef] [Green Version]
- Sequerra, E.B.; Goyal, R.; Castro, P.A.; Levin, J.B.; Borodinsky, L.N. NMDA Receptor Signaling Is Important for Neural Tube Formation and for Preventing Antiepileptic Drug-Induced Neural Tube Defects. J. Neurosci. 2018, 38, 4762–4773. [Google Scholar] [CrossRef]
- Aprea, J.; Calegari, F. Bioelectric state and cell cycle control of Mammalian neural stem cells. Stem Cells Int. 2012, 2012, 816049. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.; Prenninger, S.; Knuckles, P.; Taylor, V.; Levin, M.; Calegari, F. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev. 2011, 20, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Ribera, A.B. Potassium currents in developing neurons. Ann. N. Y. Acad. Sci. 1999, 868, 399–405. [Google Scholar] [CrossRef]
- Meier, S.D.; Kovalchuk, Y.; Rose, C.R. Properties of the new fluorescent Na+ indicator CoroNa Green: Comparison with SBFI and confocal Na+ imaging. J. Neurosci. Methods 2006, 155, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bonzanni, M.; Payne, S.L.; Adelfio, M.; Kaplan, D.L.; Levin, M.; Oudin, M.J. Defined extracellular ionic solutions to study and manipulate the cellular resting membrane potential. Biol. Open 2020, 9, bio048553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, P.S.; Gibbons, B.A.; Vereninov, A.A.; Yurinskaya, V.E.; Clements, R.J.; Model, T.A.; Model, M.A. Calibration and characterization of intracellular Asante Potassium Green probes, APG-2 and APG-4. Anal. Biochem. 2019, 567, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Funk, R. Ion Gradients in Tissue and Organ Biology. Biol. Syst. 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Blackiston, D.; Adams, D.S.; Lemire, J.M.; Lobikin, M.; Levin, M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis. Model. Mech. 2011, 4, 67–85. [Google Scholar] [CrossRef] [Green Version]
- Engels, M.; Kalia, M.; Rahmati, S.; Petersilie, L.; Kovermann, P.; van Putten, M.; Rose, C.R.; Meijer, H.G.E.; Gensch, T.; Fahlke, C. Glial Chloride Homeostasis Under Transient Ischemic Stress. Front. Cell Neurosci. 2021, 15, 735300. [Google Scholar] [CrossRef]
- Igarashi, K.; Iwai, H.; Tanaka, K.I.; Kuwahara, Y.; Kitanaka, J.; Kitanaka, N.; Kurimasa, A.; Tomita, K.; Sato, T. Neuroprotective effect of oxytocin on cognitive dysfunction, DNA damage, and intracellular chloride disturbance in young mice after cranial irradiation. Biochem. Biophys. Res. Commun. 2022, 612, 1–7. [Google Scholar] [CrossRef]
- Janach, G.M.S.; Bohm, M.; Dohne, N.; Kim, H.R.; Rosario, M.; Strauss, U. Interferon-gamma enhances neocortical synaptic inhibition by promoting membrane association and phosphorylation of GABAA receptors in a protein kinase C-dependent manner. Brain. Behav. Immun. 2022, 101, 153–164. [Google Scholar] [CrossRef]
- Yang, W.; Yuste, R. In vivo imaging of neural activity. Nat. Methods 2017, 14, 349–359. [Google Scholar] [CrossRef]
- Wong, R.O. Effects of glutamate and its analogs on intracellular calcium levels in the developing retina. Vis. Neurosci. 1995, 12, 907–917. [Google Scholar] [CrossRef]
- Rajdev, S.; Reynolds, I.J. Glutamate-induced intracellular calcium changes and neurotoxicity in cortical neurons in vitro: Effect of chemical ischemia. Neuroscience 1994, 62, 667–679. [Google Scholar] [CrossRef]
- McMillen, P.; Novak, R.; Levin, M. Toward Decoding Bioelectric Events in Xenopus Embryogenesis: New Methodology for Tracking Interplay Between Calcium and Resting Potentials In Vivo. J. Mol. Biol. 2020, 432, 605–620. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 2009, 5, 231–246. [Google Scholar] [CrossRef]
- Lagache, T.; Hanson, A.; Perez-Ortega, J.E.; Fairhall, A.; Yuste, R. Tracking calcium dynamics from individual neurons in behaving animals. PLoS Comput. Biol. 2021, 17, e1009432. [Google Scholar] [CrossRef]
- Dolensek, J.; Stozer, A.; Skelin Klemen, M.; Miller, E.W.; Slak Rupnik, M. The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLoS ONE 2013, 8, e82374. [Google Scholar] [CrossRef] [Green Version]
- Brodskiy, P.A.; Zartman, J.J. Calcium as a signal integrator in developing epithelial tissues. Phys. Biol. 2018, 15, 051001. [Google Scholar] [CrossRef]
- Adams, D.S.; Levin, M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012, 2012, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Oviedo, N.J.; Nicolas, C.L.; Adams, D.S.; Levin, M. Live Imaging of Planarian Membrane Potential Using DiBAC4(3). CSH Protoc. 2008, 2008, pdb-prot5055. [Google Scholar] [CrossRef]
- Yamada, A.; Gaja, N.; Ohya, S.; Muraki, K.; Narita, H.; Ohwada, T.; Imaizumi, Y. Usefulness and limitation of DiBAC4(3), a voltage-sensitive fluorescent dye, for the measurement of membrane potentials regulated by recombinant large conductance Ca2 + -activated K+ channels in HEK293 cells. Jpn. J. Pharmacol. 2001, 86, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Chesler, M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Rodgers, A.; Watret, L. The role of pH modulation in wound bed preparation. Diabet. Foot 2005, 8, 154. [Google Scholar]
- Bennison, L.R.; Miller, C.N.; Summers, R.J.; Minnis, A.M.B.; Sussman, G.; McGuiness, W. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Biswas, K.; Alexander, K.; Francis, M.M. Reactive Oxygen Species: Angels and Demons in the Life of a Neuron. NeuroSci 2022, 3, 130–145. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [Green Version]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Huynh, C.; Chang, C.J. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 2010, 132, 5906–5915. [Google Scholar] [CrossRef] [Green Version]
- Harsum, S.; Clarke, J.D.; Martin, P. A reciprocal relationship between cutaneous nerves and repairing skin wounds in the developing chick embryo. Dev. Biol. 2001, 238, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Baig, A.M.; Rana, Z.; Tariq, S.; Lalani, S.; Ahmad, H.R. Traced on the Timeline: Discovery of Acetylcholine and the Components of the Human Cholinergic System in a Primitive Unicellular Eukaryote Acanthamoeba spp. ACS Chem. Neurosci. 2018, 9, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric gamma-Aminobutyric acid (GABA) signalling in plants. Cell Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Erdo, S.L.; Wolff, J.R. gamma-Aminobutyric acid outside the mammalian brain. J. Neurochem. 1990, 54, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [Green Version]
- Tuszynski, J.; Tilli, T.M.; Levin, M. Ion Channel and Neurotransmitter Modulators as Electroceutical Approaches to the Control of Cancer. Curr. Pharm. Des. 2017, 23, 4827–4841. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; Vien, K.; Levin, M. Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity. NPJ Regen. Med. 2017, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Kema, I.P.; Levin, M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 2005, 15, 794–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, N.; Lal, G. Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front. Immunol. 2021, 12, 660342. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [Green Version]
- Stupack, J.; Xiong, X.P.; Jiang, L.L.; Zhang, T.; Zhou, L.; Campos, A.; Ranscht, B.; Mobley, W.; Pasquale, E.B.; Xu, H.; et al. Soluble SORLA Enhances Neurite Outgrowth and Regeneration through Activation of the EGF Receptor/ERK Signaling Axis. J. Neurosci. 2020, 40, 5908–5921. [Google Scholar] [CrossRef]
- Pool, M.; Thiemann, J.; Bar-Or, A.; Fournier, A.E. NeuriteTracer: A novel ImageJ plugin for automated quantification of neurite outgrowth. J. Neurosci. Methods 2008, 168, 134–139. [Google Scholar] [CrossRef]


| Sensor Dye | Purpose | Live/Endpoint | Works/Does Not Work | |
|---|---|---|---|---|
| Morphology | DAPI | Nuclear stain | Endpoint | √ |
| Hoechst | Nuclear stain | Endpoint | √ | |
| NeuO | Cell morphology | Live stain | ✕ | |
| Calcein AM—Green | Cell morpholgy | Live stain | √ | |
| Calcein AM—Red | Cell morphology | Live stain | √ | |
| Vmem & ion flux | DiBac | Membrane voltage | Live stain | √ |
| CoroNA AM | Intracellular Na+ | Live stain | √ | |
| APG-2 AM | Intracellular K+ | Live stain | √ | |
| Fluo4 AM | Intracellular Ca2+ | Live stain | √ | |
| MQAE | Intracellular Cl− | Live stain | ✕ | |
| Cell activity | SNARF-5F AM | Intracellular pH | Live stain | √ |
| PO1 | Intracellular ROS | Live stain | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, V.P.; Cooper, B.G.; Levin, M. Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons. Cells 2022, 11, 2470. https://doi.org/10.3390/cells11162470
Pai VP, Cooper BG, Levin M. Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons. Cells. 2022; 11(16):2470. https://doi.org/10.3390/cells11162470
Chicago/Turabian StylePai, Vaibhav P., Ben G. Cooper, and Michael Levin. 2022. "Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons" Cells 11, no. 16: 2470. https://doi.org/10.3390/cells11162470
APA StylePai, V. P., Cooper, B. G., & Levin, M. (2022). Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons. Cells, 11(16), 2470. https://doi.org/10.3390/cells11162470







