Combined Effects of Polydopamine-Assisted Copper Immobilization on 3D-Printed Porous Ti6Al4V Scaffold for Angiogenic and Osteogenic Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Porous Titanium Scaffolds
2.2. Copper Ion Coating
2.3. Characterization of TiPCu Scaffolds
2.4. In Vitro Release Profiles of Copper
2.5. Angiogenic Analysis
2.6. Cell Viability Assay and Fluorescent Staining
2.7. Osteogenesis-Related Protein Expressions
2.8. Statistical Analysis
3. Results and Discussion
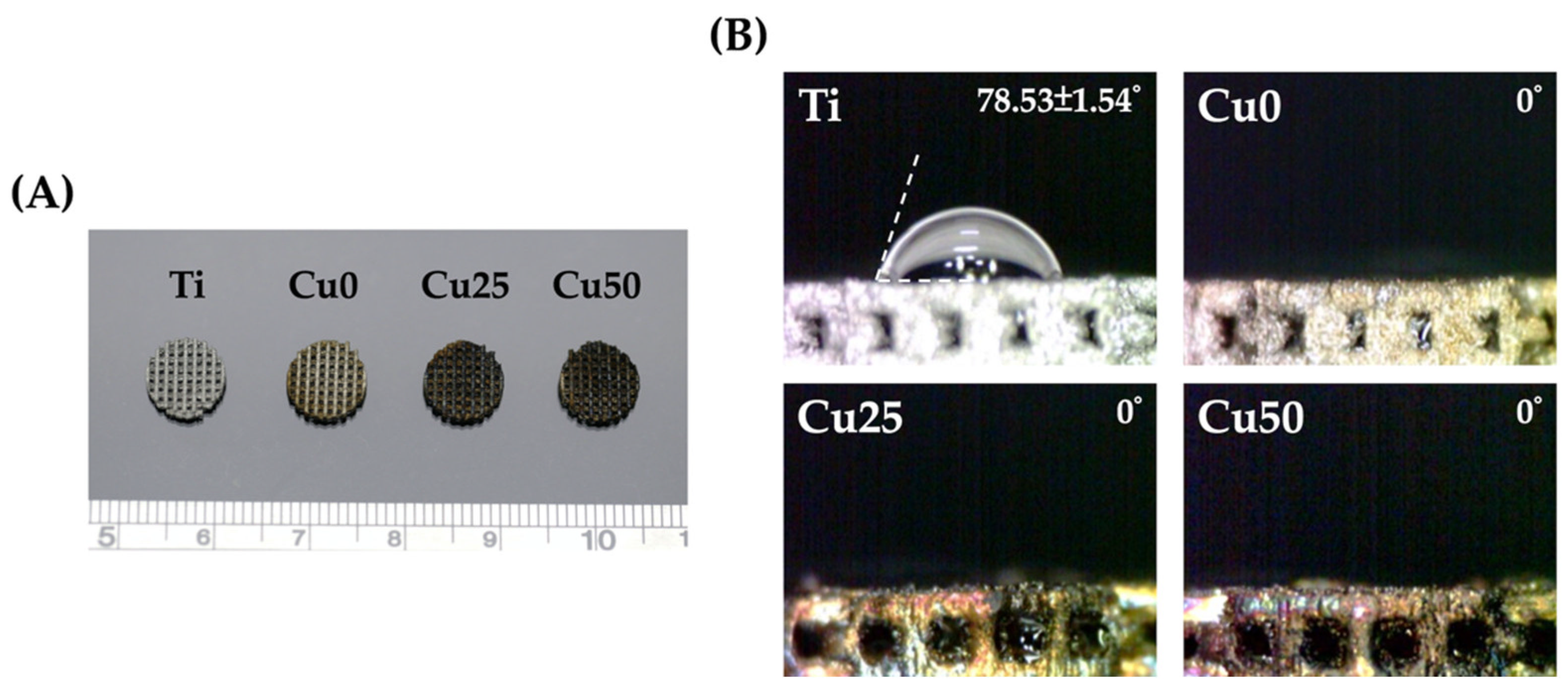
3.1. Characterization of TiPCu Scaffolds: Morphology and Contact Angle
3.2. Characterization of TiPCu Scaffolds: Chemical Composition, Bioactivity and Mechanical Test
3.3. In Vitro Release Profiles of Copper
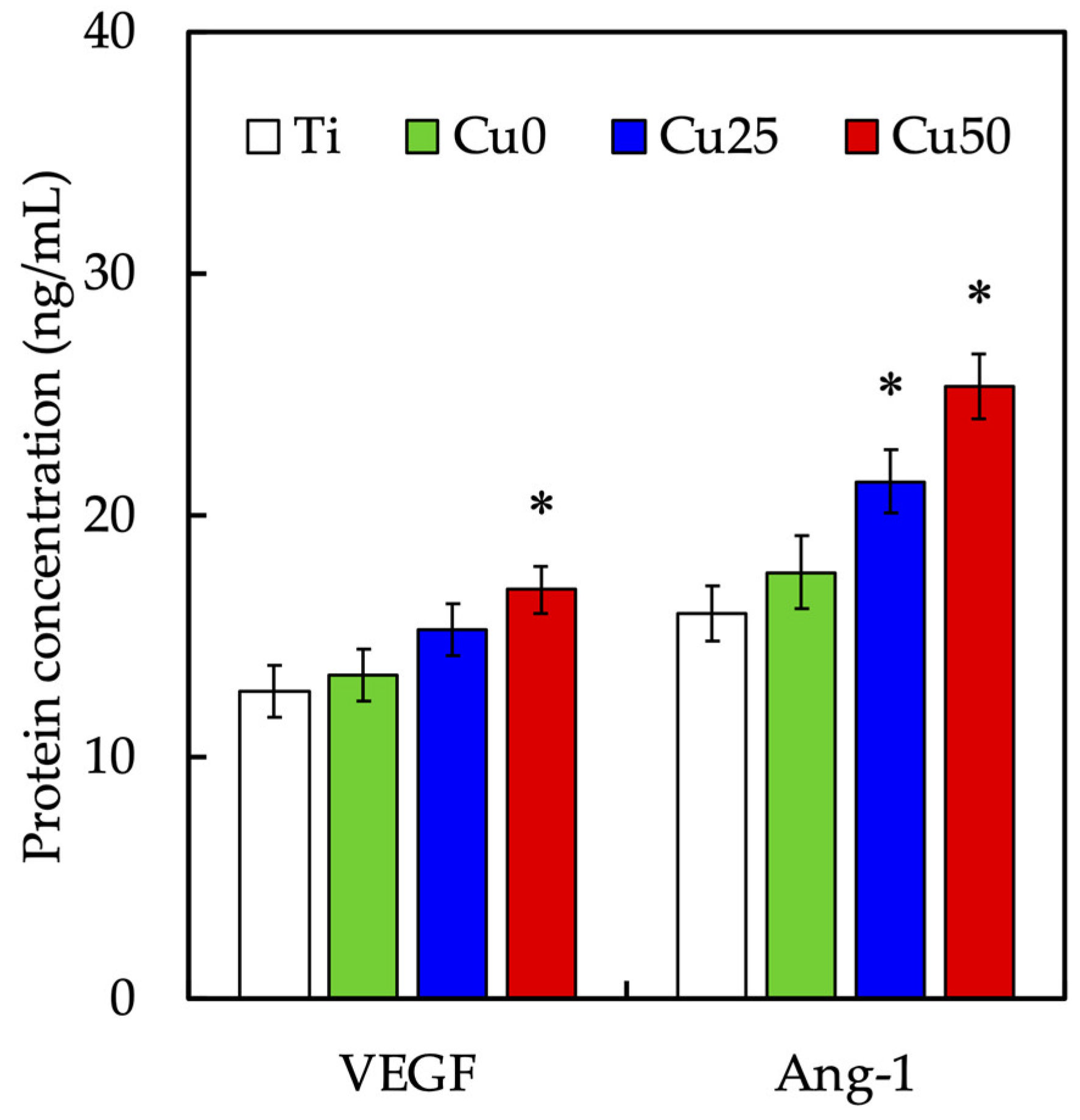
3.4. In Vitro Angiogenesis Effect of Cu/PDA Coating
3.5. Cell Viability Assay and Fluorescent Staining
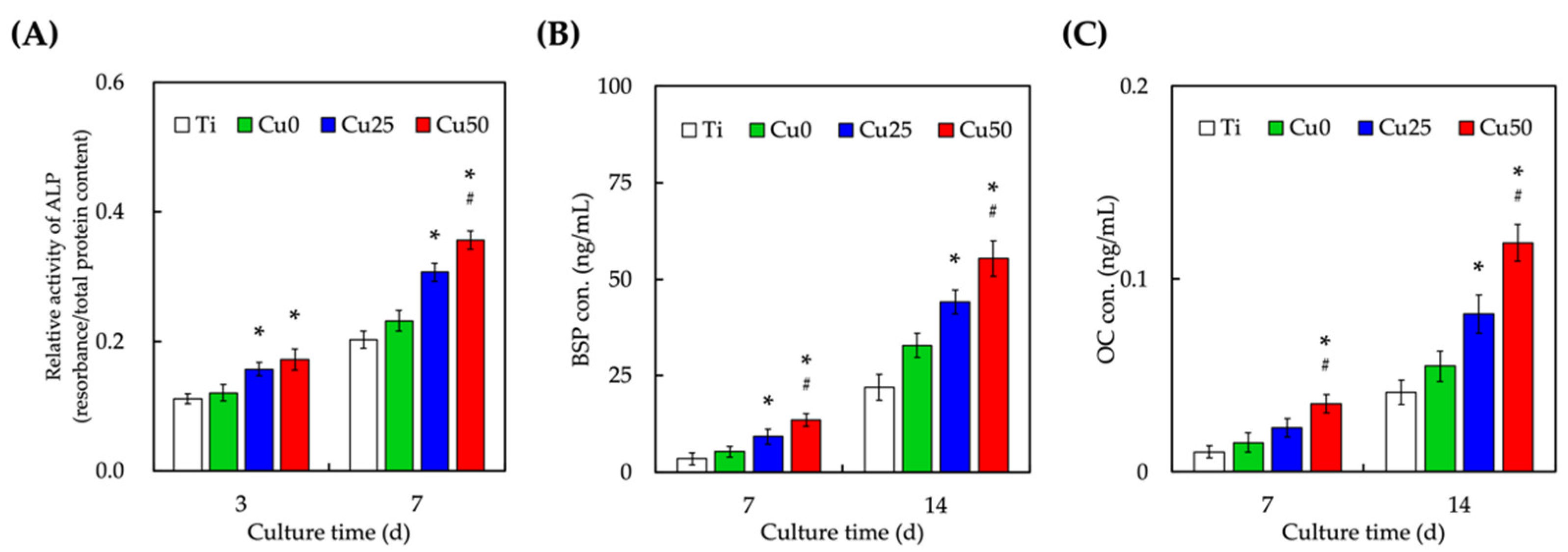
3.6. Osteogenic-Related Protein Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhardt, A.; Schneider, J.; Schroeder, A.; Papadopoulous, K.; Lopez, E.; Brückner, F.; Botzenhart, U. Surface Conditioning of Additively Manufactured Titanium Implants and Its Influence on Materials Properties and in Vitro Biocompatibility. Mater. Sci. Eng. C 2021, 119, 111631. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cao, H.; Zhao, Y.; Cheng, M.; Qin, H.; Cheng, T.; Hu, Y.; Zhang, X.; Liu, X. In Vitro and in Vivo Responses of Macrophages to Magnesium-Doped Titanium. Sci. Rep. 2017, 7, 42707–42712. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. BioMater. Adv. 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.Y.; Tai, I.C.; Ho, M.L.; Wang, J.-W.; Weng, L.W.; Wang, Y.J.; Wang, M.W.; Tseng, C.C. Controlled Release of BMP-2 from Titanium with Electrodeposition Modification Enhancing Critical Size Bone Formation. BioMater. Adv. 2019, 105, 109879. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, L.; Zhang, Z.; Dessel, J.V.; Driesen, R.B.; Lambrichts, I.; Jacobs, R.; Tian, L.; Sun, Y.; Liu, Y.; et al. BMP-2 Incorporated Biomimetic CaP Coating Functionalized 3D Printed Ti6Al4V Scaffold Induces Ectopic Bone Formation in a Dog Model. Mater. Des. 2022, 215, 110443. [Google Scholar] [CrossRef]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(Dopamine) Coating of 3D Printed Poly(Lactic Acid) Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lee, K.X.; Ho, C.C.; Fang, M.J.; Kuo, T.Y.; Shie, M.Y. The Effects of a 3D-Printed Magnesium-/Strontium-Doped Calcium Silicate Scaffold on Regulation of Bone Regeneration via Dual-Stimulation of the AKT and WNT Signaling Pathways. BioMater. Adv. 2022, 135, 112660. [Google Scholar] [CrossRef]
- Qiao, S.; Sheng, Q.; Li, Z.; Wu, D.; Zhu, Y.; Lai, H.; Gu, Y. 3D-Printed Ti6Al4V Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma as Bioactive Interface for Enhancing Osseointegration in Osteoporosis. Mater. Des. 2020, 194, 108825. [Google Scholar] [CrossRef]
- Ekoi, E.J.; Stallard, C.; Reid, I.; Dowling, D.P. Tailoring Oxide-Layer Formation on Titanium Substrates Using Microwave Plasma Treatments. Surf. Coat. Technol. 2017, 325, 299–307. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rocca, Y.D.; Oliva, S.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Diomede, F.; Pizzicannella, J. Enhanced Extracellular Matrix Deposition on Titanium Implant Surfaces: Cellular and Molecular Evidences. Biomedicines 2021, 9, 1710. [Google Scholar] [CrossRef]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z.; et al. Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. Epidermidis Infection and Enhance Osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.G.; Lee, A.K.X.; Lin, Y.H.; Huang, T.H.; Ho, C.C.; Shie, M.Y. Caffeic Acid–Coated Nanolayer on Mineral Trioxide Aggregate Potentiates the Host Immune Responses, Angiogenesis, and Odontogenesis. J. Endodont. 2020, 46, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.; Lee, J.J.; Lee, K.X.; Lin, Y.H.; Chen, J.X.; Kuo, T.Y.; Shie, M.Y. Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells 2021, 10, 2911. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Zhang, Y.; Wang, T.; Lin, K.; Liu, J. A Polydopamine-Assisted Strontium-Substituted Apatite Coating for Titanium Promotes Osteogenesis and Angiogenesis via FAK/MAPK and PI3K/AKT Signaling Pathways. BioMater. Adv. 2021, 131, 112482. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Y.; Cao, Q.; Yu, T.; Zhang, J.; Liu, Q.; Yang, X. Growth Factors Enhanced Angiogenesis and Osteogenesis on Polydopamine Coated Titanium Surface for Bone Regeneration. Mater. Des. 2020, 196, 109162. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhu, Y.; Wang, X.; Li, M.; Lin, Z.; Hu, X.; Zhang, Y.; Yin, Q.; Xia, H.; et al. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl. Mater. Interfaces 2018, 10, 127–138. [Google Scholar] [CrossRef]
- Makvandi, P.; Caccavale, C.; Sala, F.D.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of Osteoporotic Bone Regeneration by Strontium-Substituted 45S5 Bioglass via Time-Dependent Modulation of Autophagy and the Akt/MTOR Signaling Pathway. J. Mater. Chem. B 2021, 9, 3489–3501. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Agarwal, T.; Rathnam, V.S.S.; Pal, K.; Banerjee, I. Cobalt Doped Nano-Hydroxyapatite Incorporated Gum Tragacanth-Alginate Beads as Angiogenic-Osteogenic Cell Encapsulation System for Mesenchymal Stem Cell Based Bone Tissue Engineering. Int. J. Biol. Macromol. 2021, 179, 101–115. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yeh, C.H.; Shie, M.Y. Stimulatory Effects of the Fast Setting and Degradable Ca-Si-Mg Cement on Both Cementogenesis and Angiogenesis Differentiation of Human Periodontal Ligament Cells. J. Mater. Chem. B 2015, 3, 7099–7108. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Zhang, Y.; Lin, Y. Controlled Release of Dopamine Coatings on Titanium Bidirectionally Regulate Osteoclastic and Osteogenic Response Behaviors. BioMater. Adv. 2021, 129, 112376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, C.; Yang, S.; Chiu, T.W.; Wu, J.; Xiao, K.; Huang, Y.; Li, X. Enhanced Silver Loaded Antibacterial Titanium Implant Coating with Novel Hierarchical Effect. J. BioMater. Appl. 2018, 32, 1289–1299. [Google Scholar] [CrossRef]
- Yu, Q.; Han, Y.; Wang, X.; Qin, C.; Zhai, D.; Yi, Z.; Chang, J.; Xiao, Y.; Wu, C. Copper Silicate Hollow Microspheres-Incorporated Scaffolds for Chemo-Photothermal Therapy of Melanoma and Tissue Healing. ACS Nano 2018, 12, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-Doped Mesoporous Silica Nanospheres, a Promising Immunomodulatory Agent for Inducing Osteogenesis. Acta Biomater. 2016, 30, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Díez-Tercero, L.; Delgado, L.M.; Perez, R.A. Modulation of Macrophage Response by Copper and Magnesium Ions in Combination with Low Concentrations of Dexamethasone. Biomedicines 2022, 10, 764. [Google Scholar] [CrossRef]
- Wang, L.; Shang, X.; Hao, Y.; Wan, G.; Dong, L.; Huang, D.; Yang, X.; Sun, J.; Wang, Q.; Zha, G.; et al. Bi-Functional Titanium-Polydopamine-Zinc Coatings for Infection Inhibition and Enhanced Osseointegration. RSC Adv. 2019, 9, 2892–2905. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Cao, W.; Shi, C.; Zhou, P.; Li, Q.; Han, F.; Sun, J.; Xing, X.; Li, B. Mussel-Inspired Deposition of Copper on Titanium for Bacterial Inhibition and Enhanced Osseointegration in a Periprosthetic Infection Model. RSC Adv. 2017, 7, 51593–51604. [Google Scholar] [CrossRef]
- Wu, Y.H.A.; Chiu, Y.C.; Lin, Y.H.; Ho, C.C.; Shie, M.Y.; Chen, Y.W. 3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 942. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chuang, T.Y.; Chiang, W.H.; Chen, I.W.P.; Wang, K.; Shie, M.Y.; Chen, Y.W. The Synergistic Effects of Graphene-Contained 3D-Printed Calcium Silicate/Poly-ε-Caprolactone Scaffolds Promote FGFR-Induced Osteogenic/Angiogenic Differentiation of Mesenchymal Stem Cells. Mater. Sci. Eng. C 2019, 104, 109887. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chiu, Y.C.; Shen, Y.F.; Wu, Y.H.A.; Shie, M.Y. Bioactive Calcium Silicate/Poly-ε-Caprolactone Composite Scaffolds 3D Printed under Mild Conditions for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2017, 29, 11. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hung, C.H.; Kuo, C.N.; Chen, C.Y.; Peng, Y.N.; Shie, M.Y. Improved Bioactivity of 3D Printed Porous Titanium Alloy Scaffold with Chitosan/Magnesium-Calcium Silicate Composite for Orthopaedic Applications. Materials 2019, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Chen, Y.W.; Wang, K.; Shie, M.Y. Enhanced Adhesion and Differentiation of Human Mesenchymal Stem Cell inside Apatite-Mineralized/Poly(Dopamine)-Coated Poly(ε-Caprolactone) Scaffolds by Stereolithography. J. Mater. Chem. B 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The Effects of Biodentine/Polycaprolactone Three-dimensional-scaffold with Odontogenesis Properties on Human Dental Pulp Cells. Int. Endod J. 2018, 51, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile Synthesis of Novel Size-Controlled Antibacterial Hybrid Spheres Using Silver Nanoparticles Loaded with Poly-Dopamine Spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.A.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and Angiogenic Potentials of the Cell-Laden Hydrogel/Mussel-Inspired Calcium Silicate Complex Hierarchical Porous Scaffold Fabricated by 3D Bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Cheng, X.; Zheng, Y.; Lyu, M.; Di, P.; Lin, Y. MiR-181d-5p Regulates Implant Surface Roughness-Induced Osteogenic Differentiation of Bone Marrow Stem Cells. Mater. Sci. Eng. C 2021, 121, 111801. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.C.; Wang, C.Y.; Lee, A.K.X.; Yeh, C.L.; Lin, C.P. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-ε-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymer 2020, 12, 1455. [Google Scholar] [CrossRef]
- Nasulewicz, A.; Mazur, A.; Opolski, A. Role of Copper in Tumour Angiogenesis-Clinical Implications. J. Trace Elem. Med. Bio. 2004, 18, 1–8. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.Z.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-Containing Mesoporous Bioactive Glass Scaffolds with Multifunctional Properties of Angiogenesis Capacity, Osteostimulation and Antibacterial Activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Lin, K.; Li, H.; Chang, J. Synergy Effects of Copper and Silicon Ions on Stimulation of Vascularization by Copper-Doped Calcium Silicate. J. Mater. Chem. B 2014, 2, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.N.; Brandl, A.; Hiller, D.; Hoppe, A.; Gbureck, U.; Horch, R.E.; Boccaccini, A.R.; Kneser, U. Bioactive Copper-Doped Glass Scaffolds Can Stimulate Endothelial Cells in Co-Culture in Combination with Mesenchymal Stem Cells. PLoS ONE 2014, 9, e113319. [Google Scholar]
- Song, J.; Chen, Z.; Liu, Z.; Yi, Y.; Tsigkou, O.; Li, J.; Li, Y. Controllable Release of Vascular Endothelial Growth Factor (VEGF) by Wheel Spinning Alginate/Silk Fibroin Fibers for Wound Healing. Mater. Des. 2021, 212, 110231. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-Induced Vascular Endothelial Growth Factor Expression and Wound Healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821-7. [Google Scholar] [CrossRef]
- Li, S.; Xie, H.; Li, S.; Kang, Y.J. Copper Stimulates Growth of Human Umbilical Vein Endothelial Cells in a Vascular Endothelial Growth Factor-Independent Pathway. Exp. Biol. Med. 2012, 237, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Schamel, M.; Bernhardt, A.; Quade, M.; Würkner, C.; Gbureck, U.; Moseke, C.; Gelinsky, M.; Lode, A. Cu2+, Co2+ and Cr3+ Doping of a Calcium Phosphate Cement Influences Materials Properties and Response of Human Mesenchymal Stromal Cells. Mater. Sci. Eng. C 2017, 73, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-Containing Bioactive Glasses and Glass-Ceramics: From Tissue Regeneration to Cancer Therapeutic Strategies. Mater. Sci. Eng. C 2021, 121, 111741. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.S.; Lee, J.J.; Lee, A.K.X.; Ho, C.C.; Liu, Y.T.; Shie, M.Y. Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers 2020, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Chiu, Y.C.; Lee, A.K.X.; Lin, Y.H.; Huang, T.H.; Liu, Y.C.; Shie, M.Y. The Synergistic Effects of Xu Duan Combined Sr-Contained Calcium Silicate/Poly-ε-Caprolactone Scaffolds for the Promotion of Osteogenesis Marker Expression and the Induction of Bone Regeneration in Osteoporosis. Mater. Sci. Eng. C 2021, 119, 111629. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of Angiogenesis and Osteogenesis by a Specific Vessel Subtype in Bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Kesireddy, V.; Kasper, F.K. Approaches for Building Bioactive Elements into Synthetic Scaffolds for Bone Tissue Engineering. J. Mater. Chem. B 2016, 4, 6773–6786. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of Cu-Doped 45S5 Bioactive Glass on the Lipid Peroxidation-Associated Growth of Human Osteoblast-like Cells in Vitro. J. Biomed. Mater. Res. Part. A 2014, 102, 3556–3561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; He, F.; Wu, T.; Zhou, L.; Ye, J. Concentration-Dependent Osteogenic and Angiogenic Biological Performances of Calcium Phosphate Cement Modified with Copper Ions. BioMater. Adv. 2019, 99, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Liu, Y.; Xu, W.; Lu, X.; Guo, L.; Liu, Y.; Tian, S.; Liu, B.; Zhang, J.; Wen, C. Additive Manufacturing of Functionally Graded Porous Titanium Scaffolds for Dental Applications. Biomater. Adv. 2022, 139, 213018. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-Y.; Lin, Y.-H.; Lee, A.K.-X.; Kuo, T.-Y.; Tsai, C.-H.; Shie, M.-Y. Combined Effects of Polydopamine-Assisted Copper Immobilization on 3D-Printed Porous Ti6Al4V Scaffold for Angiogenic and Osteogenic Bone Regeneration. Cells 2022, 11, 2824. https://doi.org/10.3390/cells11182824
Wu H-Y, Lin Y-H, Lee AK-X, Kuo T-Y, Tsai C-H, Shie M-Y. Combined Effects of Polydopamine-Assisted Copper Immobilization on 3D-Printed Porous Ti6Al4V Scaffold for Angiogenic and Osteogenic Bone Regeneration. Cells. 2022; 11(18):2824. https://doi.org/10.3390/cells11182824
Chicago/Turabian StyleWu, Hsi-Yao, Yen-Hong Lin, Alvin Kai-Xing Lee, Ting-You Kuo, Chun-Hao Tsai, and Ming-You Shie. 2022. "Combined Effects of Polydopamine-Assisted Copper Immobilization on 3D-Printed Porous Ti6Al4V Scaffold for Angiogenic and Osteogenic Bone Regeneration" Cells 11, no. 18: 2824. https://doi.org/10.3390/cells11182824




