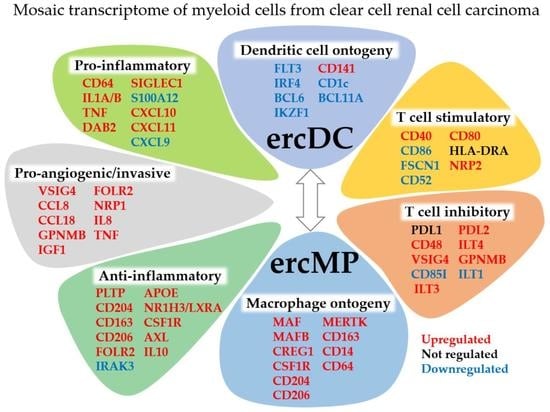
Dendritic Cells or Macrophages? The Microenvironment of Human Clear Cell Renal Cell Carcinoma Imprints a Mosaic Myeloid Subtype Associated with Patient Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissues, Cells and Cell Culture
2.2. Cell Sorting
2.3. Polychromatic Flow Cytometry
2.4. Immunofluorescence Histology and Confocal Microscopy
2.5. RNA Isolation, Microarray Hybridization
2.6. Microarray Data Preprocessing and Probe Set Filtering
2.7. Combining Microarray Studies
2.8. Hierarchical Clustering and Heatmaps
2.9. Marker Genes
2.10. ercDC Ccore in the Cancer Genome Atlas (TCGA) and the Validation (Rostock) Cohort
2.11. Public Access to Raw Data of Data Sets Analyzed in this Paper
3. Results
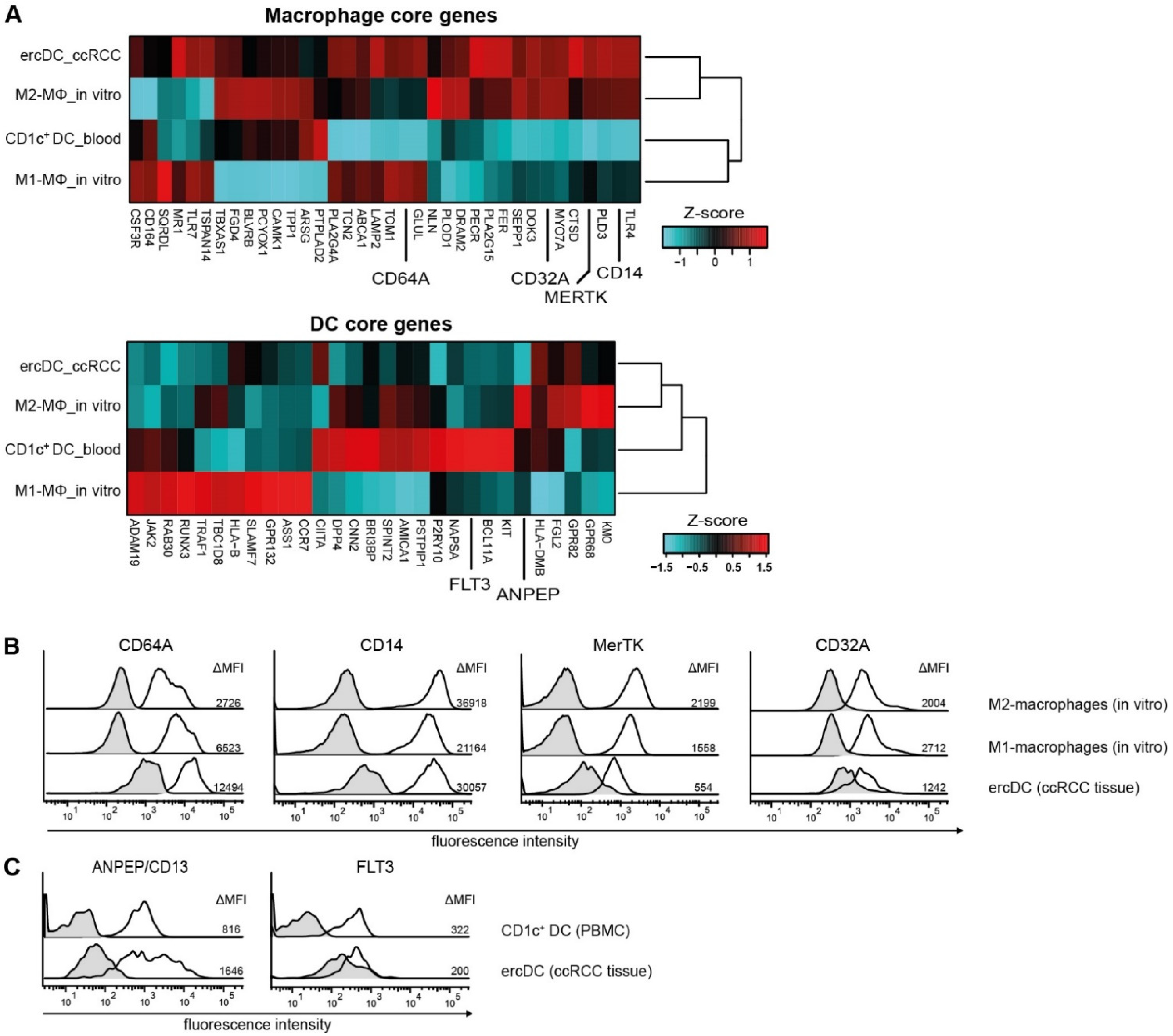
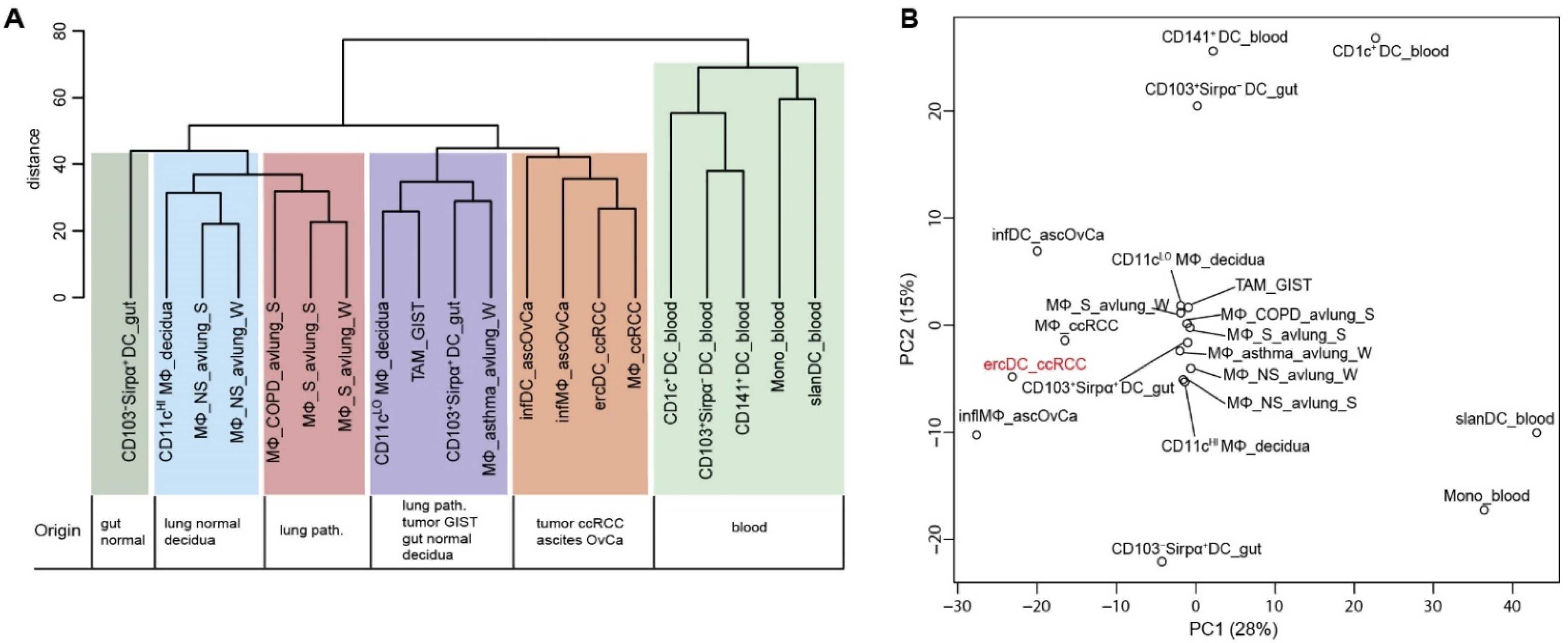
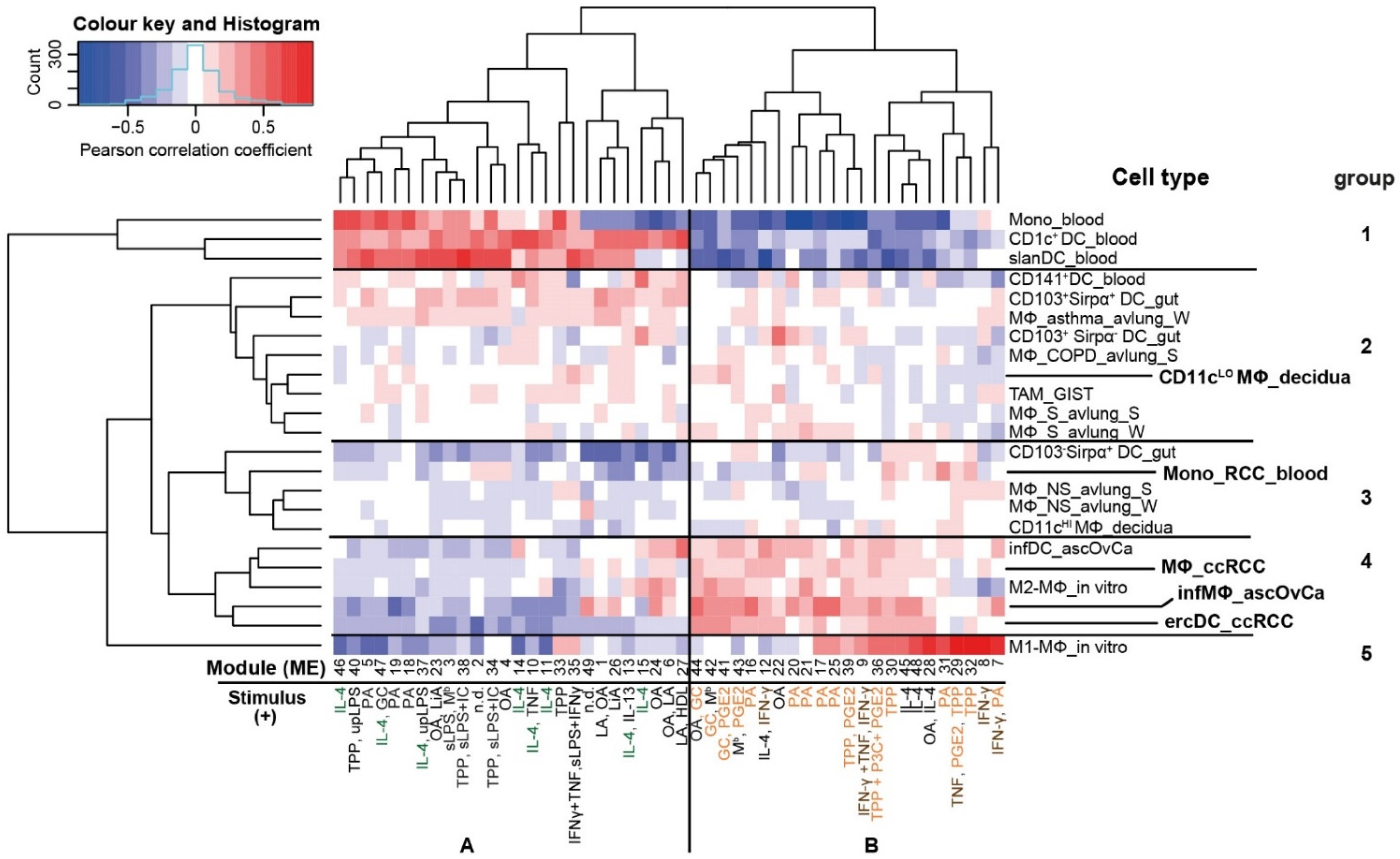
3.1. The ercDC Transcriptional Profile Identifies Them as a Unique Myeloid Subset within the Macrophage Spectrum
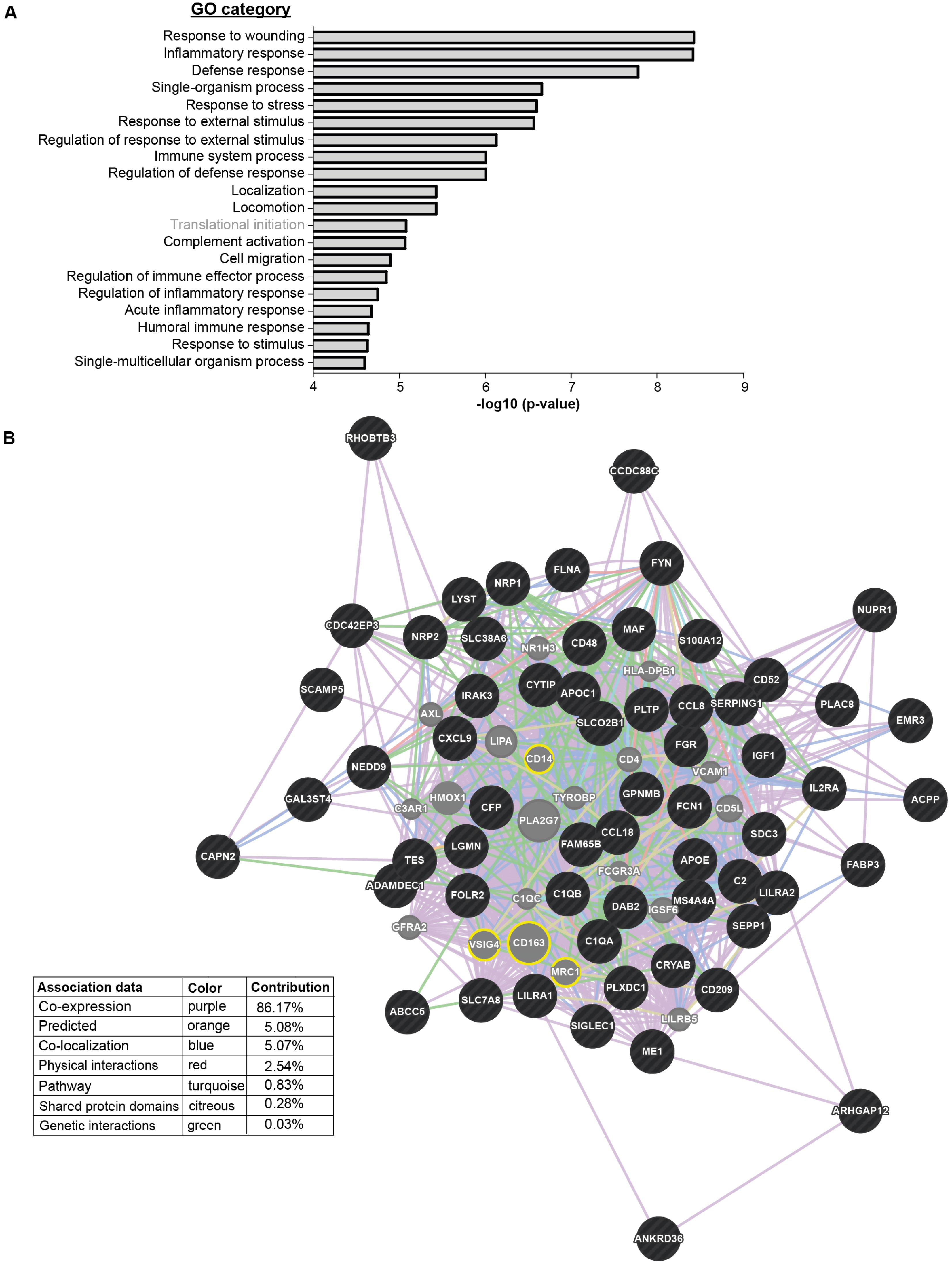
3.2. ErcDCs Have a Gene Expression Signature Similar to Inflammatory Macrophages from Ascites of Ovarian Cancer with Characteristics of Immune Tolerance
3.3. ErcDCs Are Distinct from Blood-Derived Monocytes from RCC Patients
3.4. The ercDC_ccRCC Polarization Profile Reveales Distinct Tissue Imprints
3.5. Expression of ercDC Marker Genes in ccRCC Tissue Is Predictive of Patient Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobel Lectures Physiology or Medicine 1901–1921 Ilya Mechnikov—Biographical. Available online: https://www.nobelprize.org/prizes/medicine/1908/mechnikov/biographical/ (accessed on 9 August 2022).
- Metchnikoff, O. Life of Elie Metchnikoff, 1845–1916. Nature 1922, 109, 163–166. [Google Scholar] [CrossRef]
- Merien, F. A Journey with Elie Metchnikoff: From Innate Cell Mechanisms in Infectious Diseases to Quantum Biology. Front. Public Health 2016, 4, 125. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Immunology’s foundation: The 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat. Immunol. 2008, 9, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Freeman, T. Transcriptomic analysis of mononuclear phagocyte differentiation and activation. Immunol. Rev. 2014, 262, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A.; Estrada, F.M. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-Resident Macrophage Enhancer Landscapes Are Shaped by the Local Microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef]
- Finke, J.H.; Rayman, P.; Edinger, M.; Tubbs, R.R.; Stanley, J.; Klein, E.; Bukowski, R. Characterization of a Human Renal Cell Carcinoma Specific Cytotoxic CD8+ T Cell Line (1991). J. Immunother. 1992, 11, 1–11. [Google Scholar] [CrossRef]
- Jantzer, P.; Schendel, D.J. Human renal cell carcinoma antigen-specific CTLs: Antigen-driven selection and long-term persistence in vivo. Cancer Res. 1998, 58, 3078–3086. [Google Scholar]
- Leisegang, M.; Turqueti-Neves, A.; Engels, B.; Blankenstein, T.; Schendel, D.J.; Uckert, W.; Noessner, E. T-Cell Receptor Gene–Modified T Cells with Shared Renal Cell Carcinoma Specificity for Adoptive T-Cell Therapy. Clin. Cancer Res. 2010, 16, 2333–2343. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Pagès, F.; Skliris, G.P.; Verkarre, V.; Vano, Y.; Mejean, A.; Saint-Aubert, N.; Lacroix, L.; Natario, I.; et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin. Cancer Res. 2015, 21, 3031–3040. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Petitprez, F.; Lacroix, L.; Validire, P.; Sanchez-Salas, R.; Ingels, A.; Oudard, S.; Moatti, A.; et al. Tumor-Infiltrating and Peripheral Blood T-cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 4416–4428. [Google Scholar] [CrossRef]
- Prinz, P.U.; Mendler, A.N.; Masouris, I.; Durner, L.; Oberneder, R.; Noessner, E. High DGK-α and Disabled MAPK Pathways Cause Dysfunction of Human Tumor-Infiltrating CD8+ T Cells That Is Reversible by Pharmacologic Intervention. J. Immunol. 2012, 188, 5990–6000. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Jin, Z.; Kolb, R.; Borcherding, N.; Chatzkel, J.A.; Falzarano, S.M.; Zhang, W. Updates on Immunotherapy and Immune Landscape in Renal Clear Cell Carcinoma. Cancers 2021, 13, 5856. [Google Scholar] [CrossRef]
- Mantia, C.M.; McDermott, D.F. Vascular endothelial growth factor and programmed death-1 pathway inhibitors in renal cell carcinoma. Cancer 2019, 125, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.; Miller, J.; Taylor, N.; Kyprianou, N.; Tsao, C.-K. From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells 2021, 10, 3231. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miller, C.P.; Warren, E.H.; Tykodi, S.S. Current status of antigen-specific T-cell immunotherapy for advanced renal-cell carcinoma. Hum. Vaccines Immunother. 2021, 17, 1882–1896. [Google Scholar] [CrossRef]
- Figel, A.-M.; Brech, D.; Prinz, P.U.; Lettenmeyer, U.K.; Eckl, J.; Turqueti-Neves, A.; Mysliwietz, J.; Anz, D.; Rieth, N.; Muenchmeier, N.; et al. Human Renal Cell Carcinoma Induces a Dendritic Cell Subset That Uses T-Cell Crosstalk for Tumor-Permissive Milieu Alterations. Am. J. Pathol. 2011, 179, 436–451. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Unger, W.W.; Fehres, C.M.; Kalay, H.; García-Vallejo, J.J. Glycan-based DC-SIGN targeting vaccines to enhance antigen cross-presentation. Mol. Immunol. 2012, 55, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Lukacs-Kornek, V.; Engel, D.R.; Specht, S.; Kiss, E.; Eitner, F.; Floege, J.; Groene, H.-J.; Kurts, C. Renal Dendritic Cells Stimulate IL-10 Production and Attenuate Nephrotoxic Nephritis. J. Am. Soc. Nephrol. 2008, 19, 527–537. [Google Scholar] [CrossRef]
- Soos, T.; Sims, T.; Barisoni, L.; Lin, K.; Littman, D.; Dustin, M.; Nelson, P. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006, 70, 591–596. [Google Scholar] [CrossRef]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two Unique Human Decidual Macrophage Populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Hastie, T.; Narasimhan, B.; Chu, G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc. Natl. Acad. Sci. USA 2002, 99, 6567–6572. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
- Breuer, K.; Foroushani, A.K.; Laird, M.; Chen, C.; Sribnaia, A.; Lo, R.; Winsor, G.; Hancock, R.; Brinkman, F.; Lynn, D.J. InnateDB: Systems biology of innate immunity and beyond—Recent updates and continuing curation. Nucleic Acids Res. 2013, 41, D1228–D1233. [Google Scholar] [CrossRef]
- Lynn, D.J.; Chan, C.; Naseer, M.; Yau, M.; Lo, R.; Sribnaia, A.; Ring, G.; Que, J.; Wee, K.; Winsor, G.L.; et al. Curating the innate immunity interactome. BMC Syst. Biol. 2010, 4, 117. [Google Scholar] [CrossRef]
- Lynn, D.J.; Winsor, G.; Chan, C.; Richard, N.; Laird, M.; Barsky, A.; Gardy, J.L.; Roche, F.M.; Chan, T.H.W.; Shah, N.; et al. InnateDB: Facilitating systems-level analyses of the mammalian innate immune response. Mol. Syst. Biol. 2008, 4, 218. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Büttner, F.; Winter, S.; Rausch, S.; Reustle, A.; Kruck, S.; Junker, K.; Stenzl, A.; Agaimy, A.; Hartmann, A.; Bedke, J.; et al. Survival Prediction of Clear Cell Renal Cell Carcinoma Based on Gene Expression Similarity to the Proximal Tubule of the Nephron. Eur. Urol. 2015, 68, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Y.; Şenbabaoğlu, Y.; Ciriello, G.; Yang, L.; Reznik, E.; Shuch, B.; Micevic, G.; De Velasco, G.; Shinbrot, E.; et al. Multilevel Genomics-Based Taxonomy of Renal Cell Carcinoma. Cell Rep. 2016, 14, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Maruschke, M.; Hakenberg, O.W.; Koczan, D.; Zimmermann, W.; Stief, C.G.; Buchner, A. Expression profiling of metastatic renal cell carcinoma using gene set enrichment analysis. Int. J. Urol. 2014, 21, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Maruschke, M.; Reuter, D.; Koczan, D.; Hakenberg, O.W.; Thiesen, H.-J. Gene expression analysis in clear cell renal cell carcinoma using gene set enrichment analysis for biostatistical management. Br. J. Urol. 2011, 108, E29–E35. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Hothorn, T.; Zeileis, A. Modular Toolkit for Recursive Partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. [Google Scholar]
- Therneau, T.; Grambsch, P. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. A Lego System for Conditional Inference. Am. Stat. 2006, 60, 257–263. [Google Scholar] [CrossRef]
- Hänsel, A.; Günther, C.; Ingwersen, J.; Starke, J.; Schmitz, M.; Bachmann, M.; Meurer, M.; Rieber, E.P.; Schäkel, K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong Th17/Th1 T-cell responses. J. Allergy Clin. Immunol. 2011, 127, 787–794.e9. [Google Scholar] [CrossRef]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Miller, J.C.; Brown, B.D.; Shay, T.; Gautier, E.L.; Jojic, V.; Cohain, A.; Pandey, G.; Leboeuf, M.; Elpek, K.G.; Helft, J.; et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 2012, 13, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, C.; Mjösberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Berg, G.; Sharma, S.; Buer, J.; Ernerudh, J. Gene Expression Profiling of Human Decidual Macrophages: Evidence for Immunosuppressive Phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef]
- Wang, T.; Ge, Y.; Xiao, M.; Lopez-Coral, A.; Azuma, R.; Somasundaram, R.; Zhang, G.; Wei, Z.; Xu, X.; Rauscher, F.J., 3rd; et al. Melanoma-derived conditioned media efficiently induce the differentiation of monocytes to macrophages that display a highly invasive gene signature. Pigment. Cell Melanoma Res. 2012, 25, 493–505. [Google Scholar] [CrossRef]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Zhang, X.; Mosser, D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2008, 214, 161–178. [Google Scholar] [CrossRef]
- Morris, D.; Singer, K.; Lumeng, C.N. Adipose tissue macrophages: Phenotypic plasticity and diversity in lean and obese states. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 341–346. [Google Scholar] [CrossRef]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef]
- Cao, S.; Liu, J.; Song, L.; Ma, X. The Protooncogene c-Maf Is an Essential Transcription Factor for IL-10 Gene Expression in Macrophages. J. Immunol. 2005, 174, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Schmitz, N.; Kurrer, M.O.; Bauer, M.; Hinton, H.I.; Behnke, S.; Gatto, D.; Sebbel, P.; Beerli, R.R.; Sonderegger, I.; et al. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J. Clin. Investig. 2006, 116, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.A.; Hendrycks, V.R.; Lidinsky, W.A.; Woods, M.L. PD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesion. Eur. J. Immunol. 2005, 35, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Heymann, F.; Lukacs-Kornek, V.; Boor, P.; Floege, J. Role of T cells and dendritic cells in glomerular immunopathology. Semin. Immunopathol. 2007, 29, 317–335. [Google Scholar] [CrossRef]
- Watchmaker, P.B.; Lahl, K.; Lee, M.; Baumjohann, D.; Morton, J.M.; Kim, S.J.; Zeng, R.; Dent, A.L.; Ansel, K.M.; Diamond, B.; et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat. Immunol. 2014, 15, 98–108. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B.-G.; O’Connor, T.P.; Crystal, R.G. Smoking-Dependent Reprogramming of Alveolar Macrophage Polarization: Implication for Pathogenesis of Chronic Obstructive Pulmonary Disease. J. Immunol. 2009, 183, 2867–2883. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Koth, L.L.; Yang, Y.H.; Rodriguez, M.W.; Favoreto, S.; Dolganov, G.M.; Paquet, A.C.; Erle, D.J. A Distinctive Alveolar Macrophage Activation State Induced by Cigarette Smoking. Am. J. Respir. Crit. Care Med. 2005, 172, 1383–1392. [Google Scholar] [CrossRef]
- Cavnar, M.J.; Zeng, S.; Kim, T.; Sorenson, E.C.; Ocuin, L.M.; Balachandran, V.P.; Seifert, A.M.; Greer, J.B.; Popow, R.; Crawley, M.H.; et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J. Exp. Med. 2013, 210, 2873–2886. [Google Scholar] [CrossRef]
- Segura, E.; Amigorena, S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013, 34, 440–445. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, M.; Lundberg, K.; Borrebaeck, C.A.K. Gene Family Clustering Identifies Functionally Associated Subsets of Human In Vivo Blood and Tonsillar Dendritic Cells. J. Immunol. 2005, 175, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Güttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Mittag, D.; Proietto, A.I.; Loudovaris, T.; Mannering, S.I.; Vremec, D.; Shortman, K.; Wu, L.; Harrison, L.C. Human Dendritic Cell Subsets from Spleen and Blood Are Similar in Phenotype and Function but Modified by Donor Health Status. J. Immunol. 2011, 186, 6207–6217. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Hudson, H.; Brown, C.; Lord, S.J.; Gebski, V.; De Souza, P.; Lee, C.K. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br. J. Cancer 2013, 109, 147–153. [Google Scholar] [CrossRef]
- Kawakami, T.; Lichtnekert, J.; Thompson, L.J.; Karna, P.; Bouabe, H.; Hohl, T.; Heinecke, J.W.; Ziegler, S.F.; Nelson, P.J.; Duffield, J.S. Resident Renal Mononuclear Phagocytes Comprise Five Discrete Populations with Distinct Phenotypes and Functions. J. Immunol. 2013, 191, 3358–3372. [Google Scholar] [CrossRef]
- Nelson, P.J.; Rees, A.J.; Griffin, M.; Hughes, J.; Kurts, C.; Duffield, J. The Renal Mononuclear Phagocytic System. J. Am. Soc. Nephrol. 2012, 23, 194–203. [Google Scholar] [CrossRef]
- Drabkin, H.A.; Gemmill, R.M. Obesity, Cholesterol, and Clear-Cell Renal Cell Carcinoma (RCC). Adv. Cancer Res. 2010, 107, 39–56. [Google Scholar] [CrossRef]
- Torroella-Kouri, M.; Caso, R.; Silvera, R.; Carrio, R.; Iragavarapu-Charyulu, V.; Gonzalez-Perez, R.R. Blood monocytes from mammary tumor-bearing mice: Early targets of tumor-induced immune suppression? Int. J. Oncol. 2010, 37, 891–900. [Google Scholar] [CrossRef][Green Version]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; Van den Bossche, J.; Mack, M.; Pipeleers, D.; Veld, P.I.; De Baetselier, P.; et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C(high) Monocytes. Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Chittezhath, M.; Dhillon, M.K.; Lim, J.Y.; Laoui, D.; Shalova, I.N.; Teo, Y.L.; Chen, J.; Kamaraj, R.; Raman, L.; Lum, J.; et al. Molecular Profiling Reveals a Tumor-Promoting Phenotype of Monocytes and Macrophages in Human Cancer Progression. Immunity 2014, 41, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Melé, M.; Ferreira, P.G.; Reverter, F.; DeLuca, D.S.; Monlong, J.; Sammeth, M.; Young, T.R.; Goldmann, J.M.; Pervouchine, D.D.; Sullivan, T.J.; et al. Human genomics. The human transcriptome across tissues and individuals. Science 2015, 348, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Barwe, S.P.; Lee, S.J.; Mcspadden, R.; Franco, O.E.; Hayward, S.W.; Damoiseaux, R.; Grubbs, S.S.; Petrelli, N.J.; Rajasekaran, A.K. Glucocorticoids Suppress Renal Cell Carcinoma Progression by Enhancing Na,K-ATPase Beta-1 Subunit Expression. PLoS ONE 2015, 10, e0122442. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Frilot, N.; Kim, J.; Kim, W.-J.; Daaka, Y. Prostaglandin E2 Regulates Renal Cell Carcinoma Invasion through the EP4 Receptor-Rap GTPase Signal Transduction Pathway. J. Biol. Chem. 2011, 286, 33954–33962. [Google Scholar] [CrossRef]
- Chuang, M.-J.; Sun, K.-H.; Tang, S.-J.; Deng, M.-W.; Wu, Y.-H.; Sung, J.-S.; Cha, T.-L.; Sun, G.-H. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008, 99, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Gogusev, J.; Augusti, M.; Chrétien, Y.; Droz, D. Interleukin-6 and TNFα production in human renal cell carcinoma. Kidney Int. 1993, 44, 585–592. [Google Scholar] [CrossRef]
- Hosse, J. Die Molekulare Quantifizierung von NK-Zellen im Klarzelligen Nierenzellkarzinom und die Bedeutung von CCL5 in NK-Zellen und für die Phagozytose; Ludwig-Maximilians-University Munich: Munich, Germany, 2009. [Google Scholar]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.-A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Katz, S.; Serezani, C.H.; Moore, T.A.; LeVine, A.M.; Moore, B.B. Prostaglandin E2–Induced Changes in Alveolar Macrophage Scavenger Receptor Profiles Differentially Alter Phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus Post–Bone Marrow Transplant. J. Immunol. 2013, 190, 5809–5817. [Google Scholar] [CrossRef]
- Schaer, D.J.; Boretti, F.S.; Schoedon, G.; Schaffner, A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br. J. Haematol. 2002, 119, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, Y.; Wang, F.; Zhu, J.; Zhao, Q.; Zheng, Y.; Gu, Y.; Xie, C.; Huang, Z.; Tai, Q.; et al. Liver myofibroblasts up-regulate monocyte CD163 expression via PGE2 during hepatitis B induced liver failure. J. Transl. Med. 2014, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Kim, H.L.; Belldegrun, A.S.; A Figlin, R. Immune gene therapy for kidney cancer: The search for a magic trigger. Mol. Ther. 2003, 7, 153–154. [Google Scholar] [CrossRef]
- Motzer, R.J. Renal Cell Carcinoma: A Priority Malignancy for Development and Study of Novel Therapies. J. Clin. Oncol. 2003, 21, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Stadler, W.M. Kidney cancer. Lancet 1998, 352, 1691–1696. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Braun, D.A.; Street, K.; Burke, K.P.; Cookmeyer, D.L.; Denize, T.; Pedersen, C.B.; Gohil, S.H.; Schindler, N.; Pomerance, L.; Hirsch, L.; et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell 2021, 39, 632–648.e8. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Samoilova, D.; Shitova, M.S.; Gratchev, A. Tumor Associated Macrophages in Kidney Cancer. Anal. Cell. Pathol. 2016, 2016, 9307549. [Google Scholar] [CrossRef]
- Krishna, C.; DiNatale, R.G.; Kuo, F.; Srivastava, R.M.; Vuong, L.; Chowell, D.; Gupta, S.; Vanderbilt, C.; Purohit, T.A.; Liu, M.; et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell 2021, 39, 662–677.e6. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, A.; Bocherding, N.; Chimenti, M.S.; Vishwakarma, P.; Nepple, K.; Salem, A.; Jenkins, R.W.; Zhang, W.; Zakharia, Y. Mapping the Immune Landscape of Clear Cell Renal Cell Carcinoma by Single-Cell RNA-seq. bioRxiv 2019. [Google Scholar] [CrossRef]
- Pinthus, J.H.; Whelan, K.F.; Gallino, D.; Lu, J.-P.; Rothschild, N. Metabolic features of clear-cell renal cell carcinoma: Mechanisms and clinical implications. Can. Urol. Assoc. J. 2011, 5, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.B.; Drachenberg, C.B.; Kumar, D.; Blanchaert, R.; Ord, R.A.; Ioffe, O.B.; Papadimitriou, J.C. Differential Diagnosis Between Monomorphic Clear Cell Adenocarcinoma of Salivary Glands and Renal (Clear) Cell Carcinoma. Am. J. Surg. Pathol. 1999, 23, 1532–1538. [Google Scholar] [CrossRef]
- Bagavant, H.; Fu, S.M. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2009, 21, 489–494. [Google Scholar] [CrossRef]
- Nikolic-Paterson, D.J.; Atkins, R.C. The role of macrophages in glomerulonephritis. Nephrol. Dial. Transplant. 2001, 16, 3–7. [Google Scholar] [CrossRef]
- Segerer, S.; Heller, F.; Lindenmeyer, M.; Schmid, H.; Cohen, C.; Draganovici, D.; Mandelbaum, J.; Nelson, P.; Gröne, H.-J.; Gröne, E.; et al. Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int. 2008, 74, 37–46. [Google Scholar] [CrossRef]
- Ferenbach, D.; Hughes, J. Macrophages and dendritic cells: What is the difference? Kidney Int. 2008, 74, 5–7. [Google Scholar] [CrossRef]
- Klechevsky, E.; Morita, R.; Liu, M.; Cao, Y.; Coquery, S.; Thompson-Snipes, L.; Briere, F.; Chaussabel, D.; Zurawski, G.; Palucka, A.K.; et al. Functional Specializations of Human Epidermal Langerhans Cells and CD14+ Dermal Dendritic Cells. Immunity 2008, 29, 497–510. [Google Scholar] [CrossRef]
- Krutzik, S.R.; Tan, B.; Li, H.; Ochoa, M.T.; Liu, P.T.; Sharfstein, S.E.; Graeber, T.; Sieling, P.A.; Liu, Y.-J.; Rea, T.H.; et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005, 11, 653–660. [Google Scholar] [CrossRef]
- Arazi, A.; Rao, D.A.; Berthier, C.C.; Davidson, A.; Liu, Y.; Hoover, P.J.; Chicoine, A.; Eisenhaure, T.M.; Jonsson, A.H.; Li, S.; et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019, 20, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef] [PubMed]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Chieppa, M.; Bianchi, G.; Solinas, G.; Fabbri, M.; Laskarin, G.; Mantovani, A. Engagement of the Mannose Receptor by Tumoral Mucins Activates an Immune Suppressive Phenotype in Human Tumor-Associated Macrophages. Clin. Dev. Immunol. 2010, 2010, 547179. [Google Scholar] [CrossRef]
- Bellora, F.; Castriconi, R.; Dondero, A.; Pessino, A.; Nencioni, A.; Liggieri, G.; Moretta, L.; Mantovani, A.; Moretta, A.; Bottino, C. TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur. J. Immunol. 2014, 44, 1814–1822. [Google Scholar] [CrossRef]
- Colvin, E.K. Tumor-Associated Macrophages Contribute to Tumor Progression in Ovarian Cancer. Front. Oncol. 2014, 4, 137. [Google Scholar] [CrossRef]
- Kawamura, K.; Komohara, Y.; Takaishi, K.; Katabuchi, H.; Takeya, M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol. Int. 2009, 59, 300–305. [Google Scholar] [CrossRef]
- Merogi, A.J.; Marrogi, A.J.; Ramesh, R.; Robinson, W.R.; Fermin, C.D.; Freeman, S.M. Tumor-host interaction: Analysis of cytokines, growth factors, and tumorinfiltrating lymphocytes in ovarian carcinomas. Hum. Pathol. 1997, 28, 321–331. [Google Scholar] [CrossRef]
- Giuntoli, R.L., 2nd; Webb, T.J.; Zoso, A.; Rogers, O.; Diaz-Montes, T.P.; Bristow, R.E.; Oelke, M. Ovarian cancer-associated ascites demonstrates altered immune environment: Implications for antitumor immunity. Anticancer. Res. 2009, 29, 2875–2884. [Google Scholar]
- Lane, D.; Matte, I.; Rancourt, C.; Piché, A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 2011, 11, 210. [Google Scholar] [CrossRef]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.; Wagner, U.; Müller-Brüsselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef] [PubMed]
- Gorgani, N.N.; Thathaisong, U.; Mukaro, V.R.; Poungpair, O.; Tirimacco, A.; Hii, C.S.; Ferrante, A. Regulation of CRIg Expression and Phagocytosis in Human Macrophages by Arachidonate, Dexamethasone, and Cytokines. Am. J. Pathol. 2011, 179, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.N.; Missolo-Koussou, Y.; Gerber-Ferder, Y.; Bromley, C.P.; Bugatti, M.; Núñez, N.G.; Boari, J.T.; Richer, W.; Menger, L.; Denizeau, J.; et al. Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell 2022, 185, 1189–1207.e25. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.-A.; Pai, R.; Blériot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394.e21. [Google Scholar] [CrossRef]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18. [Google Scholar] [CrossRef] [PubMed]
- Eckl, J.; Buchner, A.; Prinz, P.U.; Riesenberg, R.; Siegert, S.I.; Kammerer, R.; Nelson, P.J.; Noessner, E. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J. Mol. Med. 2012, 90, 55–66. [Google Scholar] [CrossRef]
- Fischbeck, A.J.; Ruehland, S.; Ettinger, A.; Paetzold, K.; Masouris, I.; Noessner, E.; Mendler, A.N. Tumor Lactic Acidosis: Protecting Tumor by Inhibiting Cytotoxic Activity Through Motility Arrest and Bioenergetic Silencing. Front. Oncol. 2020, 10, 589434. [Google Scholar] [CrossRef]
- Mendler, A.N.; Hu, B.; Prinz, P.U.; Kreutz, M.; Gottfried, E.; Noessner, E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer 2012, 131, 633–640. [Google Scholar] [CrossRef]
- He, J.Q.; Wiesmann, C.; Campagne, M.V.L. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol. Immunol. 2008, 45, 4041–4047. [Google Scholar] [CrossRef]
- Ikarashi, M.; Nakashima, H.; Kinoshita, M.; Sato, A.; Nakashima, M.; Miyazaki, H.; Nishiyama, K.; Yamamoto, J.; Seki, S. Distinct development and functions of resident and recruited liver Kupffer cells/macrophages. J. Leukoc. Biol. 2013, 94, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nagai, T.; Usami, M.; Hasui, K.; Takao, S.; Matsuyama, T. Phenotypic and functional profiles of CRIg (Z39Ig)-expressing macrophages in the large intestine. Innate Immun. 2012, 18, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-S.; Sato, K.; Dougherty, I.I.; Cruz, P.D.; Ariizumi, K. DC-HIL is a negative regulator of T lymphocyte activation. Blood 2007, 109, 4320–4327. [Google Scholar] [CrossRef]
- Ripoll, V.M.; Irvine, K.M.; Ravasi, T.; Sweet, M.J.; Hume, D.A. Gpnmb Is Induced in Macrophages by IFN-γ and Lipopolysaccharide and Acts as a Feedback Regulator of Proinflammatory Responses. J. Immunol. 2007, 178, 6557–6566. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, J.T.; Chen, D.; Choi, M.; Nissen, J.C.; Shroyer, K.R.; Djordevic, S.; Zachary, I.C.; Selwood, D.; Tsirka, S.E. Ablation of Neuropilin 1 from glioma-associated microglia and macrophages slows tumor progression. Oncotarget 2016, 7, 9801–9814. [Google Scholar] [CrossRef]
- Engering, A.; Geijtenbeek, T.B.H.; van Vliet, S.J.; Wijers, M.; van Liempt, E.; Demaurex, N.; Lanzavecchia, A.; Fransen, J.; Figdor, C.G.; Piguet, V.; et al. The Dendritic Cell-Specific Adhesion Receptor DC-SIGN Internalizes Antigen for Presentation to T Cells. J. Immunol. 2002, 168, 2118–2126. [Google Scholar] [CrossRef]
- Gottschalk, C.; Kurts, C. The Debate about Dendritic Cells and Macrophages in the Kidney. Front. Immunol. 2015, 6, 435. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brech, D.; Herbstritt, A.S.; Diederich, S.; Straub, T.; Kokolakis, E.; Irmler, M.; Beckers, J.; Büttner, F.A.; Schaeffeler, E.; Winter, S.; et al. Dendritic Cells or Macrophages? The Microenvironment of Human Clear Cell Renal Cell Carcinoma Imprints a Mosaic Myeloid Subtype Associated with Patient Survival. Cells 2022, 11, 3289. https://doi.org/10.3390/cells11203289
Brech D, Herbstritt AS, Diederich S, Straub T, Kokolakis E, Irmler M, Beckers J, Büttner FA, Schaeffeler E, Winter S, et al. Dendritic Cells or Macrophages? The Microenvironment of Human Clear Cell Renal Cell Carcinoma Imprints a Mosaic Myeloid Subtype Associated with Patient Survival. Cells. 2022; 11(20):3289. https://doi.org/10.3390/cells11203289
Chicago/Turabian StyleBrech, Dorothee, Anna S. Herbstritt, Sarah Diederich, Tobias Straub, Evangelos Kokolakis, Martin Irmler, Johannes Beckers, Florian A. Büttner, Elke Schaeffeler, Stefan Winter, and et al. 2022. "Dendritic Cells or Macrophages? The Microenvironment of Human Clear Cell Renal Cell Carcinoma Imprints a Mosaic Myeloid Subtype Associated with Patient Survival" Cells 11, no. 20: 3289. https://doi.org/10.3390/cells11203289
APA StyleBrech, D., Herbstritt, A. S., Diederich, S., Straub, T., Kokolakis, E., Irmler, M., Beckers, J., Büttner, F. A., Schaeffeler, E., Winter, S., Schwab, M., Nelson, P. J., & Noessner, E. (2022). Dendritic Cells or Macrophages? The Microenvironment of Human Clear Cell Renal Cell Carcinoma Imprints a Mosaic Myeloid Subtype Associated with Patient Survival. Cells, 11(20), 3289. https://doi.org/10.3390/cells11203289