The Association between Maternal Oral Inflammation and Neutrophil Phenotypes and Poly-Unsaturated Fatty Acids Composition in Human Milk: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Patient Selection
2.2. Maternal Demographic Characteristics and Dietary Intake
2.3. Oral Rinse Samples Collection and Processing
2.4. Human Milk Samples Collection and Processing
2.5. Flow Cytometry
2.6. Cell Sorting, Cytospin Preparation and Staining
2.7. Fatty Acids Analysis in Human Milk
2.8. Statistical Analysis and Sample Size Estimate
3. Results
3.1. Participants’ Descriptive Characteristics
3.2. Participants’ Oral Inflammatory Load
3.3. Maternal Dietary Intake
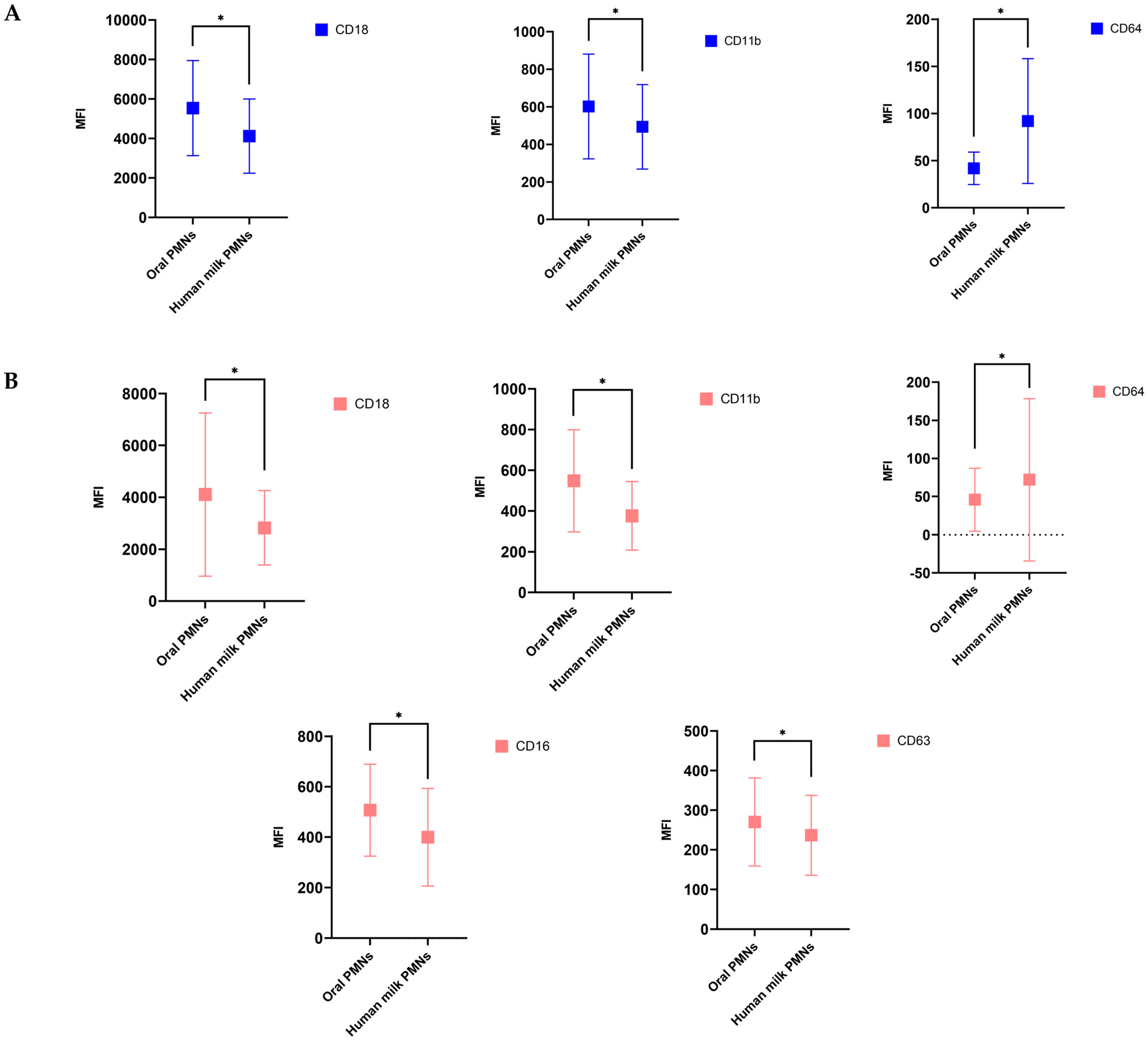
3.4. hmPMNs Had Lower Activation State Compared to oPMNs
3.5. Mothers with Moderate to Severe OIL Had hmPMNs with Higher Expression Levels of CD14 Biomarker and Lower Expression Levels of CD64 Biomarker during Lactation, Compared to Mothers with Healthy OIL
3.6. Mothers with Moderate and Severe OIL Had Higher Levels of n-6 PUFA; Docosapentaenoic Acid (DPA) in Their Human Milk at Baseline, Compared to Mothers with Healthy OIL
3.7. Mothers with Moderate to Severe OIL Had Lower Levels of C20:5n-3 (EPA) LCPUFA during Lactation, Compared to Mothers with Healthy OIL
3.8. CD64 Surface Expression on hmPMNs Was Higher in Mothers Whose Infants Had a Health Condition Compared to the Mothers of Healthy Infants
3.9. Higher Levels of Arachidonic Acid and C22:5n-6 FA Were Observed in the Human Milk of the Mothers Whose Infants Had a Health Condition Compared to Healthy Infants
3.10. Correlation between Human Milk PMNs and Their Levels of Activation and the FAs in Human Milk
4. Discussion
Study Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daalderop, L.A.; Wieland, B.V.; Tomsin, K.; Reyes, L.; Kramer, B.W.; Vanterpool, S.F.; Been, J.V. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR Clin. Transl. Res. 2018, 3, 10–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakheran, O.; Saied-Moallemi, Z.; Khademi, A. Oral Health Related Quality of Life during Pregnancy and Postpartum: A systematic review. Res. Sq. 2019, 218858094. [Google Scholar]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Tilakaratne, A.; Soory, M.; Ranasinghe, A.W.; Corea, S.M.; Ekanayake, S.L.; de Silva, M. Periodontal disease status during pregnancy and 3 months post-partum, in a rural population of Sri-Lankan women. J. Clin. Periodontol. 2000, 27, 787–792. [Google Scholar] [CrossRef]
- Teshome, A.; Yitayeh, A. Relationship between periodontal disease and preterm low birth weight: Systematic review. Pan Afr. Med. J. 2016, 24, 215. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Khoury, W.; Glogauer, J.; Tenenbaum, H.C.; Glogauer, M. Oral inflammatory load: Neutrophils as oral health biomarkers. J. Periodont. Res. 2020, 55, 594–601. [Google Scholar] [CrossRef]
- Huda, S.; Doering, H.; Tenenbaum, H.C.; Whittle, W.; Sigal, M.J.; Glogauer, M. Oral neutrophil levels: A screening test for oral inflammatory load in pregnancy in a medical setting. J. Periodontol. 2015, 86, 72–81. [Google Scholar] [CrossRef]
- Landzberg, M.; Doering, H.; Aboodi, G.M.; Tenenbaum, H.C.; Glogauer, M. Quantifying oral inflammatory load: Oral neutrophil counts in periodontal health and disease. J. Periodont. Res. 2015, 50, 330–336. [Google Scholar] [CrossRef]
- Bender, J.S.; Thang, H.; Glogauer, M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J. Periodont. Res. 2006, 41, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Delima, A.J.; Van Dyke, T.E. Origin and function of the cellular components in gingival crevice fluid. Periodontology 2000 2003, 31, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Deas, D.E.; Mackey, S.A.; McDonnell, H.T. Systemic disease and periodontitis: Manifestations of neutrophil dysfunction. Periodontology 2000 2003, 32, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Dennison, D.K.; Van Dyke, T.E. The acute inflammatory response and the role of phagocytic cells in periodontal health and disease. Periodontology 2000 1997, 14, 54–78. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Ji, A.L.; Ji, X.Y.; Li, Y.Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 9671604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fine, N.; Hassanpour, S.; Borenstein, A.; Sima, C.; Oveisi, M.; Scholey, J.; Cherney, D.; Glogauer, M. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J. Dent. Res. 2016, 95, 931–938. [Google Scholar] [CrossRef]
- Medara, N.; Lenzo, J.C.; Walsh, K.A.; Reynolds, E.C.; O’Brien-Simpson, N.M.; Darby, I.B. Peripheral neutrophil phenotypes during management of periodontitis. J. Periodont. Res. 2021, 56, 58–68. [Google Scholar] [CrossRef]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Duale, A.; Singh, P.; Al Khodor, S. Breast Milk: A Meal Worth Having. Front. Nutr. 2021, 8, 800927. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Blewett, H.J.H.; Cicalo, M.C.; Holland, C.D.; Field, C.J. The immunological components of human milk. Adv. Food Nutr. Res. 2008, 54, 45–80. [Google Scholar]
- Bobiński, R.; Bobińska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 28–40. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Cortijo, D.; Singh, P.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Breast Milk Lipids and Fatty Acids in Regulating Neonatal Intestinal Development and Protecting against Intestinal Injury. Nutrients 2020, 12, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassiotou, F.; Hepworth, A.R.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.E.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2013, 2, e3. [Google Scholar] [CrossRef]
- Riskin, A.; Almog, M.; Peri, R.; Halasz, K.; Srugo, I.; Kessel, A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatric Res. 2012, 71, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Samuel, T.M.; Zhou, Q.; Giuffrida, F.; Munblit, D.; Verhasselt, V.; Thakkar, S.K. Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front. Nutr. 2020, 7, 576133. [Google Scholar] [CrossRef]
- Peila, C.; Gazzolo, D.; Bertino, E.; Cresi, F.; Coscia, A. Influence of Diabetes during Pregnancy on Human Milk Composition. Nutrients 2020, 12, 185. [Google Scholar] [CrossRef] [Green Version]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, A.B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [Green Version]
- Laiho, K.; Lampi, A.M.; Hamalainen, M.; Moilanen, E.; Piironen, V.; Arvola, T.; Syrjänen, S.; Isolauri, E. Breast milk fatty acids, eicosanoids, and cytokines in mothers with and without allergic disease. Pediatric Res. 2003, 53, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Johansson, S.; Wold, A.E.; Sandberg, A.S. Low breast milk levels of long-chain n-3 fatty acids in allergic women, despite frequent fish intake. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2011, 41, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Amaral, Y.; Marano, D.; Abranches, A.; Silva, L.; Nehab, S.; Costa, A.C.; Moreira, M.E. Do chronic noncommunicable diseases modify the macronutrient composition of human milk? Int. J. Food Sci. Nutr. 2021, 72, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Hoppu, U.; Hämäläinen, M.; Linderborg, K.; Moilanen, E.; Isolauri, E. Breast milk fatty acids may link innate and adaptive immune regulation: Analysis of soluble CD14, prostaglandin E2, and fatty acids. Pediatric Res. 2006, 59, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Price, W.J.; Shafii, B.; Yahvah, K.M.; Bode, L.; McGuire, M.A.; McGuire, M.K. Relationships Among Microbial Communities, Maternal Cells, Oligosaccharides, and Macronutrients in Human Milk. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2017, 33, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaferi, A.A.; Schwartz, T.A.; Pawlik, T.M. STROBE Reporting Guidelines for Observational Studies. JAMA Surg. 2021, 156, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeste, A.M.; Aura, A. Rate of migration of oral leukocytes in patients with periodontitis. Scand. J. Dent. Res. 1978, 86, 43–51. [Google Scholar] [CrossRef]
- Sharma, V.; Hanafi, A.; Overgaard, C.B.; Shaibani, M.; An, K.; Dzavik, V.; Glogauer, M.; Tenenbaum, H.C. Oral inflammatory load in patients with coronary artery disease. J. Oral Sci. 2019, 61, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Lovelady, C.A.; Dewey, K.G.; Picciano, M.F.; Dermer, A. Guidelines for collection of human milk samples for monitoring and research of environmental chemicals. J. Toxicol. Environ. Health Part A 2002, 65, 1881–1891. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Pitino, M.A.; Alashmali, S.M.; Hopperton, K.E.; Unger, S.; Pouliot, Y.; Doyen, A.; O’Connor, D.L.; Bazinet, R.P. Oxylipin concentration, but not fatty acid composition, is altered in human donor milk pasteurised using both thermal and non-thermal techniques. Br. J. Nutr. 2019, 122, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.D.; Willett, J.B. (Eds.) Exploring Longitudinal Data on Change: Applied Longitudinal Data Analysis—Modeling Change and Event Occurrence; Oxford University Press Inc.: Oxford, UK, 2003. [Google Scholar]
- Wallace, D.G. Analysis of Repeated Measures Designs with Linear Mixed Models: Modeling Intraindividual Variability with Repeated Measures Data: Method and Applications; Hershberger, S.L., Moskowitz, D.S., Eds.; Erlbaum: Forest Hills, NY, USA, 2002. [Google Scholar]
- Panda, B.S.K.; Mohapatra, S.K.; Alhussien, M.N.; Dang, A.K. Amount of Milk Neutrophil Percentage and Associated CD Molecular Changes on the Compositional and Technological Properties of Milk. Open Biotechnol. J. 2019, 13, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar]
- Thorpe, L.W.; Rudloff, H.E.; Powell, L.C.; Goldman, A.S. Decreased response of human milk leukocytes to chemoattractant peptides. Pediatric Res. 1986, 20, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kipfmueller, F.; Schneider, J.; Prusseit, J.; Dimitriou, I.; Zur, B.; Franz, A.R.; Bartmann, P.; Mueller, A. Role of Neutrophil CD64 Index as a Screening Marker for Late-Onset Sepsis in Very Low Birth Weight Infants. PLoS ONE 2015, 10, e0124634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oveisi, M.; Shifman, H.; Fine, N.; Sun, C.; Glogauer, N.; Senadheera, D.; Glogauer, M. Novel Assay to Characterize Neutrophil Responses to Oral Biofilms. Infect. Immun. 2019, 87, 2. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, V.; Blufstein, A.; Behm, C.; Andrukhov, O. The Alterations in CD14 Expression in Periodontitis: A Systematic Review. Appl. Sci. 2021, 11, 2444. [Google Scholar] [CrossRef]
- De la Garza Puentes, A.; Martí Alemany, A.; Chisaguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; Garcia-Valdez, L.; Escudero-Marin, M.; Segura, M.Y.; Campoy, C.; et al. The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition-The PREOBE Follow-Up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef] [Green Version]
- Mabaya, L.; Matarira, H.T.; Tanyanyiwa, D.M.; Musarurwa, C.; Mukwembi, J.; Mudluli, T.E.; Marera, T. Polyunsaturated Fatty Acid Composition in Breast Milk Plasma of HIV-infected and Uninfected Mothers in Relation to Infant Clinical Outcomes. Nutr. Metab. Insights 2022, 15, 11786388211072768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Botolin, D.; Christian, B.; Busik, J.; Xu, J.; Jump, D.B. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 2005, 46, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Azulay Chertok, I.R.; Haile, Z.T.; Eventov-Friedman, S.; Silanikove, N.; Argov-Argaman, N. Influence of gestational diabetes mellitus on fatty acid concentrations in human colostrum. Nutrition 2017, 36, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Fat chance of immunomodulation. Immunol. Today 1998, 19, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Huynh, H.; Wan, Y. Milk lipid regulation at the maternal-offspring interface. Semin. Cell Develop. Biol. 2018, 81, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi Bernardi, J.; de Souza Escobar, R.; Ferreira, C.F.; Pelufo Silveira, P. Fetal and neonatal levels of omega-3: Effects on neurodevelopment, nutrition, and growth. Sci. World J. 2012, 2012, 202473. [Google Scholar]
- Du, J.; Li, L.; Dou, Y.; Li, P.; Chen, R.; Liu, H. Diagnostic utility of neutrophil CD64 as a marker for early-onset sepsis in preterm neonates. PLoS ONE 2014, 9, e102647. [Google Scholar] [CrossRef]
- Hashem, H.E.; El Masry, S.A.; Mokhtar, A.M.; Ismail, E.A.; Abdelaal, N.M. Valuable Role of Neutrophil CD64 and Highly Sensitive CRP Biomarkers for Diagnostic, Monitoring, and Prognostic Evaluations of Sepsis Patients in Neonatal ICUs. BioMed Res. Int. 2020, 2020, 6214363. [Google Scholar] [CrossRef]
- Ng, P.C.; Li, G.; Chui, K.M.; Chu, W.C.; Li, K.; Wong, R.P.; Chik, K.W.; Wong, E.; Fok, T.F. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatric Res. 2004, 56, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, R.M. Transmission of Infectious Diseases Through Breast Milk and Breastfeeding. Breastfeeding 2011, 406–473. [Google Scholar] [CrossRef]
- Kim, S.K.; Keeney, S.E.; Alpard, S.K.; Schmalstieg, F.C. Comparison of L-selectin and CD11b on neutrophils of adults and neonates during the first month of life. Pediatric Res. 2003, 53, 132–136. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505s–1519s. [Google Scholar] [CrossRef] [Green Version]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urwin, H.J.; Zhang, J.; Gao, Y.; Wang, C.; Li, L.; Song, P.; Man, Q.; Meng, L.; Miles, E.A.; Calder, P.C.; et al. Immune factors and fatty acid composition in human milk from river/lake, coastal and inland regions of China. Br. J. Nutr. 2013, 109, 1949–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, K.; Labéta, M.O.; Schiffrin, E.J.; Donnet-Hughes, A. Soluble CD14 in human breast milk and its role in innate immune responses. Acta Odontol. Scandinav. 2001, 59, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Blais, D.R.; Harrold, J.; Altosaar, I. Killing the messenger in the nick of time: Persistence of breast milk sCD14 in the neonatal gastrointestinal tract. Pediatric Res. 2006, 59, 371–376. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Variables | Total N (%) |
|---|---|---|
| Demographic characteristics | Age (years) | |
| (Mean ± SD) | 34.5 ± 4.4 | |
| Marital status | ||
| Married | 40 (85.1) | |
| Single/Unmarried partners | 7 (14.9) | |
| Employment status | ||
| Employed full-time | 32 (68.1) | |
| Employed part-time/Student/unemployed | 15 (31.9) | |
| Highest education level | ||
| Secondary graduate/Degree/Diploma | 23 (48.9) | |
| Post-graduate studies | 24 (51.1) | |
| Maternal and infant characteristics | Gestational age at delivery (weeks) | 39.1 ± 1.2 |
| (Mean ± SD) | ||
| BMI after delivery (kg/m2) | 25.1 ± 3.7 | |
| (Mean ± SD) | ||
| Infant feeding patterns | ||
| Exclusive BF | 32 (64.0) | |
| Predominant BF | 11 (22.0) | |
| Mixed feeding * | 4 (8.0) | |
| Infant sex | ||
| Boy | 26 (52.0) | |
| Girl | 22 (44.0) | |
| Infant health status | ||
| Healthy | 39 (78.0) | |
| Diseased/Infected | 8 (16.0) | |
| Lifestyle and dental history | Brushing at least twice a day | 32 (68.1) |
| Flossing at least once per day | 12 (25.1) | |
| Dental visit frequency | ||
| ≥1 visit a year | 38 (80.9) | |
| <1 visit a year | 5 (10.6) | |
| Only for emergency | 4 (8.5) |
| oPMN Counts (×106/10 mL Rinse) | N | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Total oPMN counts | |||||
| Baseline | 50 | 1.38 ± 1.63 | 0.65 | 0 | 6.97 |
| Follow-up | 46 | 1.93 ± 4.24 | 0.72 | 0 | 23.56 |
| Baseline | |||||
| Healthy | 21 | 0.25 ± 0.15 | 0.35 | 0 | 0.47 |
| Moderate | 8 | 0.76 ± 0.14 | 0.65 | 0.52 | 0.98 |
| Severe | 21 | 2.66 ± 1.89 | 1.89 | 1.01 | 6.97 |
| Follow-up | |||||
| Healthy | 16 | 0.22 ± 0.15 | 0.29 | 0 | 0.49 |
| Moderate | 13 | 0.78 ± 0.13 | 0.7 | 0.5 | 0.93 |
| Severe | 17 | 4.59 ± 6.38 | 1.43 | 1.1 | 23.57 |
| Neutrophils and CD Biomarkers † | Diseased OIL (n = 20) | |||
|---|---|---|---|---|
| Estimate β b (95% CI) | p-Value | Adjusted Estimate β c (95% CI) | Adjusted p-Value | |
| Absolute hmPMN counts (×106/10 mL) | 0.23 (−0.10, 0.57) | 0.168 | 0.27 (−0.11, 0.65) | 0.17 |
| CD16 | −175.72 (−419.60, 68.15) | 0.156 | −172.41 (−447.57, 102.75) | 0.216 |
| CD66a | −86.62 (−236.12, 62.88) | 0.253 | −76.77 (−270.10, 116.55) | 0.431 |
| CD11b | −6.26 (−175.29, 162.76) | 0.941 | 17.21 (−171.75, 206.17) | 0.856 |
| CD18 | 445.38 (−991.48, 1882.25) | 0.539 | 503.41 (−1000.33, 2007.16) | 0.507 |
| CD14 | 128.22 (−2.34, 258.80) | 0.054 | 151.25 (14.13, 288.36) | 0.031 |
| CD64 | −75.04 (−149.00, −1.07) | 0.047 | −95.35 (−187.69, −3.01) | 0.043 |
| CD63 | −26.44 (−145.40, 92.51) | 0.66 | −4.58 (−133.71, 124.54) | 0.944 |
| Fatty Acids in Human Milk | Diseased OIL (n = 20) | |||
|---|---|---|---|---|
| Estimate β b (95% CI) | p-Value | Adjusted Estimate β c (95% CI) | Adjusted p-Value | |
| C10:0 | −0.17 (−0.49, 0.14) | 0.269 | −0.15 (−0.50, 0.18) | 0.368 |
| C12:0 | −0.80 (−2.06, 0.46) | 0.210 | −0.91 (−2.27, 0.44) | 0.182 |
| C14:0 | −0.57 (−1.85, 0.71) | 0.376 | −0.50 (−1.80, 0.79) | 0.440 |
| C16:0 | 0.94 (−1.77, 3.65) | 0.492 | 1.63 (−1.29, 4.57) | 0.269 |
| C18:0 | 1.27 (0.10, 2.43) | 0.032 | 1.11 (−0.15, 2.39) | 0.084 |
| C20:0 | 0.01 (−0.02, 0.06) | 0.439 | 0.01 (−0.04, 0.06) | 0.739 |
| C22:0 | 0.01 (−0.04, 0.05) | 0.865 | −0.01 (−0.06, 0.04) | 0.674 |
| C23:0 | −0.03 (−0.29, 0.22) | 0.772 | −0.05 (−0.34, 0.24) | 0.719 |
| C24:0 | 0.01 (−0.03, 0.04) | 0.731 | 0.01 (−0.03, 0.04) | 0.799 |
| SFAs | 0.60 (−4.02, 5.22) | 0.796 | 0.80 (−4.32, 5.93) | 0.756 |
| C14:1 | −0.05 (−0.12, 0.02) | 0.192 | −0.01 (−0.09, 0.07) | 0.795 |
| C18:1n-7 | −2.97 (−10.66, 4.72) | 0.445 | −1.83 (−10.75, 7.08) | 0.683 |
| C18:1n-9 | 4.21 (−4.43, 12.87) | 0.335 | 2.98 (−6.76, 12.74) | 0.543 |
| C20:1n-9 | 0.05 (−0.03, 0.13) | 0.240 | 0.03 (−0.05, 0.12) | 0.469 |
| C22:1n-9 | −0.07 (−0.21, 0.05) | 0.242 | −0.11 (−0.26, 0.04) | 0.151 |
| MUFAs | 1.43 (−3.17, 6.03) | 0.539 | 1.18 (−3.64, 6.01) | 0.627 |
| C18:2n-6 (LA) | −1.22 (−6.74, 4.29) | 0.661 | −0.64 (−6.69, 5.39) | 0.831 |
| C18:3n-6 (GLA) | 0.00 (−0.04, 0.04) | 0.969 | 0.01 (−0.03, 0.04) | 0.851 |
| C20:2n-6 | 0.08 (−0.23, 0.39) | 0.604 | 0.06 (−0.28, 0.42) | 0.705 |
| C20:3n-6 | 0.05 (−0.03, 0.15) | 0.233 | 0.03 (−0.07, 0.14) | 0.501 |
| C20:4n-6 (AA) | 0.02 (−0.09, 0.14) | 0.687 | −0.01 (−0.13, 0.12) | 0.914 |
| C22:2n-6 | 0.71 (−1.18, 2.61) | 0.458 | 0.59 (−1.60, 2.80) | 0.589 |
| C22:4n-6 | −0.08 (−0.21, 0.04) | 0.188 | −0.12 (−0.26, 0.01) | 0.088 |
| C22:5n-6 | −0.05 (−0.12, 0.01) | 0.075 | −0.04 (−0.11, 0.02) | 0.166 |
| N-6 | −1.10 (−7.38, 5.18) | 0.729 | −0.50 (−7.39, 6.38) | 0.884 |
| C18:3n-3 (ALA) | 0.21 (−0.50, 0.93) | 0.558 | −0.06 (−0.85, 0.73) | 0.880 |
| C20:5n-3 (EPA) | −0.11 (−0.22, −0.01) | 0.029 | −0.12 (−0.24, 0.00) | 0.056 |
| C22:5n-3 | −0.06 (−0.19, 0.06) | 0.336 | −0.11 (−0.25, 0.03) | 0.132 |
| C22:6n-3 (DHA) | −0.04 (−0.23, 0.14) | 0.647 | −0.06 (−0.27, 0.14) | 0.536 |
| N-3 | −0.10 (−0.96, 0.75) | 0.807 | −0.42 (−1.36, 0.51) | 0.369 |
| PUFAs | −0.84 (−7.41, 5.72) | 0.799 | −0.48 (−7.62, 6.66) | 0.894 |
| N6/N3 | 4.33 (−7.19, 15.86) | 0.457 | 5.52 (−7.54, 18.59) | 0.402 |
| Neutrophils and CD Markers | Infants with a Health Condition (n = 14) c | |||
|---|---|---|---|---|
| Estimate β a (95% CI) | p-Value | Adjusted Estimate β b (95% CI) | Adjusted p-Value | |
| Absolute hmPMN counts (×106/10 mL) | −0.13 (−0.49, 0.21) | 0.288 | −0.05 (−0.30, 0.18) | 0.624 |
| CD16 | −46.23 (−306.54, 214.07) | 0.553 | 60.94 (−170.74, 292.63) | 0.599 |
| CD66a | −33.50 (−189.92, 122.91) | 0.890 | −36.03 (−155.85, 83.78) | 0.547 |
| CD11b | 10.91 (−169.38, 191.21) | 0.602 | −26.22 (−142.45, 90.00) | 0.650 |
| CD18 | 861.03 (−635.55, 2357.63) | 0.423 | 359.51 (−781.69, 1500.73) | 0.529 |
| CD14 | 81.35 (−51.31, 214.02) | 0.392 | 38.05 (−37.85, 113.95) | 0.316 |
| CD64 | 100.25 (27.69, 172.80) | 0.019 | 85.65 (21.97, 149.32) | 0.009 |
| CD63 | −4.75 (−121.62, 112.11) | 0.183 | −54.96 (−150.39, 40.47) | 0.250 |
| Fatty Acids in Human Milk | Infants with a Health Condition (n = 14) | |||
|---|---|---|---|---|
| Estimate β a (95% CI) | p-Value | Adjusted Estimate β b (95% CI) | Adjusted p-Value | |
| C10:0 | −0.14 (−0.49, 0.20) | 0.257 | −0.16 (−0.43, 0.11) | 0.241 |
| C12:0 | −0.79 (−2.21, 0.63) | 0.219 | −0.63 (−1.76, 0.49) | 0.267 |
| C14:0 | −0.99 (−2.42, 0.43) | 0.121 | −0.45 (−1.51, 0.61) | 0.399 |
| C16:0 | −2.61 (−5.58, 0.35) | 0.057 | −1.51 (−3.85, 0.82) | 0.200 |
| C18:0 | −0.17 (−1.49, 1.14) | 0.415 | −0.92 (−1.95, 0.11) | 0.081 |
| C20:0 | −0.02 (−0.07, 0.02) | 0.564 | −0.02 (−0.06, 0.01) | 0.193 |
| C22:0 | 0.03 (−0.01, 0.08) | 0.055 | 0.02 (−0.01, 0.06) | 0.152 |
| C23:0 | 0.00 (−0.26, 0.26) | 0.492 | −0.01 (−0.22, 0.19) | 0.902 |
| C24:0 | 0.01 (−0.01, 0.03) | 0.003 | 0.03 (0.01, 0.06) | 0.004 |
| SFAs | −4.42 (−9.34, 0.49) | 0.028 | −3.34 (−7.39, 0.69) | 0.103 |
| C14:1 | −0.01 (−0.09, 0.07) | 0.216 | −0.01 (−0.07, 0.04) | 0.646 |
| C18:1n-7 | −2.52 (−10.96, 5.91) | 0.326 | −2.17 (−7.53, 3.17) | 0.417 |
| C18:1n-9 | 0.29 (−9.19, 9.79) | 0.575 | −0.11 (−6.60, 6.37) | 0.971 |
| C20:1n-9 | −0.04 (−0.14, 0.05) | 0.845 | −0.01 (−0.08, 0.07) | 0.898 |
| C22:1n-9 | −0.01 (−0.16, 0.12) | 0.512 | 0.08 (−0.04, 0.20) | 0.182 |
| MUFAs | −2.14 (−7.01, 2.71) | 0.540 | −2.39 (−5.76, 0.97) | 0.160 |
| C18:2n-6 (LA) | 3.01 (−2.58, 8.62) | 0.140 | 2.86 (−1.10, 6.83) | 0.153 |
| C18:3n-6 (GLA) | −0.01 (−0.05, 0.03) | 0.762 | −0.01 (−0.04, 0.02) | 0.664 |
| C20:2n-6 | 0.21 (−0.11, 0.54) | 0.138 | 0.19 (−0.09, 0.47) | 0.180 |
| C20:3n-6 | −0.01 (−0.12, 0.08) | 0.773 | 0.01 (−0.07, 0.10) | 0.719 |
| C20:4n-6 (AA) | 0.09 (−0.03, 0.22) | 0.009 | 0.12 (0.02, 0.21) | 0.013 |
| C22:2n-6 | 0.09 (−1.96, 2.15) | 0.683 | −0.14 (−1.96, 1.66) | 0.873 |
| C22:4n-6 | 0.04 (−0.07, 0.16) | 0.041 | 0.08 (−0.01, 0.16) | 0.065 |
| C22:5n-6 | 0.09 (0.02, 0.16) | 0.010 | 0.06 (0.01, 0.12) | 0.015 |
| N-6 | 4.60 (−1.78, 10.99) | 0.155 | 3.73 (−0.83, 8.30) | 0.107 |
| C18:3n-3 (ALA) | 0.78 (0.05, 1.52) | 0.036 | 0.44 (−0.17, 1.07) | 0.155 |
| C20:5n-3 (EPA) | −0.04 (−0.15, 0.06) | 0.902 | −0.04 (−0.12, 0.04) | 0.319 |
| C22:5n-3 | −0.01 (−0.14, 0.11) | 0.155 | 0.06 (−0.04, 0.16) | 0.264 |
| C22:6n-3 (DHA) | 0.11 (−0.09, 0.31) | 0.06 | 0.11 (−0.04, 0.27) | 0.171 |
| N-3 | 0.83 (−0.03, 1.70) | 0.014 | 0.67 (−0.02, 1.37) | 0.059 |
| PUFAs | 5.21 (−1.28, 11.71) | 0.060 | 4.27 (−0.46, 9.01) | 0.076 |
| N6/N3 | −5.51 (−18.27, 7.24) | 0.321 | −4.07 (−13.48, 5.33) | 0.388 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badewy, R.; Azarpazhooh, A.; Tenenbaum, H.; Connor, K.L.; Lai, J.Y.; Sgro, M.; Bazinet, R.P.; Fine, N.; Watson, E.; Sun, C.; et al. The Association between Maternal Oral Inflammation and Neutrophil Phenotypes and Poly-Unsaturated Fatty Acids Composition in Human Milk: A Prospective Cohort Study. Cells 2022, 11, 4110. https://doi.org/10.3390/cells11244110
Badewy R, Azarpazhooh A, Tenenbaum H, Connor KL, Lai JY, Sgro M, Bazinet RP, Fine N, Watson E, Sun C, et al. The Association between Maternal Oral Inflammation and Neutrophil Phenotypes and Poly-Unsaturated Fatty Acids Composition in Human Milk: A Prospective Cohort Study. Cells. 2022; 11(24):4110. https://doi.org/10.3390/cells11244110
Chicago/Turabian StyleBadewy, Rana, Amir Azarpazhooh, Howard Tenenbaum, Kristin L. Connor, Jim Yuan Lai, Michael Sgro, Richard P. Bazinet, Noah Fine, Erin Watson, Chunxiang Sun, and et al. 2022. "The Association between Maternal Oral Inflammation and Neutrophil Phenotypes and Poly-Unsaturated Fatty Acids Composition in Human Milk: A Prospective Cohort Study" Cells 11, no. 24: 4110. https://doi.org/10.3390/cells11244110





