Human iPSC-Derived Renal Cells Change Their Immunogenic Properties during Maturation: Implications for Regenerative Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Human Cells Lines
2.3. Differentiation of hiPSCs into Renal Cell Types
2.4. Human Primary Cells
2.5. Immunofluorescence Staining of Adherent Cells
2.6. Transcriptome Analysis
2.7. Functional Uptake Assays
2.8. Fluorescence Labeling of PBMCs
2.9. Flow Cytometry
2.10. Immune Cell Proliferation Assay
2.11. Cytokine Detection
2.12. Statistical Analysis
3. Results
3.1. Generation and Maintenance of hiPSC-Derived Renal Cells
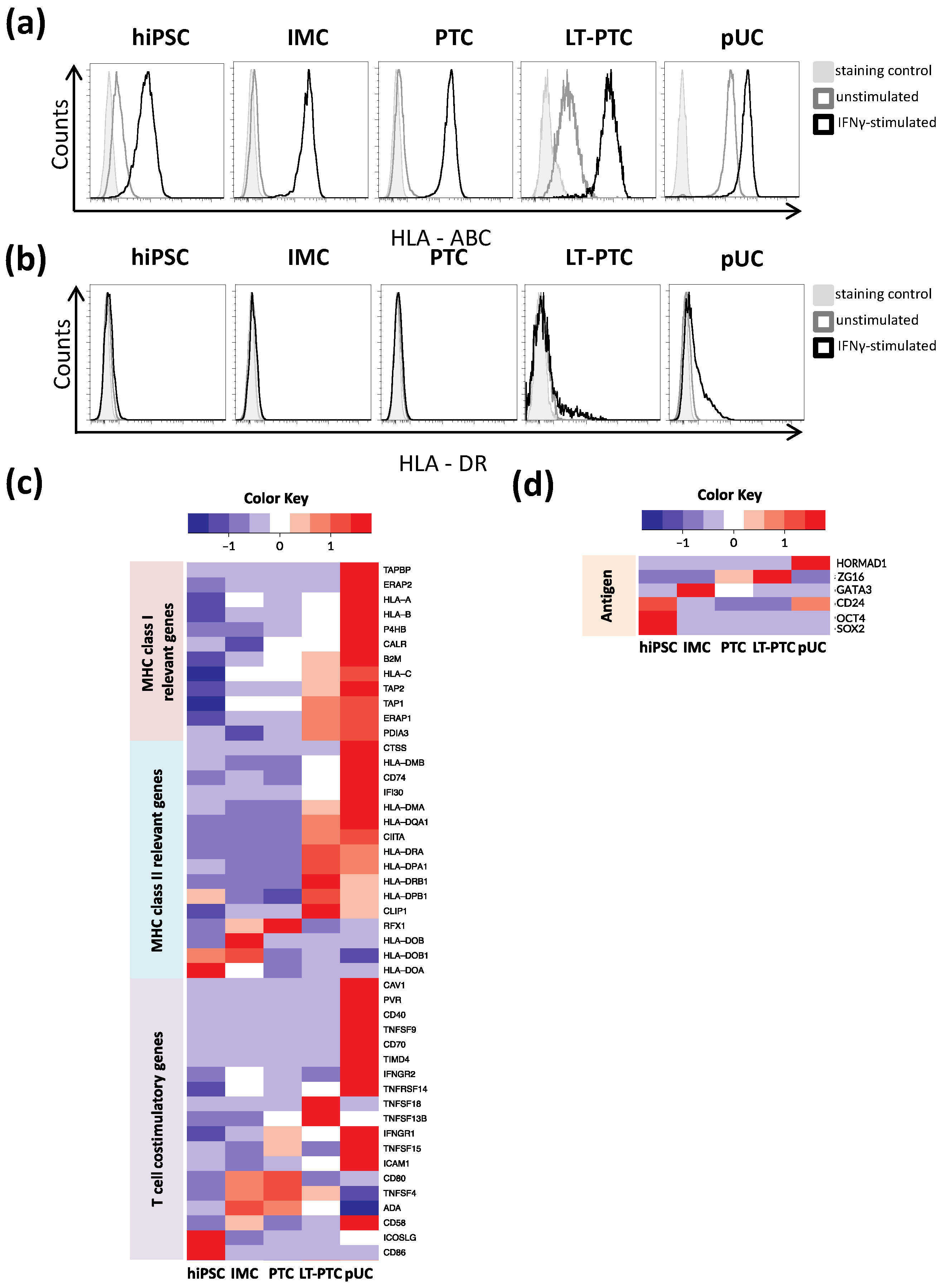
3.2. Immune Phenotype of hiPSCs and hiPSC-Derived Renal Cells
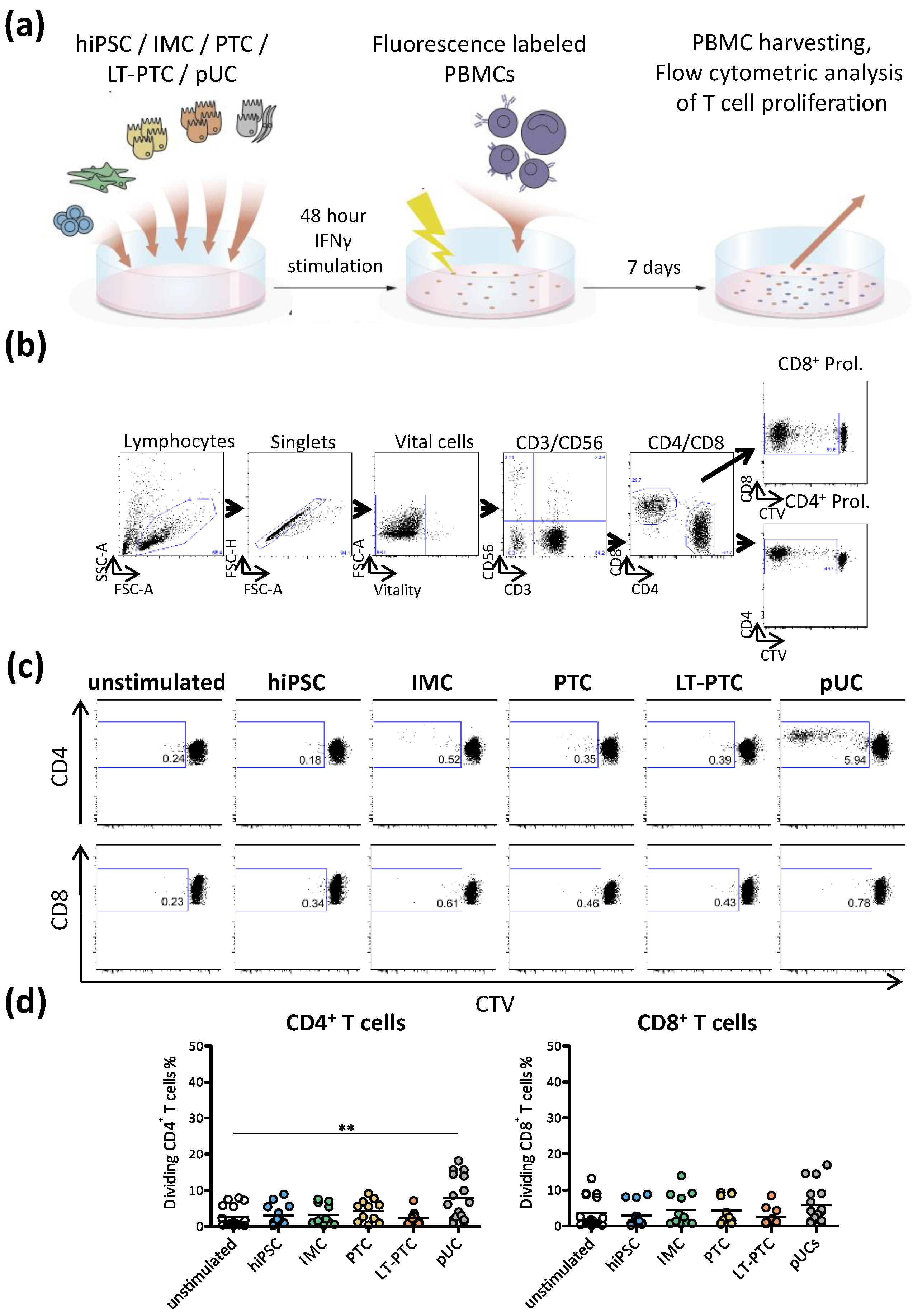
3.3. Autologous T Cell Response against hiPSC-Derived Renal Cells
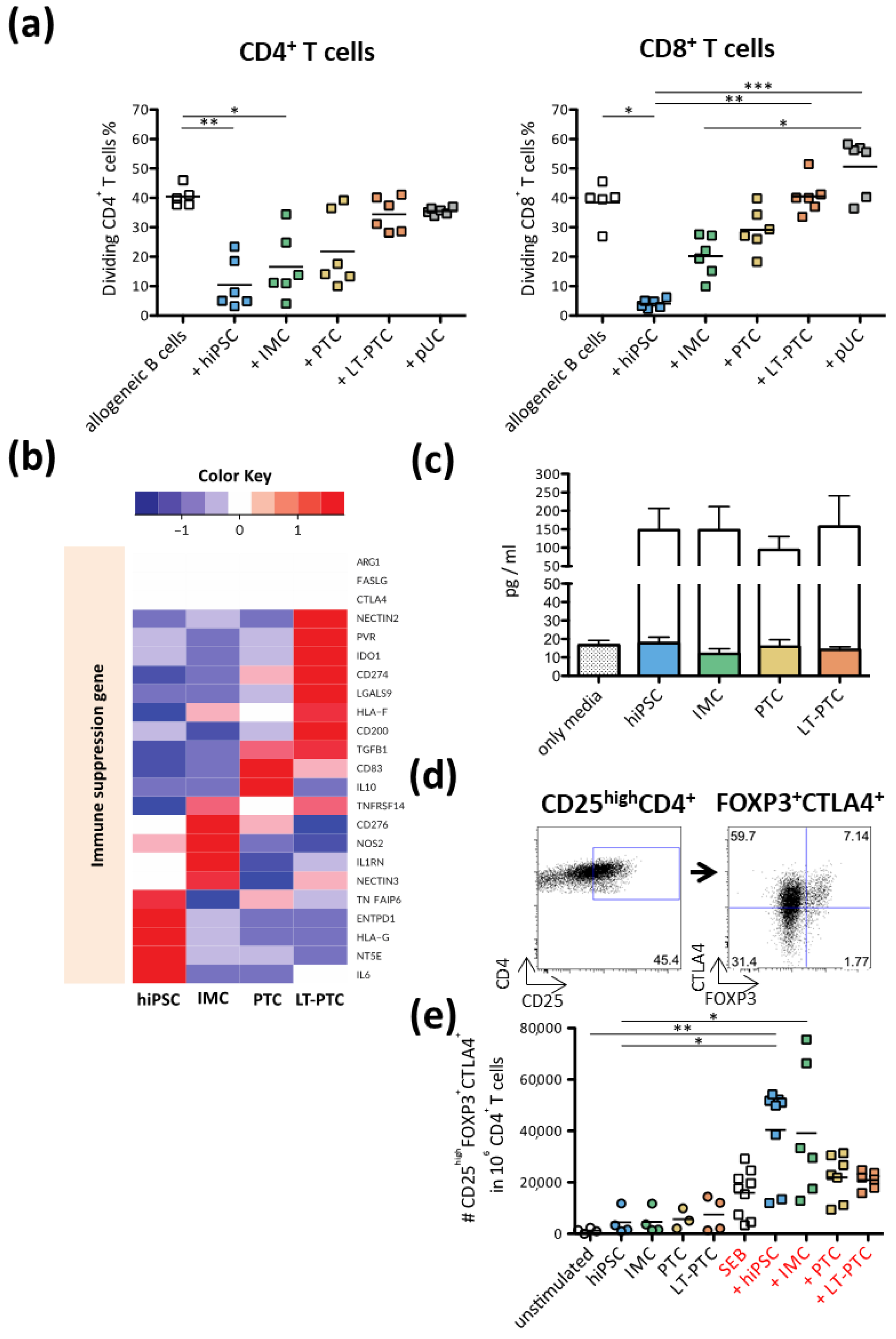
3.4. Allogeneic T Cell Response against hiPSC-Derived Renal Cells
3.5. Immunomodulatory Properties of hiPSCs, IMCs, PTCs and LT-PTCs
3.6. Autologous and Allogeneic NK Cell Responses to hiPSCs and hiPSC-Derived Renal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Mora, C.; Serzanti, M.; Consiglio, A.; Memo, M.; Dell’Era, P. Clinical potentials of human pluripotent stem cells. Cell Biol. Toxicol. 2017, 33, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Y.; Zeng, F. Integration-free methods for generating induced pluripotent stem cells. Genom. Proteom. Bioinform. 2013, 11, 284–287. [Google Scholar] [CrossRef] [Green Version]
- Totonchi, M.; Taei, A.; Seifinejad, A.; Tabebordbar, M.; Rassouli, H.; Farrokhi, A.; Gourabi, H.; Aghdami, N.; Hosseini-Salekdeh, G.; Baharvand, H. Feeder- and serum-free establishment and expansion of human induced pluripotent stem cells. Int. J. Dev. Biol. 2010, 54, 877–886. [Google Scholar] [CrossRef]
- De Almeida, P.E.; Meyer, E.H.; Kooreman, N.G.; Diecke, S.; Dey, D.; Sanchez-Freire, V.; Hu, S.; Ebert, A.; Odegaard, J.; Mordwinkin, N.M.; et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat. Commun. 2014, 5, 3903. [Google Scholar] [CrossRef]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef]
- Guha, P.; Morgan, J.W.; Mostoslavsky, G.; Rodrigues, N.P.; Boyd, A.S. Lack of Immune Response to Differentiated Cells Derived from Syngeneic Induced Pluripotent Stem Cells. Cell Stem Cell 2013, 12, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Zhang, Z.N.; Westenskow, P.D.; Todorova, D.; Hu, Z.; Lin, T.; Rong, Z.; Kim, J.; He, J.; Wang, M.; et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Yan, Y.T.; Ye, L.; Xie, F.; Beyer, A.I.; Muench, M.O.; Wang, J.; Chen, Z.; Liu, H.; Chen, S.J. Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc. Natl. Acad. Sci. USA 2018, 115, 2180–2185. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef]
- Taylor, C.J.; Peacock, S.; Chaudhry, A.N.; Bradley, J.A.; Bolton, E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 2012, 11, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Lam, A.Q.; Freedman, B.S.; Morizane, R.; Lerou, P.H.; Valerius, M.T.; Bonventre, J.V. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 2014, 25, 1211–1225. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, K.; Stachelscheid, H.; Rossbach, B.; Oh, S.J.; Mah, N.; Schmidt-Ott, K.; Kurtz, A.; Reinke, P. Parallel generation of easily selectable multiple nephronal cell types from human pluripotent stem cells. Cell Mol. Life Sci. 2019, 76, 179–192. [Google Scholar] [CrossRef]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.L.; Jardine, M.; et al. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef]
- Lee, P.Y.; Chien, Y.; Chiou, G.Y.; Lin, C.H.; Chiou, C.H.; Tarng, D.C. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012, 21, 2569–2585. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Tian, S.F.; Guo, Y.; Niu, X.; Hu, B.; Guo, S.C.; Wang, N.S.; Wang, Y. Transplantation of induced pluripotent stem cell-derived renal stem cells improved acute kidney injury. Cell Biosci. 2015, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Rossbach, B.; Hildebrand, L.; El-Ahmad, L.; Stachelscheid, H.; Reinke, P.; Kurtz, A. Generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using an integration free vector. Stem Cell Res. 2016, 16, 314–317. [Google Scholar] [CrossRef]
- Rossbach, B.; Hildebrand, L.; El-Ahmad, L.; Stachelscheid, H.; Reinke, P.; Kurtz, A. Generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using integration free Sendai virus technology. Stem Cell Res. 2017, 21, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, M.; Zentz, C.; Mayr, C.; Wimmer, R.; Hammerschmidt, W.; Zeidler, R.; Moosmann, A. Conditional immortalization of human B cells by CD40 ligation. PLoS ONE 2008, 3, e1461. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of induced pluripotent stem cells from urine samples. J. Am. Soc. Nephrol. 2011, 22, 1221–1228. [Google Scholar] [CrossRef]
- Drukker, M.; Katz, G.; Urbach, A.; Schuldiner, M.; Markel, G.; Itskovitz-Eldor, J.; Reubinoff, B.; Mandelboim, O.; Benvenisty, N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9864–9869. [Google Scholar] [CrossRef] [Green Version]
- Dörrenhaus, A.; Müller, J.I.; Golka, K.; Jedrusik, P.; Schulze, H.; Föllmann, W. Cultures of exfoliated epithelial cells from different locations of the human urinary tract and the renal tubular system. Arch. Toxicol. 2000, 74, 618–626. [Google Scholar] [CrossRef]
- Seliger, B.; Hohne, A.; Knuth, A.; Bernhard, H.; Meyer, T.; Tampe, R.; Momburg, F.; Huber, C. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: Evidence for deficiencies associated with transformation and progression. Cancer Res. 1996, 56, 1756–1760. [Google Scholar]
- Dhodapkar, K.M.; Gettinger, S.N.; Das, R.; Zebroski, H.; Dhodapkar, M.V. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology 2013, 2, e25205. [Google Scholar] [CrossRef] [Green Version]
- Dhodapkar, K.M.; Feldman, D.; Matthews, P.; Radfar, S.; Pickering, R.; Turkula, S.; Zebroski, H.; Dhodapkar, M.V. Natural immunity to pluripotency antigen OCT4 in humans. Proc. Natl. Acad. Sci. USA 2010, 107, 8718–8723. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.E.; Yu, C.W.; Chang, K.W.; Chou, W.H.; Lu, C.Y.; Ghelfi, E.; Wu, F.C.; Jan, P.S.; Huang, M.C.; Allard, P.; et al. Comparative global immune-related gene profiling of somatic cells, human pluripotent stem cells and their derivatives: Implication for human lymphocyte proliferation. Exp. Mol. Med. 2017, 49, e376. [Google Scholar] [CrossRef] [Green Version]
- Labastie, M.C.; Catala, M.; Gregoire, J.; Peault, B. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int. 1995, 47, 1597–1603. [Google Scholar] [CrossRef] [Green Version]
- Burrack, A.L.; Landry, L.G.; Siebert, J.; Coulombe, M.; Gill, R.G.; Nakayama, M. Simultaneous Recognition of Allogeneic MHC and Cognate Autoantigen by Autoreactive T Cells in Transplant Rejection. J. Immunol. 2018, 200, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Yachimovich-Cohen, N.; Even-Ram, S.; Shufaro, Y.; Rachmilewitz, J.; Reubinoff, B. Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. J. Immunol. 2010, 184, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Fändrich, F.; Lin, X.; Chai, G.X.; Schulze, M.; Ganten, D.; Bader, M.; Holle, J.; Huang, D.; Parwaresch, R.; Zavazava, N.; et al. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat. Med. 2002, 8, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Li, M.O. TGF-β: Guardian of T cell function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, N.; Yopp, A.C.; Chen, D.; Mao, M.; Chen, D.; Zhang, H.; Ding, Y.; Bromberg, J.S. TGF-beta induces Foxp3+ T-regulatory cells from CD4+ CD25—precursors. Am. J. Transplant. 2004, 4, 1614–1627. [Google Scholar] [CrossRef]
- Kruse, V.; Hamann, C.; Monecke, S.; Cyganek, L.; Elsner, L.; Hübscher, D.; Walter, L.; Streckfuss-Bömeke, K.; Guan, K.; Dressel, R. Human Induced Pluripotent Stem Cells Are Targets for Allogeneic and Autologous Natural Killer (NK) Cells and Killing Is Partly Mediated by the Activating NK Receptor DNAM-1. PLoS ONE 2015, 10, e0125544. [Google Scholar] [CrossRef]
- Dressel, R.; Nolte, J.; Elsner, L.; Novota, P.; Guan, K.; Streckfuss-Bömeke, K.; Hasenfuss, G.; Jaenisch, R.; Engel, W. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J. 2010, 24, 2164–2177. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Barber, D.F.; Ljunggren, H.; O Long, E. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2005, 202, 1001–1012. [Google Scholar] [CrossRef]
- Ma, M.; Ding, S.; Lundqvist, A.; San, H.; Fang, F.; Konoplyannikov, M.; Berry, C.; Beltran, L.E.; Chen, G.; Kovacic, J.C.; et al. Major histocompatibility complex-I expression on embryonic stem cell-derived vascular progenitor cells is critical for syngeneic transplant survival. Stem Cells 2010, 28, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, S.; Gore, A.; Li, Z.; Panopoulos, A.D.; Montserrat, N.; Fung, H.; Giorgetti, A.; Bilic, J.; Batchelder, E.M.; Zaehres, H.; et al. Analysis of protein-coding mutations in hiPSCs and their possible role during somatic cell reprogramming. Nat. Commun. 2013, 4, 1382. [Google Scholar] [CrossRef] [Green Version]
- Martins-Taylor, K.; Nisler, B.S.; Taapken, S.M.; Compton, T.; Crandall, L.; Montgomery, K.D.; Lalande, M.; Xu, R. Recurrent copy number variations in human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 488–491. [Google Scholar] [CrossRef]
- Espinosa, J.R.; Samy, K.P.; Kirk, A.D. Memory T cells in organ transplantation: Progress and challenges. Nat. Rev. Nephrol. 2016, 12, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Valujskikh, A.; Li, X.C. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J. Am. Soc. Nephrol. 2007, 18, 2252–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljung, K.; Simonson, O.E.; Felldin, U.; Wärdell, E.; Ibarra, C.; Antonsson, L.; Kumagai-Braesch, M.; Hovatta, O.; Lampela, R.; Grinnemo, K.; et al. Costimulation blockade induces foxp3(+) regulatory T cells to human embryonic stem cells. Biores. Open Access 2013, 2, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Lui, K.O.; Boyd, A.S.; Cobbold, S.P.; Waldmann, H.; Fairchild, P.J. A role for regulatory T cells in acceptance of ESC-derived tissues transplanted across an major histocompatibility complex barrier. Stem Cells 2010, 28, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Brook, F.A.; Gardner, R.L.; Cobbold, S.P.; Waldmann, H.; Fairchild, P.J. Embryonic stem cell- derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 20920–20925. [Google Scholar] [CrossRef] [Green Version]
- Sugita, S.; Kamao, H.; Iwasaki, Y.; Okamoto, S.; Hashiguchi, T.; Iseki, K.; Hayashi, N.; Mandai, M.; Takahashi, M. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1051–1062. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 15, 1875–1886. [Google Scholar] [CrossRef]
- Chen, Q.; Kim, Y.C.; Laurence, A.; Punkosdy, G.A.; Shevach, E.M. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J. Immunol. 2011, 186, 6329–6337. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.G.; Wang, J.; Wang, J.; Wang, P.; Gray, J.D.; Horwitz, D.A. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007, 178, 2018–2027. [Google Scholar] [CrossRef] [Green Version]
- Wahl, P.; Schoop, R.; Bilic, G.; Neuweiler, J.; Hir, M.L.; Yoshinaga, S.K.; Wüthrich, R.P. Renal tubular epithelial expression of the costimulatory molecule B7RP-1 (inducible costimulator ligand). J. Am. Soc. Nephrol. 2002, 13, 1517–1526. [Google Scholar] [CrossRef]
- Steimle, V.; Siegrist, C.A.; Mottet, A.; Lisowska-Grospierre, B.; Mach, B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 1994, 265, 106–109. [Google Scholar] [CrossRef]
- Früh, K.; Yang, Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr. Opin. Immunol. 1999, 11, 76–81. [Google Scholar] [CrossRef]
- Martín-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Yamada, Y.; Aoyama, A.; Madsen, J.C. Natural killer cells in rejection and tolerance of solid organ allografts. Curr. Opin. Organ Transplant. 2011, 16, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strowig, T.; Chijioke, O.; Carrega, P.; Arrey, F.; Meixlsperger, S.; Rämer, P.C.; Ferlazzo, G.; Münz, C. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood 2010, 116, 4158–4167. [Google Scholar] [CrossRef] [Green Version]
- Andre, M.C.; Erbacher, A.; Gille, C.; Schmauke, V.; Goecke, B.; Hohberger, A.; Mang, P.; Wilhelm, A.; Mueller, I.; Herr, W.; et al. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J. Immunol. 2010, 185, 2710–2720. [Google Scholar] [CrossRef] [Green Version]
- Kooreman, N.G.; de Almeida, P.E.; Stack, J.P.; Nelakanti, R.V.; Diecke, S.; Shao, N.; Swijnenburg, R.; Sanchez-Freire, V.; Matsa, E.; Liu, C.; et al. Alloimmune Responses of Humanized Mice to Human Pluripotent Stem Cell Therapeutics. Cell Rep. 2017, 20, 1978–1990. [Google Scholar] [CrossRef] [Green Version]
- Bain, B.; Vas, M.R.; Lowenstein, L. The development of large immature mononuclear cells in mixed leukocyte cultures. Blood 1964, 23, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Hirschhorn, K.; Bach, F.; Kolodny, R.L.; Firschein, R.L.; Hashem, N. Immune response and mitosis of human peripheral blood lymphocytes in vitro. Science 1964, 143, 1185–1187. [Google Scholar] [CrossRef]
- Najafian, N.; Salama, A.D.; Fedoseyeva, E.V.; Benichou, G.; Sayegh, M.H. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: Potential novel assay for prediction of outcomes for renal transplant recipients. J. Am. Soc. Nephrol. 2002, 13, 252–259. [Google Scholar] [CrossRef]
- De Sousa, P.A.; Steeg, R.; Wachter, E.; Bruce, K.; King, J.; Hoeve, M.; Khadun, S.; McConnachie, G.; Holder, J.; Kurtz, A.; et al. Rapid establishment of the European Bank for induced Pluripotent Stem Cells (EBiSC)—The Hot Startexperience. Stem Cell Res. 2017, 20, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Miyagawa, S.; Fukushima, S.; Maeda, A.; Kashiyama, N.; Kawamura, A.; Miki, K.; Okita, K.; Yoshida, Y.; Shiina, T.; et al. Cardiomyocytes Derived from MHC-Homozygous Induced Pluripotent Stem Cells Exhibit Reduced Allogeneic Immunogenicity in MHC-Matched Non- human Primates. Stem Cell Rep. 2016, 6, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.G.; Xu, Y.; et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Hou, J.; Fujino, M.; Zhang, Q.; Ichimaru, N.; Takahara, S.; Araki, R.; Lu, L.; Chen, J.M.; Zhuang, J.; et al. iPSC-Derived Regulatory Dendritic Cells Inhibit Allograft Rejection by Generating Alloantigen-Specific Regulatory T Cells. Stem Cell Rep. 2017, 8, 1174–1189. [Google Scholar] [CrossRef] [Green Version]
- Deuse, T.; Hu, X.; Gravin, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossbach, B.; Hariharan, K.; Mah, N.; Oh, S.-J.; Volk, H.-D.; Reinke, P.; Kurtz, A. Human iPSC-Derived Renal Cells Change Their Immunogenic Properties during Maturation: Implications for Regenerative Therapies. Cells 2022, 11, 1328. https://doi.org/10.3390/cells11081328
Rossbach B, Hariharan K, Mah N, Oh S-J, Volk H-D, Reinke P, Kurtz A. Human iPSC-Derived Renal Cells Change Their Immunogenic Properties during Maturation: Implications for Regenerative Therapies. Cells. 2022; 11(8):1328. https://doi.org/10.3390/cells11081328
Chicago/Turabian StyleRossbach, Bella, Krithika Hariharan, Nancy Mah, Su-Jun Oh, Hans-Dieter Volk, Petra Reinke, and Andreas Kurtz. 2022. "Human iPSC-Derived Renal Cells Change Their Immunogenic Properties during Maturation: Implications for Regenerative Therapies" Cells 11, no. 8: 1328. https://doi.org/10.3390/cells11081328





