NFAT Factors Are Dispensable for the Development but Are Critical for the Maintenance of Foxp3+ Regulatory T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibodies, Cell Sorting and Flow Cytometry
2.3. Neonatal Thymic Organ Culture (NTOC)
2.4. Intracellular Foxp3 and Bcl-2 Staining
2.5. Confocal Microscopy
2.6. Cell Death Analysis
2.7. Statistics
3. Results
3.1. Constitutive Calcineurin/NFAT Activity Does Not Affect the Development of Foxp3+ nTreg Cells in the Thymus
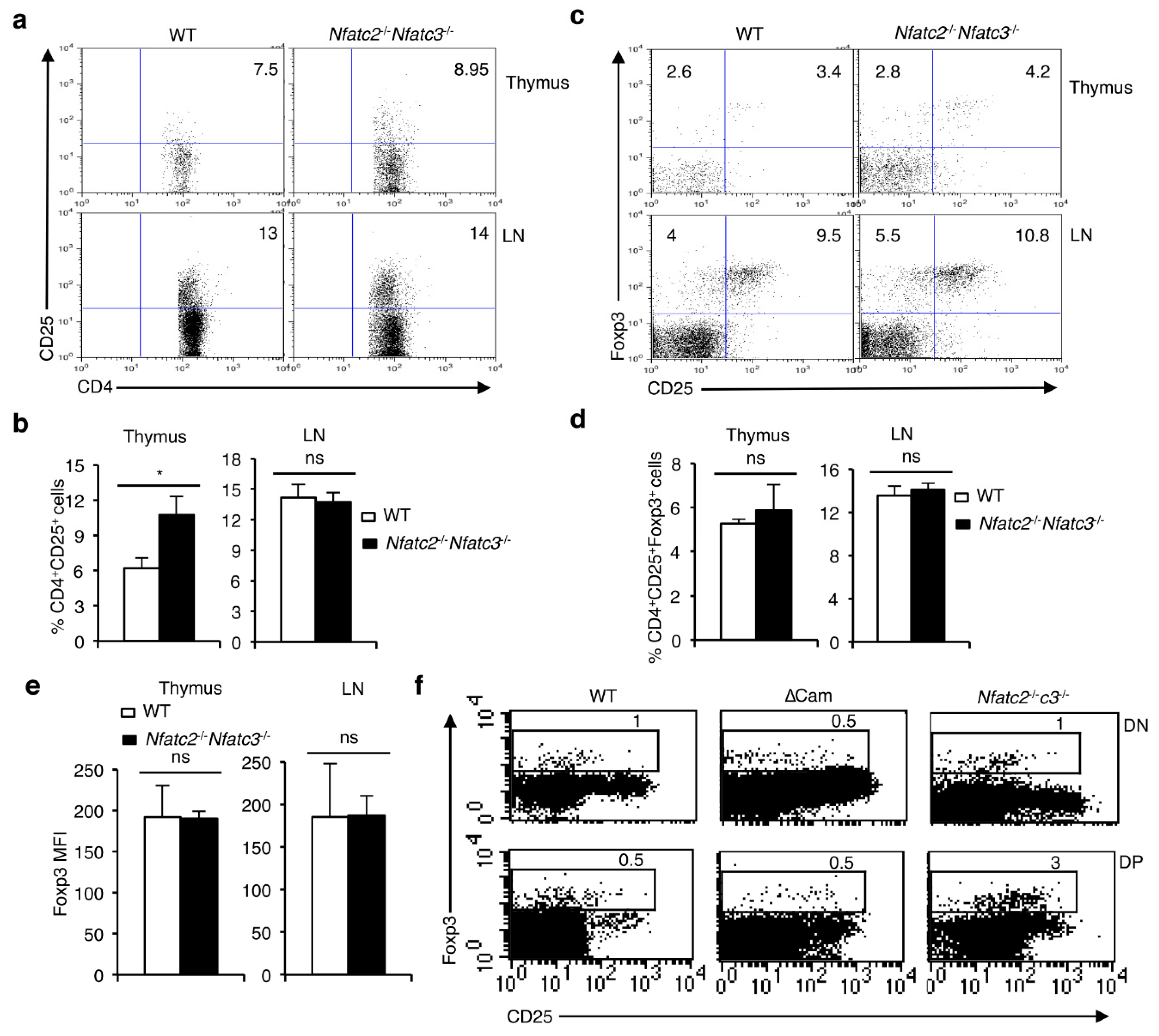
3.2. Deficiency in NFATc2 and NFATc3 Does Not Affect Thymic Development of Foxp3+ nTreg Cells
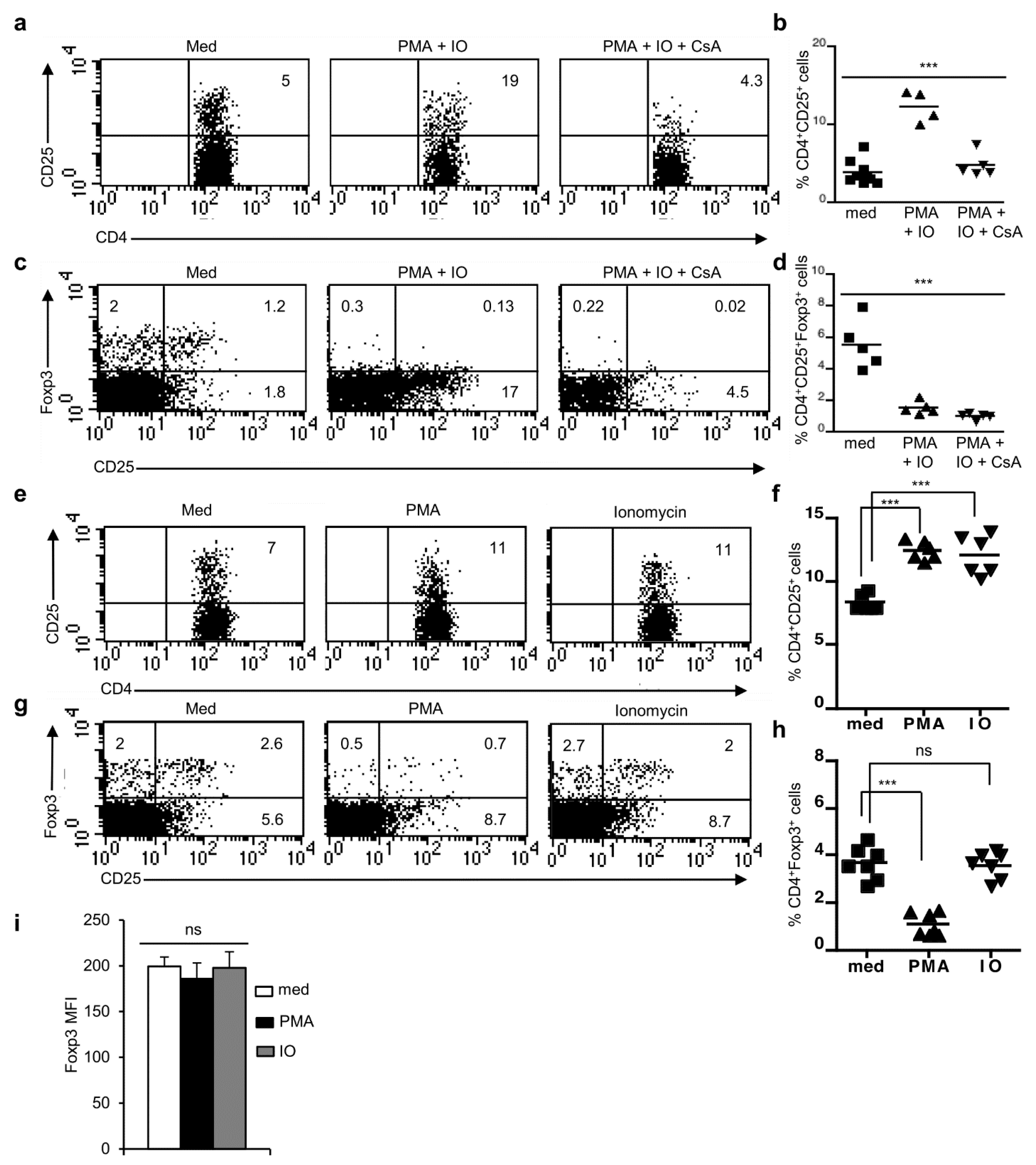
3.3. Cyclosporin A (CsA) Treatment Reduces the Population of Foxp3+ Treg Cells Both in the Thymus and in the Periphery
3.4. PMA Signals Negatively Regulate the Development of Foxp3+ nTreg Cells in the Thymus
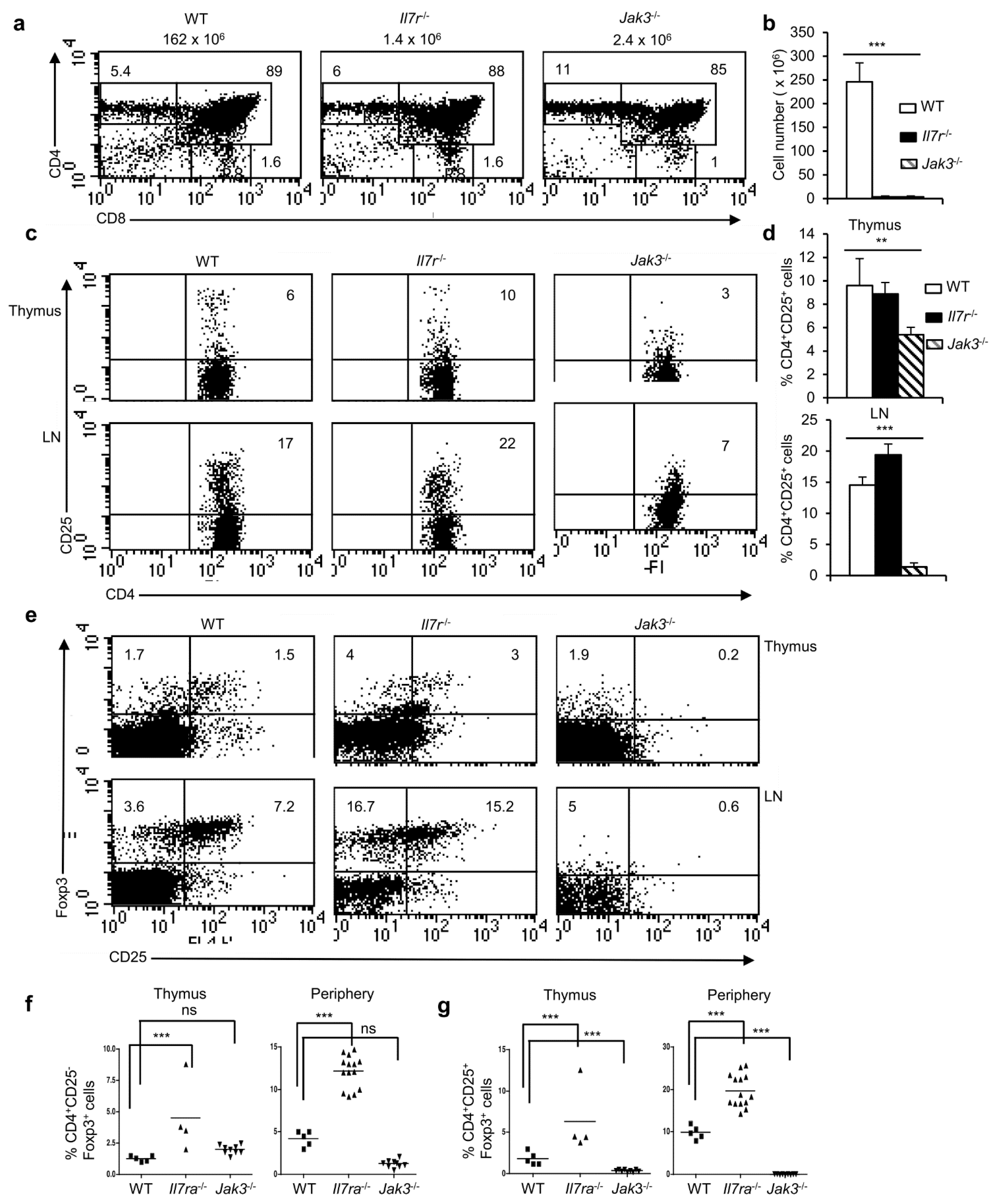
3.5. NFAT Signals Are Not Essential for the Generation of Foxp3+ nTreg Cells
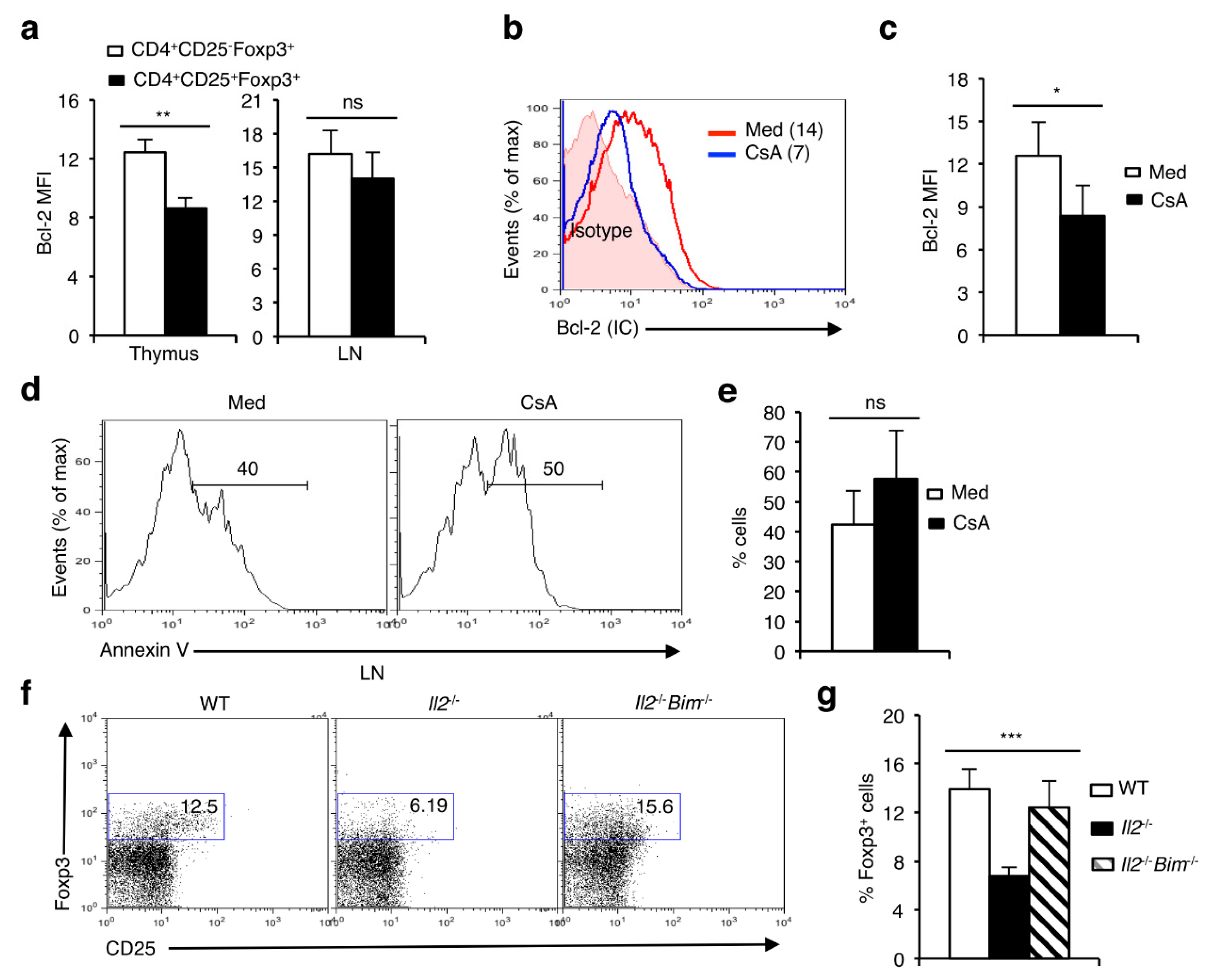
3.6. Lack of NFAT Activity Compromises the Survival of nTreg cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Fantini, M.C.; Becker, C.; Monteleone, G.; Pallone, F.; Galle, P.R.; Neurath, M.F. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004, 172, 5149–5153. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, K.; Apostolou, I.; Hawiger, D.; Khazaie, K.; Nussenzweig, M.C.; Von Boehmer, H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005, 6, 1219–1227. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Sun, C.-M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Takahashi, T.; Sakaguchi, N.; Kuniyasu, Y.; Shimizu, J.; Otsuka, F.; Sakaguchi, S. Thymus and autoimmunity: Production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999, 162, 5317–5326. [Google Scholar]
- Shimizu, J.; Yamazaki, S.; Takahashi, T.; Ishida, Y.; Sakaguchi, S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002, 3, 135–142. [Google Scholar] [CrossRef]
- Hori, S.; Haury, M.; Coutinho, A.; Demengeot, J. Specificity requirements for selection and effector functions of CD25+ 4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8213–8218. [Google Scholar] [CrossRef] [Green Version]
- Bonomo, A.; Kehn, P.J.; Payer, E.; Rizzo, L.; Cheever, A.W.; Shevach, E.M. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J. Immunol. 1995, 154, 6602–6611. [Google Scholar]
- Asano, M.; Toda, M.; Sakaguchi, N.; Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996, 184, 387–396. [Google Scholar] [CrossRef]
- Marie, J.C.; Letterio, J.J.; Gavin, M.; Rudensky, A.Y. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005, 201, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Marie, J.C.; Liggitt, D.; Rudensky, A.Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 2006, 25, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.O.; Sanjabi, S.; Flavell, R.A. Transforming Growth Factor-β Controls Development, Homeostasis, and Tolerance of T Cells by Regulatory T Cell-Dependent and -Independent Mechanisms. Immunity 2006, 25, 455–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Serfling, E.; Klein-Hessling, S.; Palmetshofer, A.; Bopp, T.; Stassen, M.; Schmitt, E. NFAT transcription factors in control of peripheral T cell tolerance. Eur. J. Immunol. 2006, 36, 2837–2843. [Google Scholar] [CrossRef]
- Patra, A.; Avots, A.; Zahedi, R.; Schüler, T.; Sickmann, A.; Bommhardt, U.; Serfling, E. An alternative NFAT-activation pathway mediated by IL-7 is critical for early thymocyte development. Nat. Immunol. 2013, 14, 127–135. [Google Scholar] [CrossRef]
- Klein-Hessling, S.; Rudolf, R.; Muhammad, K.; Knobeloch, K.-P.; Maqbool, M.A.; Cauchy, P.; Andrau, J.-C.; Avots, A.; Talora, C.; Ellenrieder, V.; et al. A threshold level of NFATc1 activity facilitates thymocyte differentiation and opposes notch-driven leukaemia development. Nat. Commun. 2016, 7, 11841. [Google Scholar] [CrossRef]
- Wu, Y.; Borde, M.; Heissmeyer, V.; Feuerer, M.; Lapan, A.D.; Stroud, J.; Bates, D.L.; Guo, L.; Han, A.; Ziegler, S.F.; et al. FOXP3 Controls Regulatory T Cell Function through Cooperation with NFAT. Cell 2006, 126, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Vaeth, M.; Schliesser, U.; Müller, G.; Reissig, S.; Satoh, K.; Tuettenberg, A.; Jonuleit, H.; Waisman, A.; Müller, M.R.; Serfling, E.; et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16258–16263. [Google Scholar] [CrossRef] [Green Version]
- Bopp, T.; Palmetshofer, A.; Serfling, E.; Heib, V.; Schmitt, S.; Richter, C.; Klein, M.; Schild, H.; Schmitt, E.; Stassen, M. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J. Exp. Med. 2005, 201, 181–187. [Google Scholar] [CrossRef] [Green Version]
- de la Pompa, J.L.; Timmerman, L.A.; Takimoto, H.; Yoshida, H.; Elia, A.J.; Samper, E.; Potter, J.; Wakeham, A.; Marengere, L.; Langille, B.L.; et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 1998, 392, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Langenhorst, D.; Beilhack, A.; Serfling, E.; Patra, A.K. Interleukin-2 critically regulates bone marrow erythropoiesis and prevents anemia development. Eur. J. Immunol. 2015, 45, 3362–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, A.K.; Drewes, T.; Engelmann, S.; Chuvpilo, S.; Kishi, H.; Hünig, T.; Serfling, E.; Bommhardt, U.H. PKB Rescues Calcineurin/NFAT-Induced Arrest of Rag Expression and Pre-T Cell Differentiation. J. Immunol. 2006, 177, 4567–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranger, A.M.; Oukka, M.; Rengarajan, J.; Glimcher, L.H. Inhibitory Function of Two NFAT Family Members in Lymphoid Homeostasis and Th2 Development. Immunity 1998, 9, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Lahl, K.; Sparwasser, T. In Vivo Depletion of FoxP3+ Tregs Using the DEREG Mouse Model. Methods Mol. Biol. 2011, 707, 157–172. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Rasmussen, J.; Williams, L.M.; Dooley, J.; Farr, A.G.; Rudensky, A.Y. Regulatory T Cell Lineage Specification by the Forkhead Transcription Factor Foxp3. Immunity 2005, 22, 329–341. [Google Scholar] [CrossRef]
- Schuh, K.; Twardzik, T.; Kneitz, B.; Heyer, J.; Schimpl, A.; Serfling, E. The Interleukin 2 Receptor α Chain/CD25 Promoter Is a Target for Nuclear Factor of Activated T Cells. J. Exp. Med. 1998, 188, 1369–1373. [Google Scholar] [CrossRef] [Green Version]
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151. [Google Scholar] [CrossRef]
- D’Cruz, L.M.; Klein, L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 2005, 6, 1152–1159. [Google Scholar] [CrossRef]
- Cante-Barrett, K.; Gallo, E.M.; Winslow, M.M.; Crabtree, G.R. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of bim [corrected]. J. Immunol. 2006, 176, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Fry, T.J.; Mackall, C.L. The Many Faces of IL-7: From Lymphopoiesis to Peripheral T Cell Maintenance. J. Immunol. 2005, 174, 6571–6576. [Google Scholar] [CrossRef] [PubMed]
- Peschon, J.J.; Morrissey, P.J.; Grabstein, K.H.; Ramsdell, F.J.; Maraskovsky, E.; Gliniak, B.C.; Park, L.S.; Ziegler, S.F.; E Williams, D.; Ware, C.B.; et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994, 180, 1955–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosaka, T.; van Deursen, J.M.A.; Tripp, R.A.; Thierfelder, W.E.; Witthuhn, B.A.; McMickle, A.P.; Doherty, P.C.; Grosveld, G.C.; Ihle, J.N. Defective Lymphoid Development in Mice Lacking Jak3. Science 1995, 270, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Saijo, K.; Takahashi, T.; Osawa, M.; Arase, H.; Hirayama, N.; Miyake, K.; Nakauchi, H.; Shirasawa, T.; Saito, T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 1995, 3, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Thomis, D.C.; Gurniak, C.B.; Tivol, E.; Sharpe, A.H.; Berg, L.J. Defects in B Lymphocyte Maturation and T Lymphocyte Activation in Mice Lacking Jak3. Science 1995, 270, 794–797. [Google Scholar] [CrossRef]
- Peffault de Latour, R.; Dujardin, H.C.; Mishellany, F.; Burlen-Defranoux, O.; Zuber, J.; Marques, R.; Di Santo, J.; Cumano, A.; Vieira, P.; Bandeira, A. Ontogeny, function, and peripheral homeostasis of regulatory T cells in the absence of interleukin-7. Blood 2006, 108, 2300–2306. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; de St Groth, B.F.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.; Luo, C.; Hogan, P.G. Transcription Factors of the NFAT Family: Regulation and Function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef]
- Jain, J.; Loh, C.; Rao, A. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 1995, 7, 333–342. [Google Scholar] [CrossRef]
- Lord, J.D.; McIntosh, B.C.; Greenberg, P.D.; Nelson, B. The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J. Immunol. 1998, 161, 4627–4633. [Google Scholar]
- Miyazaki, T.; Liu, Z.-J.; Kawahara, A.; Minami, Y.; Yamada, K.; Tsujimoto, Y.; Barsoumian, E.L.; Perlmutter, R.M.; Taniguchi, T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell 1995, 81, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Adachi, M.; Torigoe, T.; Takayama, S.; Imai, K. BAG-1 and Bcl-2 in IL-2 Signaling. Leuk. Lymphoma 1998, 30, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Giampaolo, S.; Wojcik, G.; Klein-Hessling, S.; Serfling, E.; Patra, A.K. NFAT-mediated defects in erythropoiesis cause anemia in Il2(−/−) mice. Oncotarget 2018, 9, 9632–9644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chougnet, C.A.; Tripathi, P.; Lages, C.S.; Raynor, J.; Sholl, A.; Fink, P.; Plas, D.R.; Hildeman, D. A Major Role for Bim in Regulatory T Cell Homeostasis. J. Immunol. 2011, 186, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, X.; Erman, B.; Alag, A.; Mu, J.; Kimura, M.; Katz, G.; Guinter, T.; McCaughtry, T.; Etzensperger, R.; Feigenbaum, L.; et al. Foxp3 Transcription Factor Is Proapoptotic and Lethal to Developing Regulatory T Cells unless Counterbalanced by Cytokine Survival Signals. Immunity 2013, 38, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Josefowicz, S.; Kas, A.; Chu, T.-T.; Gavin, M.A.; Rudensky, A.Y. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 2007, 445, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.K.; Schwartz, R.H.; Pardoll, D.M. Effects of cyclosporine A on T cell development and clonal deletion. Science 1988, 241, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.-K.; Lo, D.; Cheney, R.; Kanagawa, O.; Sprent, J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature 1988, 336, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Oukka, M.; Ho, I.-C.; de la Brousse, F.C.; Hoey, T.; Grusby, M.J.; Glimcher, L.H. The Transcription Factor NFAT4 Is Involved in the Generation and Survival of T Cells. Immunity 1998, 9, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Ono, K.; Morimoto, T.; Akao, M.; Iwai-Kanai, E.; Wada, H.; Sowa, N.; Kita, T.; Hasegawa, K. Endothelin-1–Dependent Nuclear Factor of Activated T Lymphocyte Signaling Associates with Transcriptional Coactivator p300 in the Activation of the B Cell Leukemia-2 Promoter in Cardiac Myocytes. Circ. Res. 2004, 94, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Kanno, Y.; Kerenyi, M.; Stephens, G.; Durant, L.; Watford, W.T.; Laurence, A.; Robinson, G.W.; Shevach, E.M.; Moriggl, R.; et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 2007, 109, 4368–4375. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barahona de Brito, C.; Patra, A.K. NFAT Factors Are Dispensable for the Development but Are Critical for the Maintenance of Foxp3+ Regulatory T Cells. Cells 2022, 11, 1397. https://doi.org/10.3390/cells11091397
Barahona de Brito C, Patra AK. NFAT Factors Are Dispensable for the Development but Are Critical for the Maintenance of Foxp3+ Regulatory T Cells. Cells. 2022; 11(9):1397. https://doi.org/10.3390/cells11091397
Chicago/Turabian StyleBarahona de Brito, Carlotta, and Amiya Kumar Patra. 2022. "NFAT Factors Are Dispensable for the Development but Are Critical for the Maintenance of Foxp3+ Regulatory T Cells" Cells 11, no. 9: 1397. https://doi.org/10.3390/cells11091397




