Multinucleated Retinal Pigment Epithelial Cells Adapt to Vision and Exhibit Increased DNA Damage Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Genotyping
2.3. Fundus Photography and Fluorescein Angiography
2.4. Mouse Retina Protein Extraction and Western Blot Analysis
2.5. Histology, Immunohistochemistry and Immunofluorescence
2.6. Animals’ RPE Flat Mount Immunofluorescence
2.7. Comet Assay
2.8. Primary Cell Culture
2.9. Cell Proliferation Assay
2.10. Measurement of Intracellular ROS Levels
2.11. Mitochondrial Membrane Potential
2.12. Statistical Analysis
3. Results
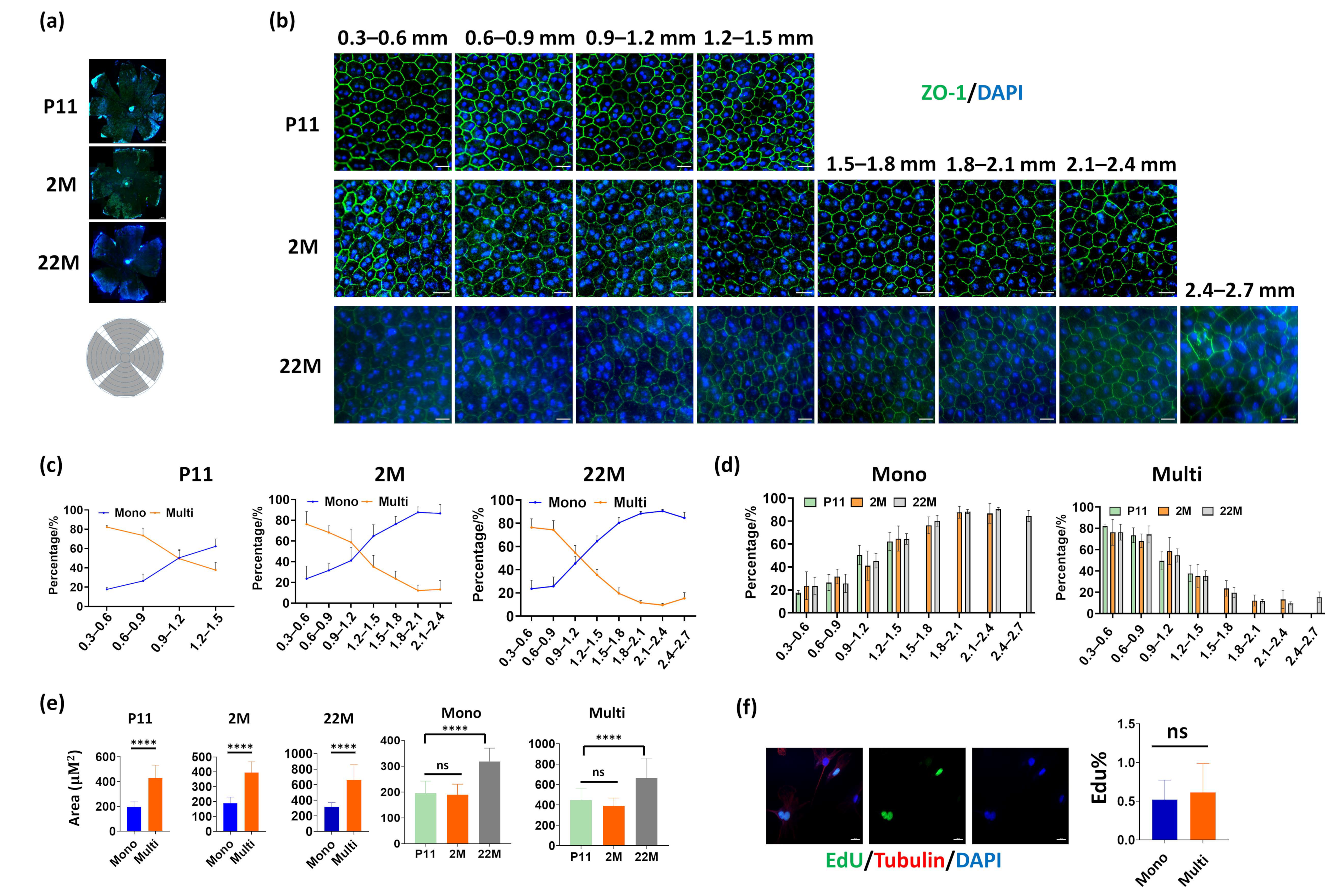
3.1. Distribution of Mononucleated and Multinucleated Cells in Mouse RPE
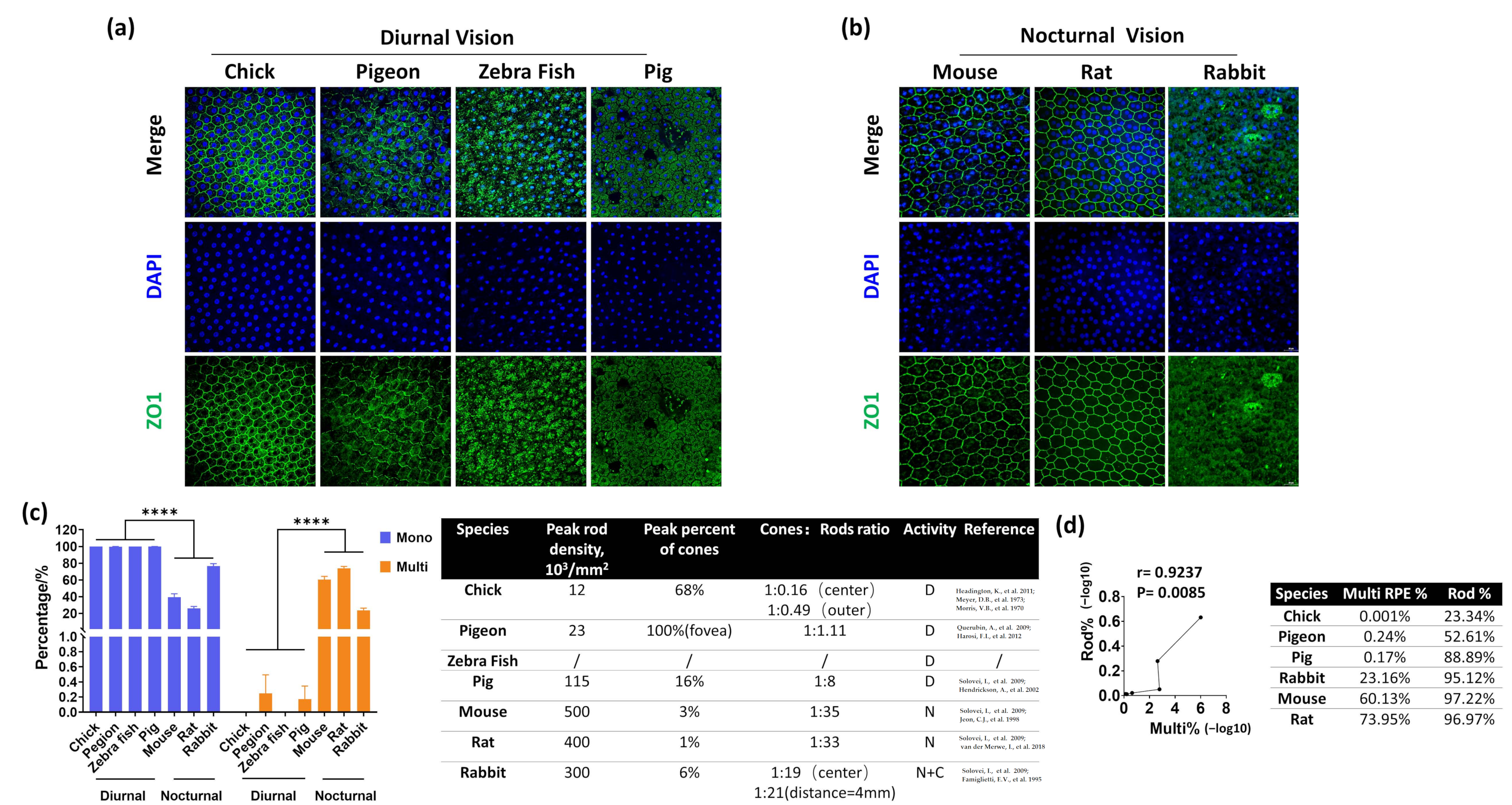
3.2. A High Percentage of Multinucleated RPE Correlates with Nocturnal Vision
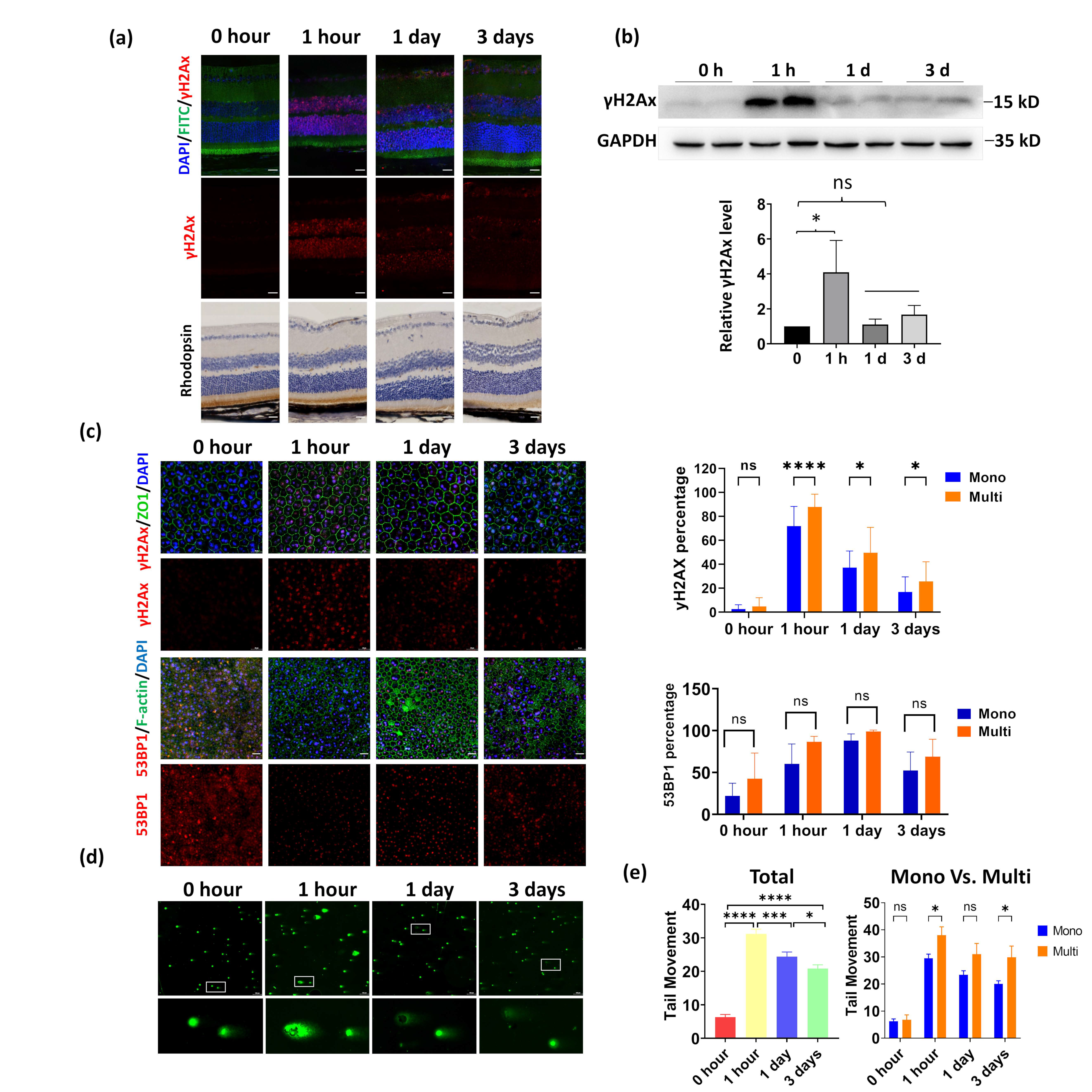
3.3. Multinucleated RPE Exhibits Increased DNA Damage Compared to Mononucleated RPE
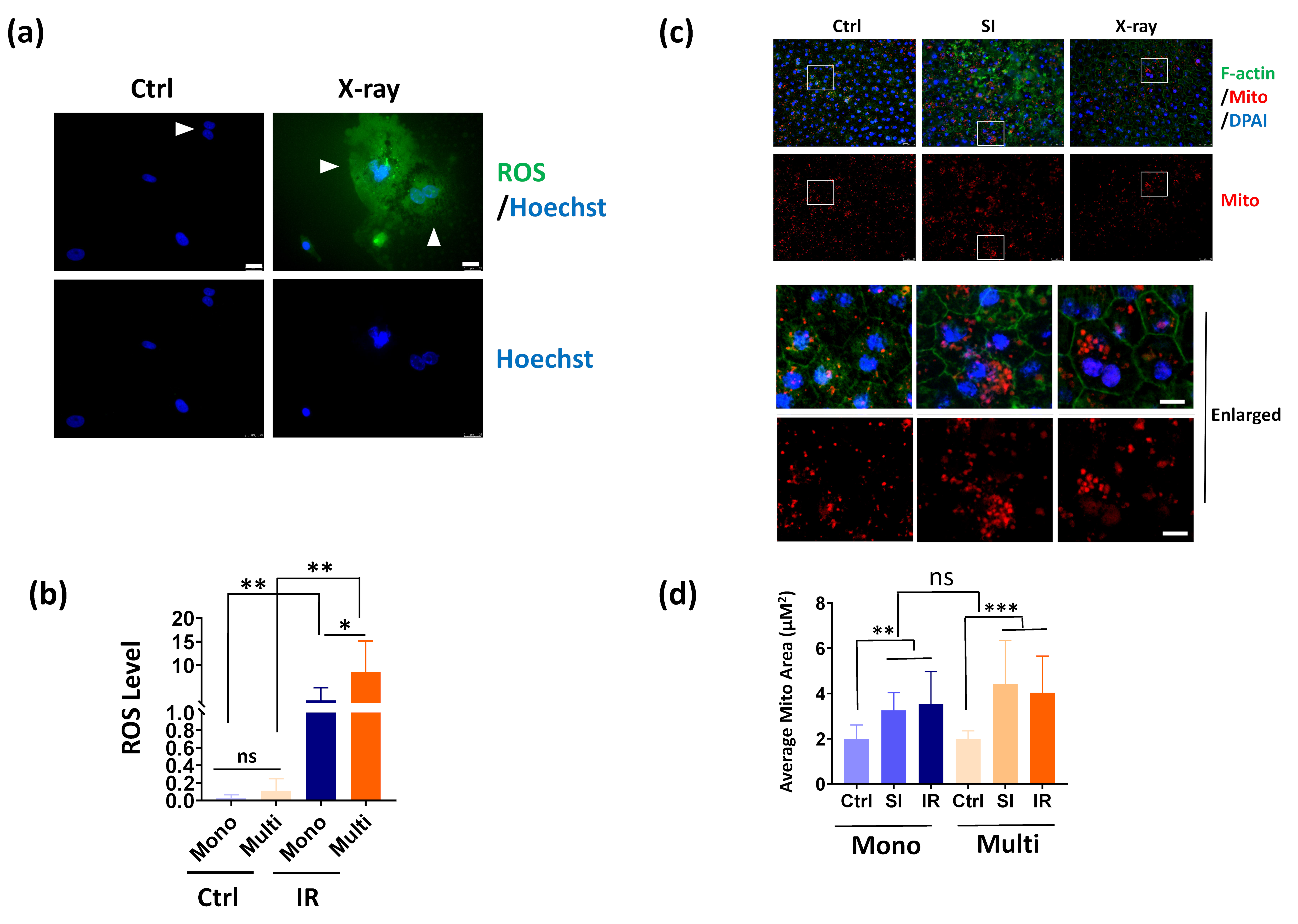
3.4. Multinucleated RPE Cells Show Increased ROS Production after IR Exposure
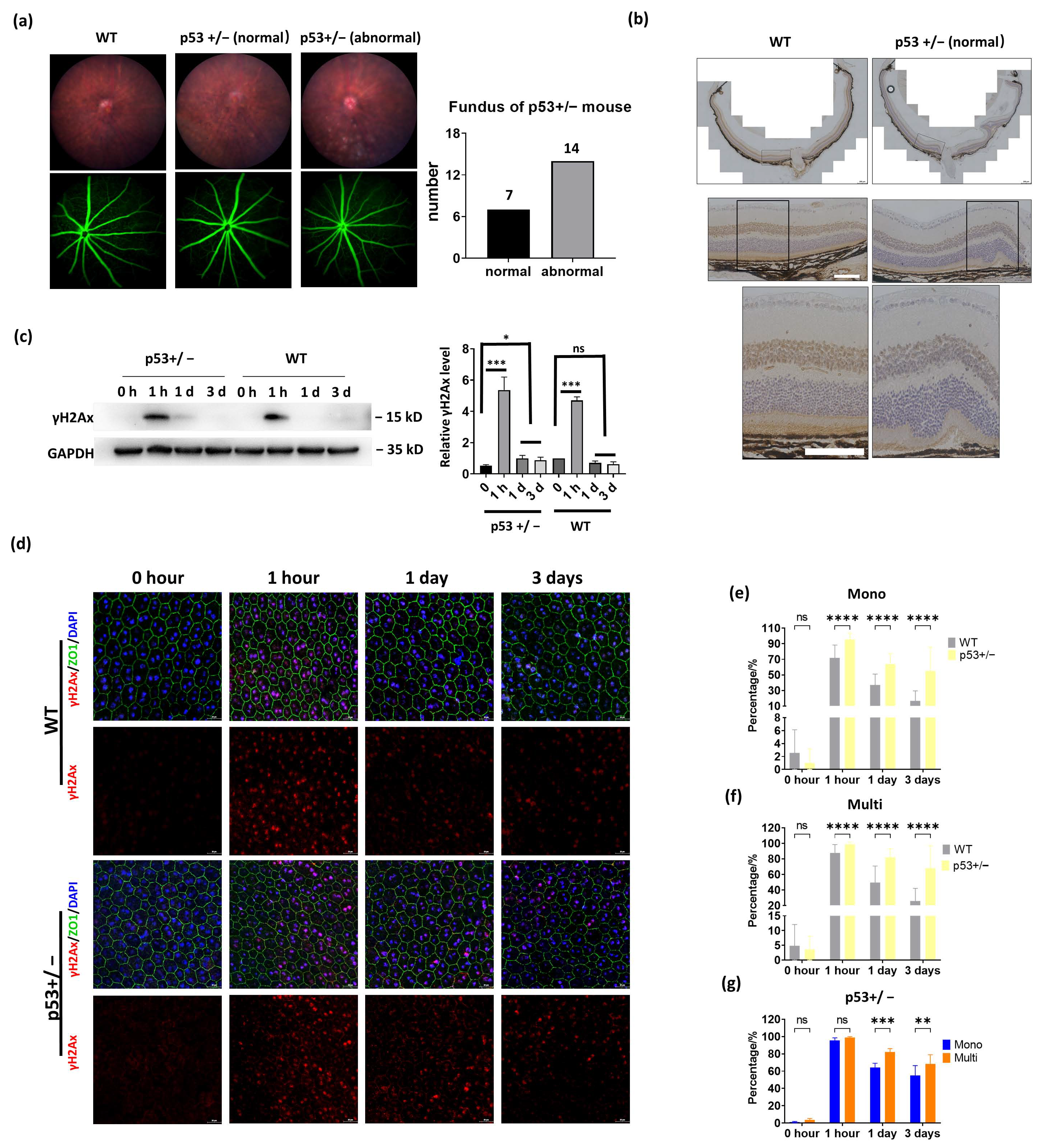
3.5. p53 Haploinsufficiency Leads to Increased DNA Damage in the RPE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strauss, O. The role of retinal pigment epithelium in visual functions. Ophthalmologe 2009, 106, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular Endothelial Growth Factor Expression in the Retinal Pigment Epithelium Is Essential for Choriocapillaris Development and Visual Function. Am. J. Pathol. 2005, 167, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, G.M.; Kijlstra, A.; Peek, R.; de Vos, A.F. Retinal pigment epithelium-immune system interactions: Cytokine production and cytokine-induced changes. Prog. Retin. Eye Res. 2001, 20, 29–48. [Google Scholar] [CrossRef]
- Fuhrmann, S.; Zou, C.J.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Bose, D.; Maminishkis, A.; Bharti, K. Retinal Pigment Epithelium Replacement Therapy for Age-Related Macular Degeneration: Are We There Yet? Annu. Rev. Pharmacol. Toxicol. 2020, 60, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Orr-Weaver, T.L. When bigger is better: The role of polyploidy in organogenesis. Trends Genet. 2015, 31, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfelder, K.P.; Fox, D.T. The expanding implications of polyploidy. J. Cell Biol. 2015, 209, 485–491. [Google Scholar] [CrossRef]
- Ts’o, M.O.; Friedman, E. The retinal pigment epithelium. I. Comparative histology. Arch. Ophthalmol. 1967, 78, 641–649. [Google Scholar] [CrossRef]
- Starnes, A.C.; Huisingh, C.; McGwin, G., Jr.; Sloan, K.R.; Ablonczy, Z.; Smith, R.T.; Curcio, C.A.; Ach, T. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis. Neurosci. 2016, 33, e001. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Rajapakse, D.; Fraczek, M.; Luo, C.; Forrester, J.V.; Xu, H. Retinal pigment epithelial cell multinucleation in the aging eye—A mechanism to repair damage and maintain homoeostasis. Aging Cell 2016, 15, 436–445. [Google Scholar] [CrossRef]
- Lin, H.; Xu, H.; Liang, F.Q.; Liang, H.; Gupta, P.; Havey, A.N.; Boulton, M.E.; Godley, B.F. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.; Blasiak, J.; Niittykoski, M.; Kinnunen, K.; Kauppinen, A.; Salminen, A.; Kaarniranta, K. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells—Implications for age-related macular degeneration (AMD). Ageing Res. Rev. 2017, 36, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Piechota, M.; Pawlowska, E.; Szatkowska, M.; Sikora, E.; Kaarniranta, K. Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role? Oxidative Med. Cell. Longev. 2017, 2017, 5293258. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gong, L.; Qi, R.; Qing, W.; Zou, M.; Ke, Q.; Zhang, L.; Tang, X.; Nie, Q.; Yang, Y.; et al. Oxidative stress-induced KLF4 activates inflammatory response through IL17RA and its downstream targets in retinal pigment epithelial cells. Free Radic. Biol. Med. 2020, 147, 271–281. [Google Scholar] [CrossRef]
- Volland, S.; Esteve-Rudd, J.; Hoo, J.; Yee, C.; Williams, D.S. A Comparison of Some Organizational Characteristics of the Mouse Central Retina and the Human Macula. PLoS ONE 2015, 10, e0125631. [Google Scholar] [CrossRef]
- Kaskan, P.M.; Franco, E.C.; Yamada, E.S.; Silveira, L.C.; Darlington, R.B.; Finlay, B.L. Peripheral variability and central constancy in mammalian visual system evolution. Proc. R. Soc. B Boil. Sci. 2005, 272, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Headington, K.; Choi, S.S.; Nickla, D.; Doble, N. Single cell imaging of the chick retina with adaptive optics. Curr. Eye Res. 2011, 36, 947–957. [Google Scholar] [CrossRef]
- Meyer, D.B.; May, H.C., Jr. The topographical distribution of rods and cones in the adult chicken retina. Exp. Eye Res. 1973, 17, 347–355. [Google Scholar] [CrossRef]
- Morris, V.B. Symmetry in a receptor mosaic demonstrated in the chick from the frequencies, spacing and arrangement of the types of retinal receptor. J. Comp. Neurol. 1970, 140, 359–398. [Google Scholar] [CrossRef]
- Querubin, A.; Lee, H.R.; Provis, J.M.; O′Brien, K.M. Photoreceptor and ganglion cell topographies correlate with information convergence and high acuity regions in the adult pigeon (Columba livia) retina. J. Comp. Neurol. 2009, 517, 711–722. [Google Scholar] [CrossRef]
- Harosi, F.I.; Novales Flamarique, I. Functional significance of the taper of vertebrate cone photoreceptors. J. Gen. Physiol. 2012, 139, 159–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solovei, I.; Kreysing, M.; Lanctot, C.; Kosem, S.; Peichl, L.; Cremer, T.; Guck, J.; Joffe, B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 2009, 137, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickson, A.; Hicks, D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp. Eye Res. 2002, 74, 435–444. [Google Scholar] [CrossRef]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The major cell populations of the mouse retina. J. Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Merwe, I.; Lukats, A.; Blahova, V.; Oosthuizen, M.K.; Bennett, N.C.; Nemec, P. The topography of rods, cones and intrinsically photosensitive retinal ganglion cells in the retinas of a nocturnal (Micaelamys namaquensis) and a diurnal (Rhabdomys pumilio) rodent. PLoS ONE 2018, 13, e0202106. [Google Scholar] [CrossRef]
- Famiglietti, E.V.; Sharpe, S.J. Regional topography of rod and immunocytochemically characterized "blue" and "green" cone photoreceptors in rabbit retina. Vis. Neurosci. 1995, 12, 1151–1175. [Google Scholar] [CrossRef]
- Frohns, A.; Frohns, F.; Naumann, S.C.; Layer, P.G.; Lobrich, M. Inefficient double-strand break repair in murine rod photoreceptors with inverted heterochromatin organization. Curr. Biol. 2014, 24, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Hawes, N.L.; Chang, B.; Avery, C.S.; Smith, R.S.; Nishina, P.M. Severe ocular abnormalities in C57BL/6 but not in 129/Sv p53-deficient mice. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1874–1878. [Google Scholar]
- Millay, D.P.; O′Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Rios, A.C.; Fu, N.Y.; Jamieson, P.R.; Pal, B.; Whitehead, L.; Nicholas, K.R.; Lindeman, G.J.; Visvader, J.E. Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat. Commun. 2016, 7, 11400. [Google Scholar] [CrossRef]
- Pandit, S.K.; Westendorp, B.; de Bruin, A. Physiological significance of polyploidization in mammalian cells. Trends Cell Biol. 2013, 23, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Daniele, L.L.; Lillo, C.; Lyubarsky, A.L.; Nikonov, S.S.; Philp, N.; Mears, A.J.; Swaroop, A.; Williams, D.S.; Pugh, E.N., Jr. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2156–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, M.; Adams, S.D.; Draviam, V.M. Multinucleation associated DNA damage blocks proliferation in p53-compromised cells. Commun. Biol. 2021, 4, 451. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Q.; Gong, L.; Zhu, X.; Qi, R.; Zou, M.; Chen, B.; Liu, W.; Huang, S.; Liu, Y.; Li, D.W.-C. Multinucleated Retinal Pigment Epithelial Cells Adapt to Vision and Exhibit Increased DNA Damage Response. Cells 2022, 11, 1552. https://doi.org/10.3390/cells11091552
Ke Q, Gong L, Zhu X, Qi R, Zou M, Chen B, Liu W, Huang S, Liu Y, Li DW-C. Multinucleated Retinal Pigment Epithelial Cells Adapt to Vision and Exhibit Increased DNA Damage Response. Cells. 2022; 11(9):1552. https://doi.org/10.3390/cells11091552
Chicago/Turabian StyleKe, Qin, Lili Gong, Xingfei Zhu, Ruili Qi, Ming Zou, Baoxin Chen, Wei Liu, Shan Huang, Yizhi Liu, and David Wan-Cheng Li. 2022. "Multinucleated Retinal Pigment Epithelial Cells Adapt to Vision and Exhibit Increased DNA Damage Response" Cells 11, no. 9: 1552. https://doi.org/10.3390/cells11091552





