An Integrative Study on the Inhibition of Bone Loss via Osteo-F Based on Network Pharmacology, Experimental Verification, and Clinical Trials in Postmenopausal Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Set Construction and Network Analysis
2.2. Preparation of Osteo-F
2.3. Mineralized Matrix Formation Assay
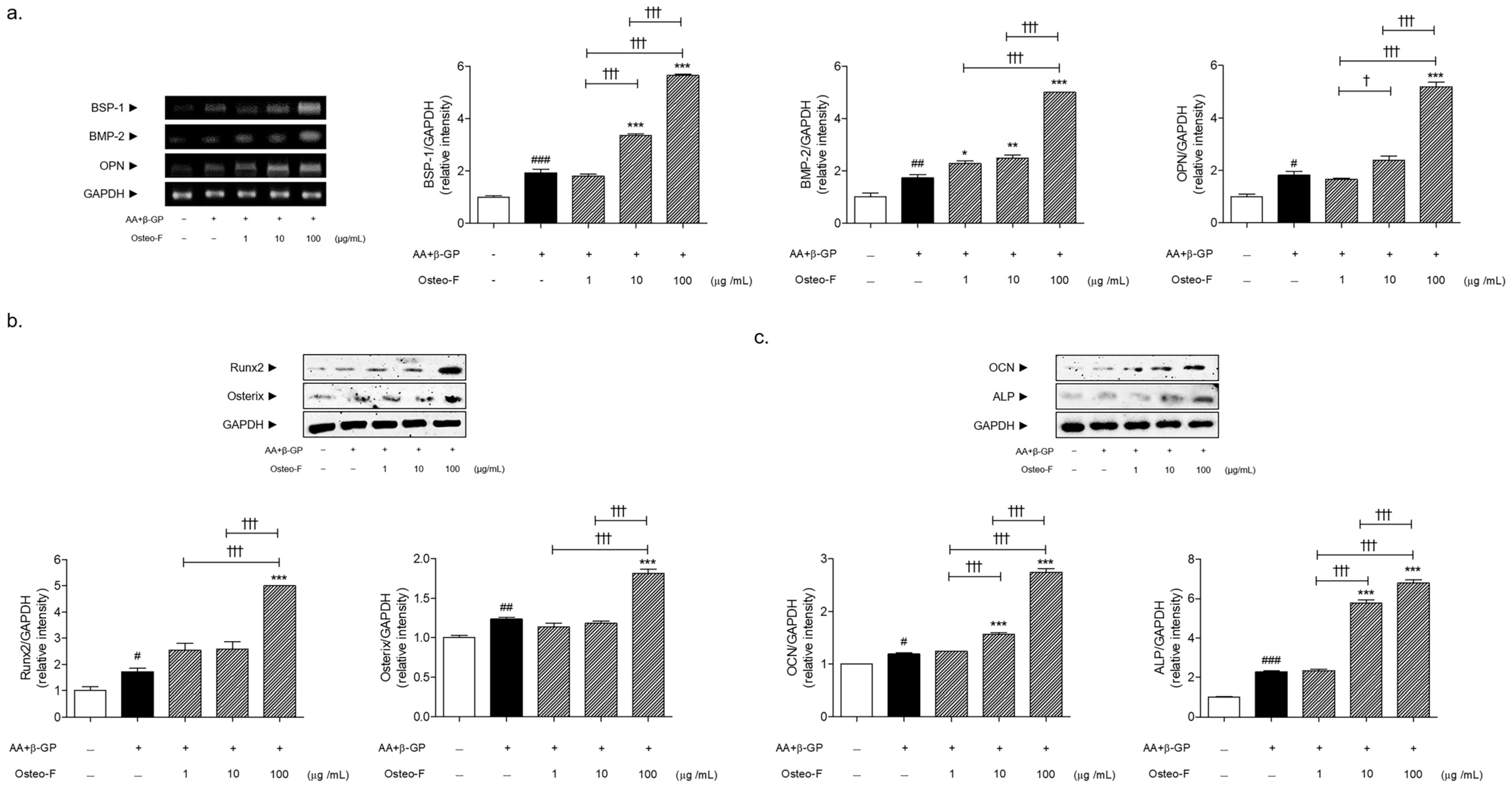
2.4. Bone Formation-Related Markers Content by Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.5. Bone Formation-Related Markers Content by Western Blotting Analysis
2.6. Animal Experiment
2.7. Bone Mineral Density Determined by Dual Energy X-ray Absorptiometry
2.8. Osteocalcin Concentration in Serum
2.9. Osteocalcin+ Expression in Femoral Bone Tissues by Immunofluorescence
2.10. Study Design for Randomized, Double-Blind, Placebo-Controlled Clinical Trial
2.11. Measurement of BMD and Serum OCN, Ca, and PTH Levels in Humans
2.12. Statistical Analysis
3. Results
3.1. Gene Comparison between Osteo-F and Osteoporosis
3.2. Functional Enrichment Analysis of the Osteo-F Network
3.3. Osteogenic Potential of Osteo-F in Mineralized Osteoblasts
3.4. Increase of Bone Formation-Related Markers Expressions by Osteo-F in Mineralized Osteoblasts
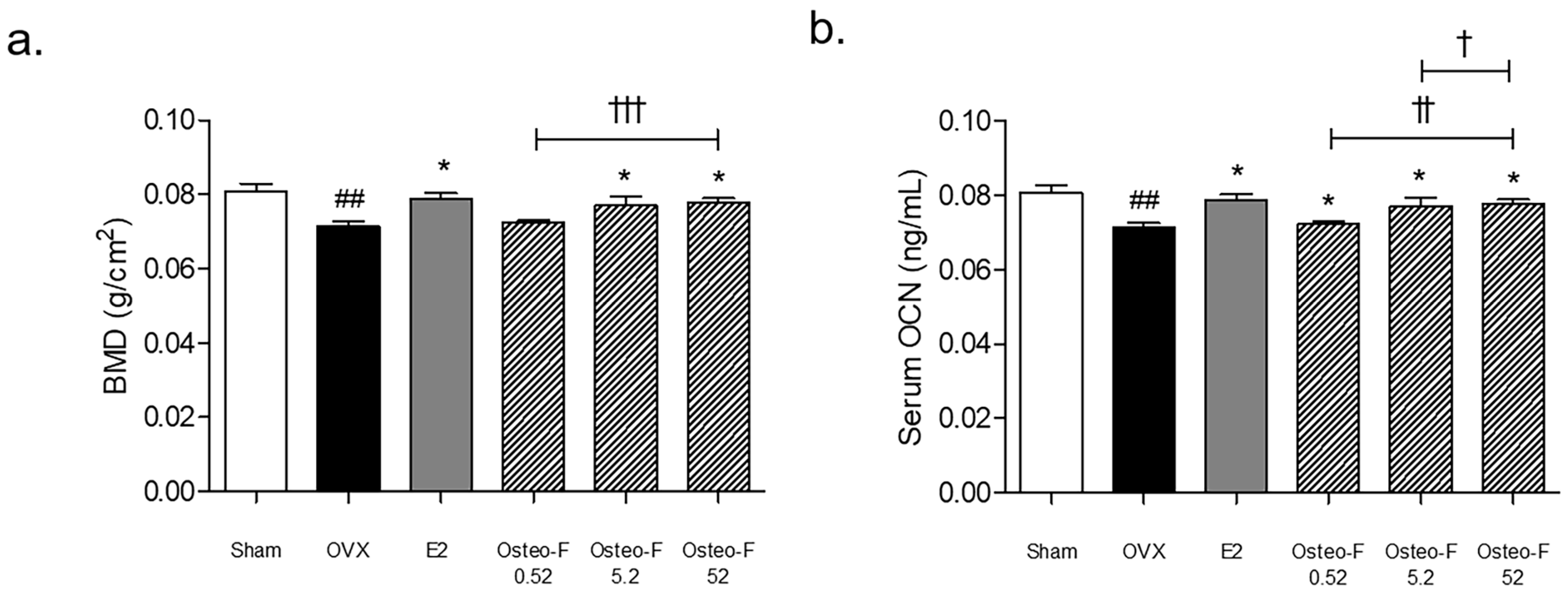
3.5. Recovery of the BMD Level by Osteo-F in OVX-Induced Osteoporotic Mice
3.6. Serum OCN and OCN+ Expression of Femoral Bone Tissues by Osteo-F in OVX-Induced Osteoporotic Mice
3.7. Change in Biochemical Bone Markers including the Z-Score and T-Score by Osteo-F in Postmenopausal Women
3.8. Change in Serum Biomarkers including the PTH and PTH/Ca Ratio by Osteo-F in Postmenopausal Women
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 2096–2097. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Aubry-Rozier, B.; Kaouri, S.; Lamy, O. Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J. Bone Min. Res. 2017, 32, 1291–1296. [Google Scholar] [CrossRef] [Green Version]
- Jolette, J.; Attalla, B.; Varela, A.; Long, G.G.; Mellal, N.; Trimm, S.; Smith, S.Y.; Ominsky, M.S.; Hattersley, G. Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1-34). Regul. Toxicol. Pharmacol. 2017, 86, 356–365. [Google Scholar] [CrossRef]
- Cosman, F. Anabolic Therapy and Optimal Treatment Sequences for Patients With Osteoporosis at High Risk for Fracture. Endocr. Pract. 2020, 26, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Oida, H.; Kobayashi, T.; Maruyama, T.; Tanaka, M.; Katayama, T.; Yamaguchi, K.; Segi, E.; Tsuboyama, T.; Matsushita, M.; et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA 2002, 99, 4580–4585. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.H.; Choi, Y.; Yang, W.M. Ameliorative effects of Osteo-F, a newly developed herbal formula, on osteoporosis via activation of bone formation. J. Ethnopharmacol. 2021, 268, 113590. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, M.H.; Choi, H.; Yang, W.M. Effects of Osteo-F, a new herbal formula, on osteoporosis via up-regulation of Runx2 and Osterix. RSC Adv. 2017, 7, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Abd-Algaleel, S.A.; Metwally, A.A.; Abdel-Bar, H.M.; Kassem, D.H.; Hathout, R.M. Synchronizing In Silico, In Vitro, and In Vivo Studies for the Successful Nose to Brain Delivery of an Anticancer Molecule. Mol. Pharm. 2021, 18, 3763–3776. [Google Scholar] [CrossRef] [PubMed]
- De Hao, C.; Xiao, P.G. Network pharmacology: A Rosetta Stone for traditional Chinese medicine. Drug Dev. Res. 2014, 75, 299–312. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duxin, S.; Wei, G.; Hongxiang, H.; Simon, Z. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part. B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Corrado, A.; Sanpaolo, E.R.; Di Bello, S.; Cantatore, F.P. Osteoblast as a target of anti-osteoporotic treatment. Postgrad. Med. 2017, 129, 858–865. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Tanphiriyakun, T.; Rojanasthien, S.; Khumrin, P. Bone mineral density response prediction following osteoporosis treatment using machine learning to aid personalized therapy. Sci. Rep. 2021, 11, 13811. [Google Scholar] [CrossRef]
- Wendlova, J. Differences in distribution of T-scores and Z-scores among bone densitometry tests in postmenopausal women (a comparative study). Wien. Med. Wochenschr. 2002, 152, 591–595. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Blake, G.M. T-scores and Z-scores. J. Clin. Densitom. 2007, 10, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Licata, A.A. Diagnosing primary osteoporosis: It’s more than a T score. Cleve Clin. J. Med. 2006, 73, 473–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Harris, M.A.; Rossini, G.; Dunstan, C.R.; Dallas, S.L.; Feng, J.Q.; Mundy, G.R.; Harris, S.E. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif. Tissue Int. 1997, 60, 283–290. [Google Scholar] [CrossRef]
- Siggelkow, H.; Rebenstorff, K.; Kurre, W.; Niedhart, C.; Engel, I.; Schulz, H.; Atkinson, M.J.; Hufner, M. Development of the osteoblast phenotype in primary human osteoblasts in culture: Comparison with rat calvarial cells in osteoblast differentiation. J. Cell Biochem. 1999, 75, 22–35. [Google Scholar]
- Manolagas, S.C. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020, 16, e1008714. [Google Scholar] [CrossRef]
- Delmas, P.D.; Eastell, R.; Garnero, P.; Seibel, M.J.; Stepan, J.; Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos. Int. 2000, 11, S2–S17. [Google Scholar] [CrossRef]
- Nikel, O.; Laurencin, D.; McCallum, S.A.; Gundberg, C.M.; Vashishth, D. NMR investigation of the role of osteocalcin and osteopontin at the organic-inorganic interface in bone. Langmuir 2013, 29, 13873–13882. [Google Scholar] [CrossRef] [Green Version]
- De Toni, L.; Jawich, K.; De Rocco Ponce, M.; Di Nisio, A.; Foresta, C. Osteocalcin: A Protein Hormone Connecting Metabolism, Bone and Testis Function. Protein Pept. Lett. 2020, 27, 1268–1275. [Google Scholar] [CrossRef]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.J.; Coxam, V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging 2015, 19, 77–86. [Google Scholar] [CrossRef]
- Jiang, D.; Franceschi, R.T.; Boules, H.; Xiao, G. Parathyroid hormone induction of the osteocalcin gene. Requirement for an osteoblast-specific element 1 sequence in the promoter and involvement of multiple-signaling pathways. J. Biol. Chem. 2004, 279, 5329–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Wei, D.; Wang, D.; Phimphilai, M.; Krebsbach, P.H.; Franceschi, R.T. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J. Bone Min. Res. 2003, 18, 705–715. [Google Scholar] [CrossRef]
- Villa, I.; Senesi, P.; Montesano, A.; Ferraretto, A.; Vacante, F.; Spinello, A.; Bottani, M.; Bolamperti, S.; Rubinacci, A.; Luzi, L.; et al. Betaine promotes cell differentiation of human osteoblasts in primary culture. J. Transl. Med. 2017, 15, 132. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, H.; Wu, Y.; Han, Y.; Wang, X. Pinoresinol Diglucoside Relieves Osteoporosis Through Enhancing Osteogenic Differentiation via Activating Phosphatidylinositol-3-Kinase/Protein Kinase B Signaling Pathway. J. Biomater. Tissue Eng. 2020, 10, 709–718. [Google Scholar] [CrossRef]
- Ni, S.; Qian, Z.; Yuan, Y.; Li, D.; Zhong, Z.; Ghorbani, F.; Zhang, X.; Zhang, F.; Zhang, Z.; Liu, Z.; et al. Schisandrin A restrains osteoclastogenesis by inhibiting reactive oxygen species and activating Nrf2 signalling. Cell Prolif. 2020, 53, e12882. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Song, C.; Kang, H.; Guo, Q.; Dong, Y.; Zhang, Y.; Peng, R.; Guan, H.; Li, F. Schisandrin B Inhibits Osteoclastogenesis and Protects Against Ovariectomy-Induced Bone Loss. Front. Pharmacol. 2020, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Shen, Y.; Chen, X.; Zhou, F.; Peng, D. Schisantherin A suppresses osteoclast formation and wear particle-induced osteolysis via modulating RANKL signaling pathways. Biochem. Biophys. Res. Commun. 2014, 449, 344–350. [Google Scholar] [CrossRef]
- Steinmetz, K.L.; Spack, E.G. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 2009, 9, S2. [Google Scholar] [CrossRef] [Green Version]




| Variables | OSTEO-F (n = 39) | Placebo (n = 43) | p-Value ‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Change | p-Value † | Baseline | Follow-Up | Change | p-Value † | ||
| Bone mineral density | |||||||||
| Lumbar spine | |||||||||
| BMD (g/cm2) | 1.03 ± 0.13 | 1.03 ± 0.13 | 0.00 ± 0.02 | 0.572 | 1.02 ± 0.12 | 1.02 ± 0.12 | −0.01 ± 0.02 | 0.103 | 0.113 |
| BMC (g) | 52.38 ± 11.31 | 52.41 ± 11.27 | 0.03 ±1.27 | 0.875 | 53.56 ± 11.57 | 53.33 ± 11.57 | −0.23 ± 1.39 | 0.296 | 0.395 |
| T-score | −0.95 ± 1.09 | −0.93 ± 1.09 | 0.02 ± 0.19 | 0.633 | −1.02 ± 1.00 | −1.06 ± 1.00 | −0.04 ± 0.18 | 0.153 | 0.164 |
| Z-score | −0.23 ± 1.08 | −0.16 ± 1.08 | 0.07 ± 0.20 | 0.045 | −0.32 ± 0.96 | −0.34 ± 0.97 | −0.02 ± 0.19 | 0.544 | 0.048 |
| Femur total | |||||||||
| BMD (g/cm2) | 0.95 ± 0.09 | 0.95 ± 0.09 | 0.00 ± 0.01 | 0.063 | 0.95 ± 0.11 | 0.95 ± 0.11 | 0.00 ± 0.01 | 0.058 | 0.794 |
| BMC (g) | 28.07 ± 3.29 | 28.02 ± 3.35 | −0.05 ± 0.50 | 0.555 | 28.29 ± 3.36 | 28.18 ± 3.34 | −0.11 ± 0.44 | 0.103 | 0.538 |
| T-score | −0.18 ± 0.76 | −0.22 ± 0.78 | −0.03 ± 0.09 | 0.044 | −0.18 ± 0.96 | −0.21 ± 0.95 | −0.03 ± 0.12 | 0.096 | 0.988 |
| Z-score | 0.00 ± 0.78 | 0.02 ± 0.80 | 0.02 ± 0.12 | 0.438 | 0.00 ± 0.88 | 0.00 ± 0.88 | 0.00 ± 0.12 | 0.898 | 0.495 |
| Biochemical bone markers | |||||||||
| OCN (ng/mL) | 18.44± 6.14 | 19.23 ± 5.89 | 0.79 ± 3.02 | 0.110 | 21.41 ± 7.21 | 21.24 ±5.50 | −0.10 ±2.58 | 0.813 | 0.042 |
| PTH (pg/mL) | 39.97 ± 10.99 | 34.58 ± 9.13 | −5.39 ± 9.18 | 0.001 | 36.00 ± 10.61 | 35.30 ± 13.15 | −0.70 ± 10.17 | 0.655 | 0.031 |
| PTH/ Ca ratio | 4.38 ± 1.24 | 3.76 ± 0.99 | −0.62 ± 1.03 | 0.001 | 3.92 ± 1.23 | 3.85 ± 1.47 | −0.07 ± 1.16 | 0.675 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Bok, M.; Lim, H.; Yang, W.M. An Integrative Study on the Inhibition of Bone Loss via Osteo-F Based on Network Pharmacology, Experimental Verification, and Clinical Trials in Postmenopausal Women. Cells 2023, 12, 1992. https://doi.org/10.3390/cells12151992
Kim MH, Bok M, Lim H, Yang WM. An Integrative Study on the Inhibition of Bone Loss via Osteo-F Based on Network Pharmacology, Experimental Verification, and Clinical Trials in Postmenopausal Women. Cells. 2023; 12(15):1992. https://doi.org/10.3390/cells12151992
Chicago/Turabian StyleKim, Mi Hye, Minkyung Bok, Hyunjung Lim, and Woong Mo Yang. 2023. "An Integrative Study on the Inhibition of Bone Loss via Osteo-F Based on Network Pharmacology, Experimental Verification, and Clinical Trials in Postmenopausal Women" Cells 12, no. 15: 1992. https://doi.org/10.3390/cells12151992







