In Vitro Study of a Novel Vibrio alginolyticus-Based Collagenase for Future Medical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adipose Tissue Collection
2.2. Production and Purification of Vibrio alginolyticus Collagenase
2.3. Adipose Tissue Enzymatic Digestion
2.4. Cellular Yield
2.5. Clonogenic Capacity
2.6. Proliferation Capacity
2.7. Immunophenotyping
2.8. Differentiation Assay
2.9. Cellular Viability Test
2.10. Statistical Analyses
3. Results
3.1. Optimization of the Collagenase Fidia Adipose Tissue Digestion Process
3.1.1. Cellular Yield, Clonogenic Potential, and Proliferation Capacity
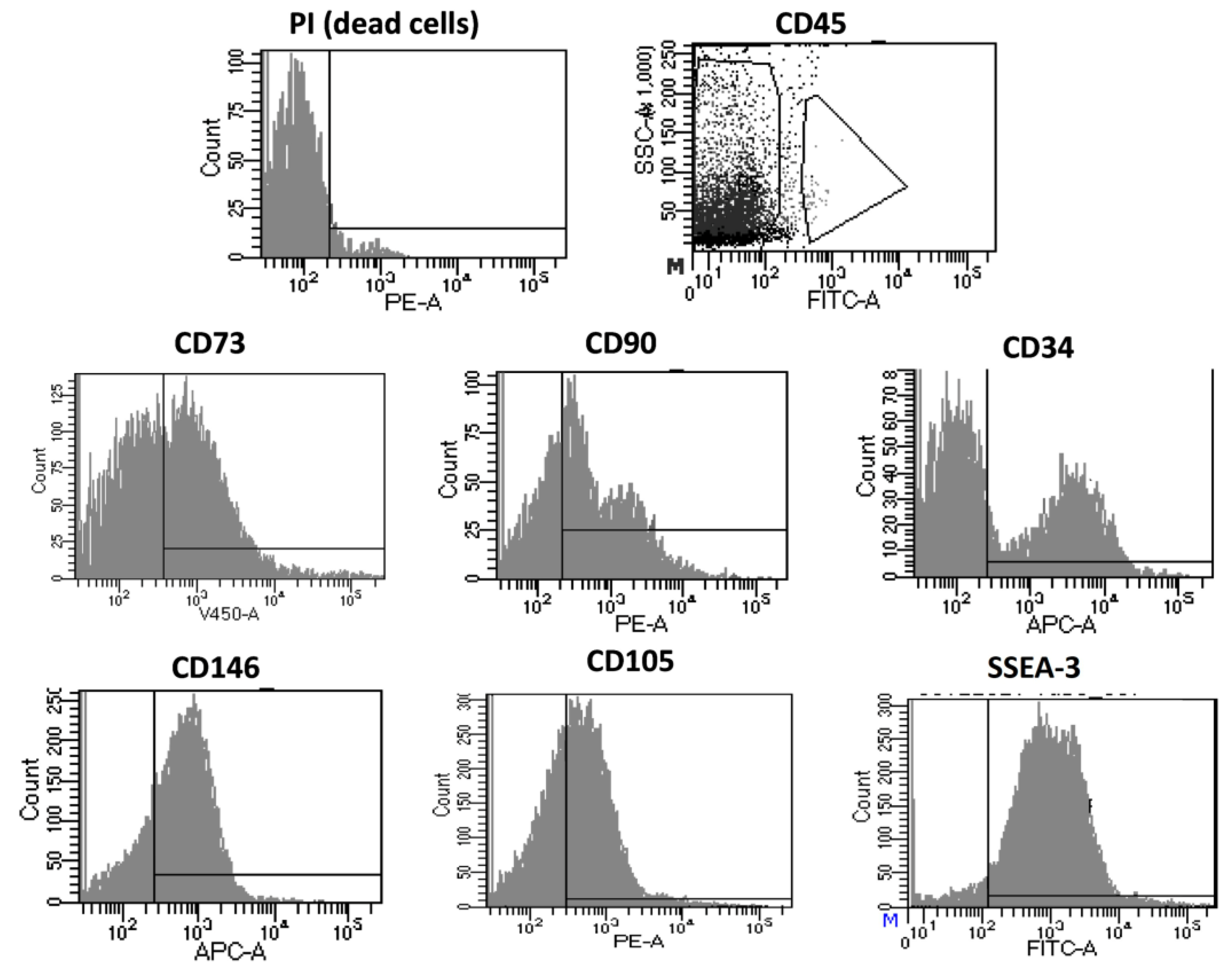
3.1.2. Immunophenotyping of Optimized Fidia Collagenase Method
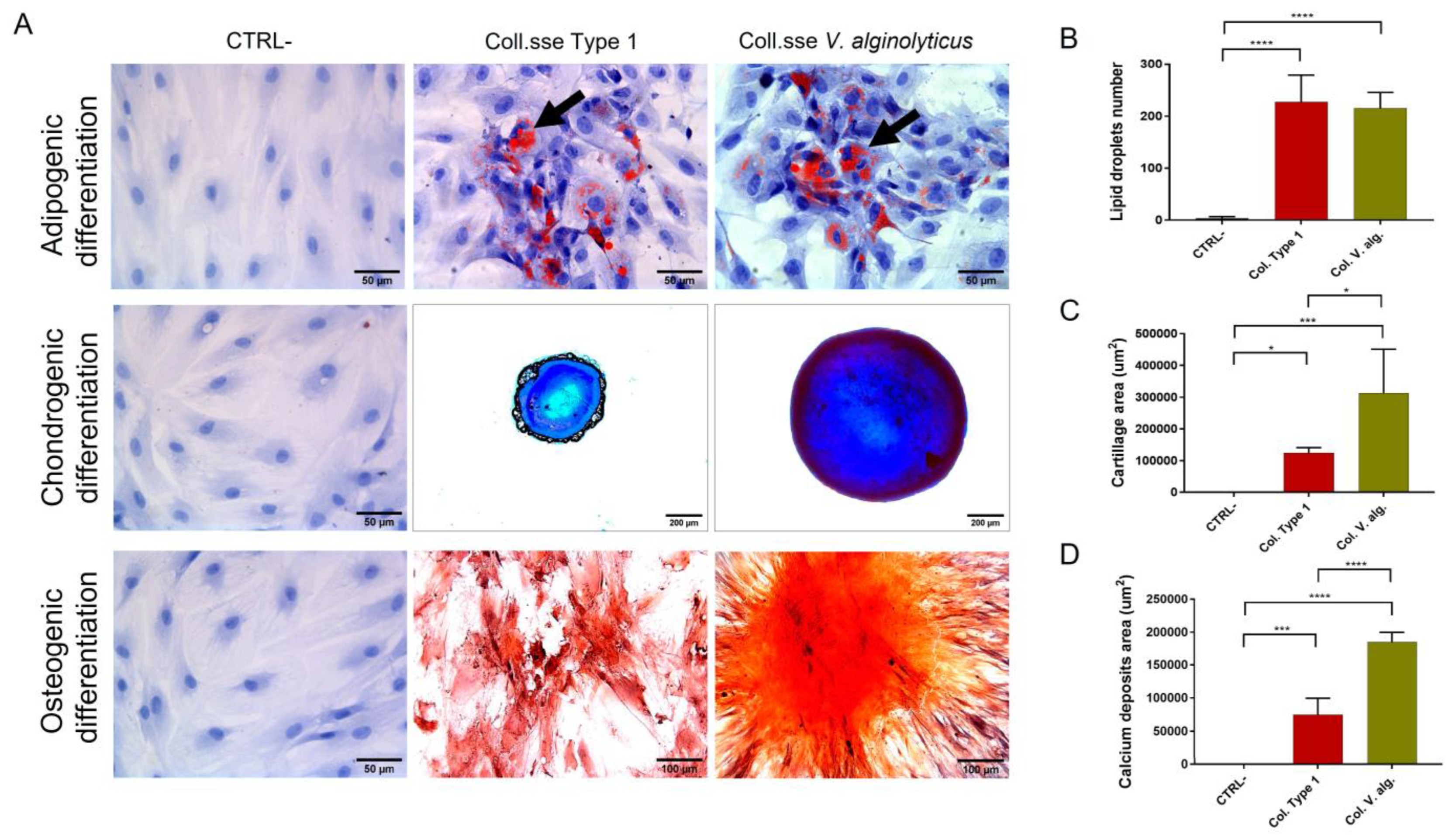
3.1.3. Analysis of Multipotency
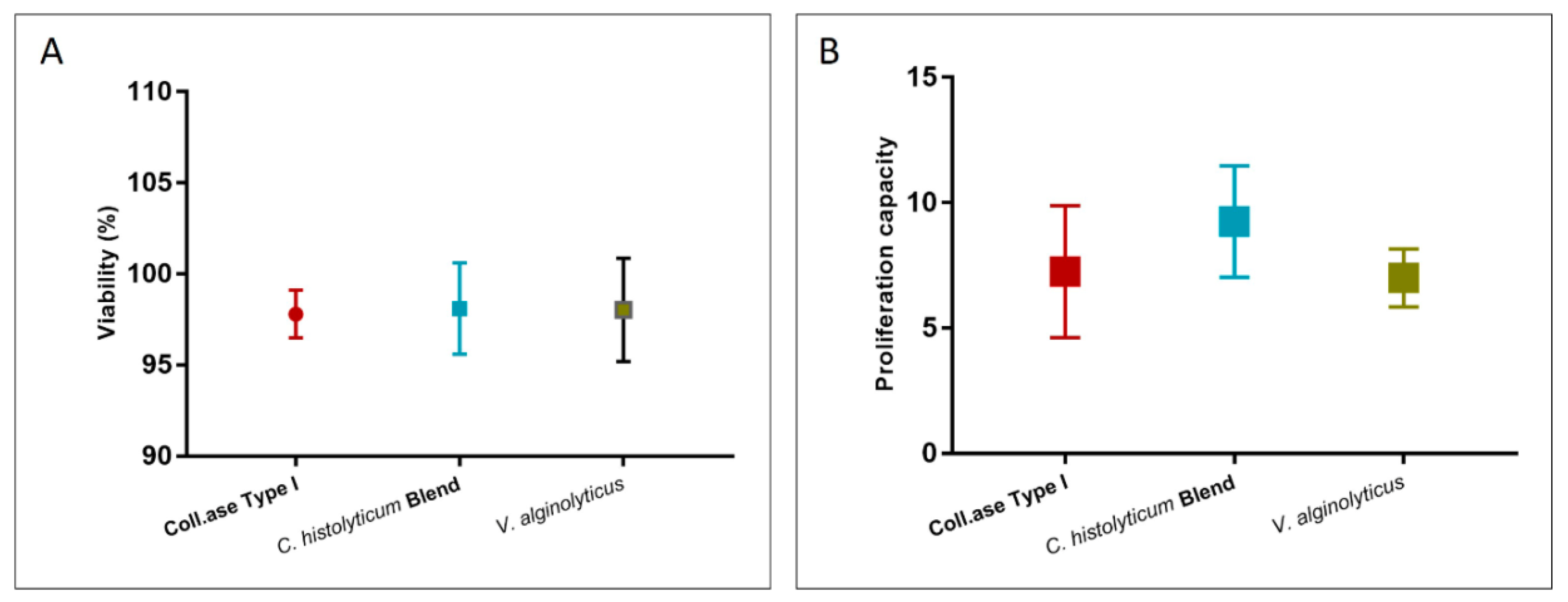
3.2. Comparison of the Optimized Protocol with Commercial Collagenases
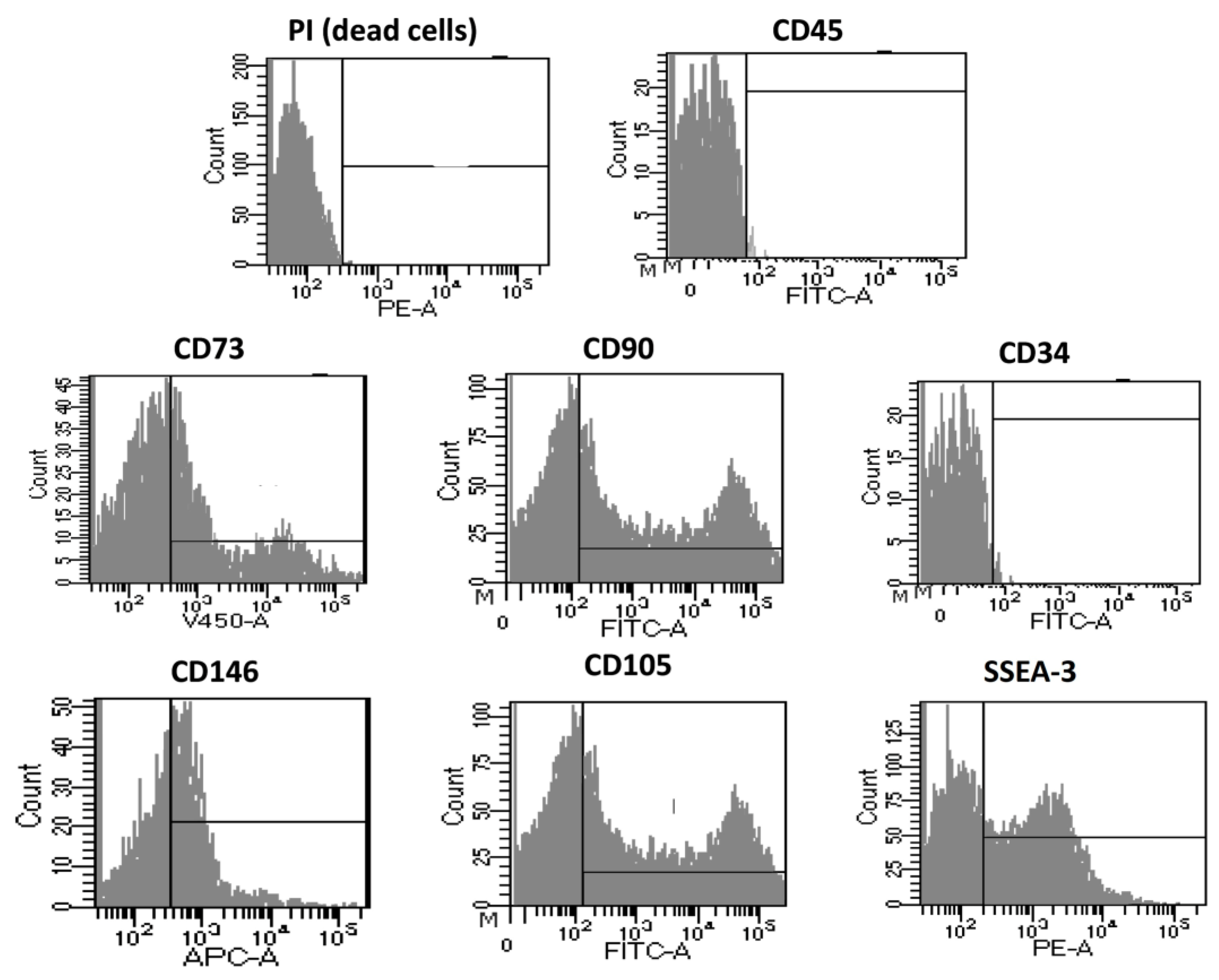
3.2.1. Cellular Yield, Clonogenic Potential, and Proliferation Capacity

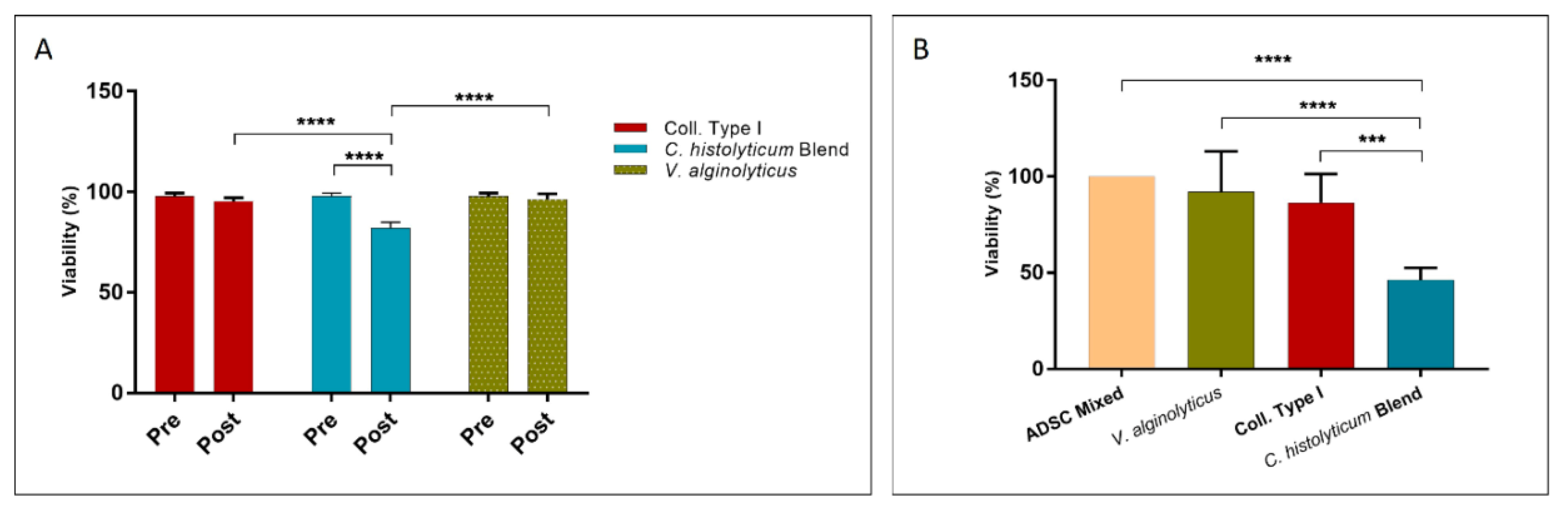
3.2.2. Cellular Integrity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koch, T.G.; Berg, L.C.; Betts, D.H. Current and future regenerative medicine-Principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Can. Vet. J. 2009, 50, 155–165. [Google Scholar] [PubMed]
- Alzate-Correa, D.; Lawrence, W.R.; Salazar-Puerta, A.; Higuita-Castro, N.; Gallego-Perez, D. Nanotechnology-Driven Cell-Based Therapies in Regenerative Medicine. AAPS J. 2022, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, Z.; Sun, M.; Xu, H.; Gao, Y.; Liu, J.; Li, M. Characterization and therapeutic applications of mesenchymal stem cells for regenerative medicine. Tissue Cell 2020, 64, 101330. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Jalali, S.A.; Varedi, M. Isolation of adipose tissue mesenchymal stem cells without tissue destruction: A non-enzymatic method. Tissue Cell 2014, 46, 54–58. [Google Scholar] [CrossRef]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of human mesenchymal stem cells in regenerative therapy. Cells 2021, 10, 54. [Google Scholar] [CrossRef]
- Prasai, A.; El Ayadi, A.; Mifflin, R.C.; Wetzel, M.D.; Andersen, C.R.; Redl, H.; Herndon, D.N.; Finnerty, C.C. Characterization of Adipose-Derived Stem Cells Following Burn Injury. Stem Cell Rev. Rep. 2017, 13, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.E.; Pagliara, D.; Locatelli, F. Mesenchymal stromal cell therapy: A revolution in Regenerative Medicine? Bone Marrow Transplant. 2012, 47, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, L.S.; Condé-Green, A.; Naaldijk, Y.; Lee, E.S.; Rameshwar, P. An enzyme-free method for isolation and expansion of human adipose-derived mesenchymal stem cells. J. Vis. Exp. 2019, 2019, e59419. [Google Scholar]
- De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. Int. J. Mol. Sci. 2021, 22, 10197. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of Multi-Lineage Cells from Human Adipose Tissue and Bone Marrow. Cells Tissues Organs. 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Nakagami, H.; Morishita, R.; Maeda, K.; Kikuchi, Y.; Ogihara, T.; Kaneda, Y. Adipose Tissue-Derived Stromal Cells as a Novel Option for Regenerative Cell Therapy. J. Atheroscler. Thromb. 2006, 13, 77–81. [Google Scholar] [CrossRef] [Green Version]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Hassani, N.; Taurin, S.; Alshammary, S. Meta-Analysis: The Clinical Application of Autologous Adult Stem Cells in the Treatment of Stroke. Stem Cells Cloning Adv. Appl. 2021, 14, 81–91. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.-Z.; Lin, Y.-H.; Su, L.-J.; Wu, M.-S.; Jeng, H.-Y.; Chang, H.-C.; Huang, Y.-H.; Ling, T.-Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef]
- Alstrup, T.; Eijken, M.; Bohn, A.B.; Møller, B.; Damsgaard, T.E. Isolation of Adipose Tissue-Derived Stem Cells: Enzymatic Digestion in Combination with Mechanical Distortion to Increase Adipose Tissue-Derived Stem Cell Yield from Human Aspirated Fat. Curr. Protoc. Stem Cell Biol. 2019, 48, e68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, A.; Riccio, V.; Riccio, M.; Sbarbati, A. Simple and Rapid Non-Enzymatic Procedure Allows the Isolation of Structurally Preserved Connective Tissue Micro-Fragments Enriched with SVF. Cells 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A.; et al. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purpura, V.; Bondioli, E.; Melandri, D.; Parodi, P.C.; Valenti, L.; Riccio, M. The Collection of Adipose Derived Stem Cells using Water–Jet Assisted Lipoplasty for their Use in Plastic and Reconstructive Surgery: A Preliminary Study. Front. Cell Dev. Biol. 2016, 4, 136. [Google Scholar] [CrossRef] [Green Version]
- Raposio, E.; Bertozzi, N. How to isolate a ready-to-use adipose-derived stem cells pellet for clinical application. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4252–4260. [Google Scholar]
- Coccè, V.; Brini, A.; Giannì, A.B.; Sordi, V.; Berenzi, A.; Alessandri, G.; Tremolada, C.; Versari, S.; Bosetto, A.; Pessina, A. A Nonenzymatic and Automated Closed-Cycle Process for the Isolation of Mesenchymal Stromal Cells in Drug Delivery Applications. Stem Cells Int. 2018, 2018, 4098140. [Google Scholar]
- De Francesco, F.; Mannucci, S.; Conti, G.; Dai Prè, E.; Sbarbati, A.; Riccio, M. A Non-Enzymatic Method to Obtain a Fat Tissue Derivative Highly Enriched in Adipose Stem Cells (ASCs) from Human Lipoaspirates: Preliminary Results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [CrossRef] [Green Version]
- Dai Prè, E.; Busato, A.; Mannucci, S.; Vurro, F.; De Francesco, F.; Riccio, V.; Solito, S.; Biswas, R.; Bernardi, P.; Riccio, M.; et al. In Vitro Characterization of Adipose Stem Cells Non-Enzymatically Extracted from the Thigh and Abdomen. Int. J. Mol. Sci. 2020, 21, 3081. [Google Scholar] [CrossRef]
- De Francesco, F.; Riccio, V.; Biswas, R.; Busato, A.; Di Bella, C.; Serri, E.; Sbarbati, A.; Zavan, B.; Riccio, M.; Palumbo Piccionello, A. In Vitro Characterization of Canine Microfragmented Adipose Tissue Non-Enzymatically Extracted from the Thigh and Lumbar Regions. Animals 2021, 11, 3231. [Google Scholar] [CrossRef]
- De Francesco, F.; Guastafierro, A.; Nicoletti, G.; Razzano, S.; Riccio, M.; Ferraro, G. The Selective Centrifugation Ensures a Better In Vitro Isolation of ASCs and Restores a Soft Tissue Regeneration In Vivo. Int. J. Mol. Sci. 2017, 18, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero Sierra, L.A.; Biswas, R.; Conti, A.; Busato, A.; Ossanna, R.; Zingaretti, N.; Parodi, P.C.; Conti, G.; Riccio, M.; Sbarbati, A.; et al. Highly Pluripotent Adipose-Derived Stem Cell–Enriched Nanofat: A Novel Translational System in Stem Cell Therapy. Cell Transplant. 2023, 32, 096368972311759. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Breast Reconstruction with Enhanced Stromal Vascular Fraction Fat Grafting. Plast Reconstr. Surg—Glob. Open 2015, 3, e406. [Google Scholar] [CrossRef]
- Grasys, J.; Kim, B.-S.; Pallua, N. Content of Soluble Factors and Characteristics of Stromal Vascular Fraction Cells in Lipoaspirates from Different Subcutaneous Adipose Tissue Depots. Aesthetic Surg. J. 2016, 36, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Daboor, S.M.; Budge, S.M.; Ghaly, A.E.; Brooks, S.-L.; Dave, D. Extraction and Purification of Collagenase Enzymes: A Critical Review. Am. J. Biochem. Biotechnol. 2010, 6, 239–263. [Google Scholar] [CrossRef] [Green Version]
- Kirshen, C.; Woo, K.; Ayello, E.A.; Sibbald, R.G. Debridement: A vital component of wound bed preparation. Adv. Skin Wound Care 2006, 19, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Ramundo, J.; Gray, M. Enzymatic Wound Debridement. J. Wound Ostomy Cont. Nurs. 2008, 35, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, L.C.; Badalamente, M.A.; Hentz, V.R.; Hotchkiss, R.N.; Kaplan, F.T.D.; Meals, R.A.; Smith, T.M.; Rodzvilla, J.; CORD I Study Group. Injectable Collagenase Clostridium histolyticum for Dupuytren’s Contracture. N. Engl. J. Med. 2009, 361, 968–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, F.; Thomas, A.N.; Singh, S.; Kolluru, V.; Emeigh Hart, S.G.; Bayat, A. In Vitro Study of Novel Collagenase (XIAFLEX®) on Dupuytren’s Disease Fibroblasts Displays Unique Drug Related Properties. PLoS ONE 2012, 7, e31430. [Google Scholar] [CrossRef] [Green Version]
- Martín-Ferrero, M.Á.; Simón-Pérez, C.; Rodríguez-Mateos, J.I.; García-Medrano, B.; Hernández-Ramajo, R.; Brotat-García, M. Tratamiento de la enfermedad de Dupuytren mediante la colagenasa del Clostridium histolyticum. Rev. Esp. Cir. Ortop. Traumatol. 2013, 57, 398–402. [Google Scholar] [CrossRef]
- Riccio, M.; Marchesini, A.; Riccio, V.; Orlando, F.; Warwick, D.; Costa, A.L.; Zavan, B.; De Francesco, F. A novel collagenase from Vibrio Alginolyticus: Experimental study for Dupuytren’s disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 18–28. [Google Scholar]
- Gelbard, M.; Lipshultz, L.I.; Tursi, J.; Smith, T.; Kaufman, G.; Levine, L.A. Phase 2b Study of the Clinical Efficacy and Safety of Collagenase Clostridium histolyticum in Patients With Peyronie Disease. J. Urol. 2012, 187, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, F.M.; Towe, M.; Yafi, F.A. Management of Peyronie’s disease with collagenase Clostridium histolyticum in the acute phase. World J. Urol. 2020, 38, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, R.; Vaccaro, S.; Caputo, M.; Cuppari, C.; Caruso, S.; Catania, A.; Messina, L. Collagenase-assisted wound bed preparation: An in vitro comparison between Vibrio alginolyticus and Clostridium histolyticum collagenases on substrate specificity. Int. Wound J. 2019, 16, 1013–1023. [Google Scholar] [PubMed]
- Van Wart, H.E. Clostridium Collagenases. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 607–611. [Google Scholar]
- Fukushima, J.; Shimura, Y.; Okuda, K. Vibrio Collagenase. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 604–606. [Google Scholar]
- Salamone, M.; Nicosia, A.; Ghersi, G.; Tagliavia, M. Vibrio Proteases for Biomedical Applications: Modulating the Proteolytic Secretome of V. alginolyticus and V. parahaemolyticus for Improved Enzymes Production. Microorganisms 2019, 7, 387. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, D343–D350. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Shibano, Y.; Morihara, K.; Fukushima, J.; Inami, S.; Keil, B.; Gilles, A.M.; Kawamoto, S.; Okuda, K. Structural gene and complete amino acid sequence of Vibrio alginolyticus collagenase. Biochem. J. 1992, 281, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Onesti, M.G.; Fioramonti, P.; Carella, S.; Fino, P.; Sorvillo, V.; Scuderi, N. A new association between hyaluronic acid and collagenase in wound repair: An open study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 210–216. [Google Scholar]
- Riley, K.N.; Herman, I.M. Collagenase promotes the cellular responses to injury and wound healing in vivo. J. Burns Wounds 2005, 4, e8. [Google Scholar]
- De Francesco, F.; De Francesco, M.; Riccio, M. Hyaluronic Acid/Collagenase Ointment in the Treatment of Chronic Hard-to-Heal Wounds: An Observational and Retrospective Study. J. Clin. Med. 2022, 11, 537. [Google Scholar] [CrossRef]
- Bassetto, F.; Maschio, N.; Abatangelo, G.; Zavan, B.; Scarpa, C.; Vindigni, V. Collagenase From Vibrio alginolyticus Cultures: Experimental Study and Clinical Perspectives. Surg. Innov. 2016, 23, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.K.; Mckenney, S.; Jarrell, B.E. Collagenase Lot Selection and Purification for Adipose Tissue Digestion. Cell Transplant. 1995, 4, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.P.; Gimble, J.M.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Xenofree Enzymatic Products for the Isolation of Human Adipose-Derived Stromal/Stem Cells. Tissue Eng. Part C Methods 2013, 19, 473–478. [Google Scholar] [CrossRef]
- Agostini, F.; Rossi, F.M.; Aldinucci, D.; Battiston, M.; Lombardi, E.; Zanolin, S.; Massarut, S.; Parodi, P.C.; Da Ponte, A.; Tessitori, G.; et al. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res. Ther. 2018, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Sensebé, L.; Bourin, P.; Tarte, K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum. Gene Ther. 2011, 22, 19–26. [Google Scholar] [CrossRef]
- Vaccaro, S.; Caputo, M.; Cuppari, C.; Gennari, G. New Process for the Production and Purification of the Collagenase Enzyme from Vibrio alginolyticus. WO 2013/156525 A1, 24 October 2013. [Google Scholar]
- Kang, N.; Sivakumar, B.; Sanders, R.; Nduka, C.; Gault, D. Intra-lesional injections of collagenase are ineffective in the treatment of keloid and hypertrophic scars. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 693–699. [Google Scholar] [CrossRef]
- Patry, J.; Blanchette, V. Enzymatic debridement with collagenase in wounds and ulcers: A systematic review and meta-analysis. Int. Wound J. 2017, 14, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Collier, Z.; Fang, M.; Howell, A.; Gillenwater, T. The role of collagenase ointment in acute burns: A systematic review and meta-analysis. J. Wound Care 2019, 28, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Kaufman-Janette, J.A.; Bass, L.S.; Xiang, Q.; McLane, M.P.; Kirby, M.T.; Vijayan, S. Efficacy, Safety, and Durability of Response of Collagenase Clostridium histolyticum-aaes for Treating Cellulite. Plast. Reconstr. Surg.—Glob. Open 2020, 8, e3316. [Google Scholar] [CrossRef]
- Graivier, M.; Hill, D.; Katz, B.; Boehm, K.A.; Fisher, J.; Battista, C. Collagenase Clostridium histolyticum for the Treatment of Cellulite in the Buttocks and Thigh: Early Insights From Clinical Practice. Aesthetic Surg. J. Open Forum 2022, 4, ojac057. [Google Scholar] [CrossRef]
- Frederick, R.E.; Bearden, R.; Jovanovic, A.; Jacobson, N.; Sood, R.; Dhall, S. Clostridium Collagenase Impact on Zone of Stasis Stabilization and Transition to Healthy Tissue in Burns. Int. J. Mol. Sci. 2021, 22, 8643. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Ciliberti, R. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; De Francesco, F.; Pinton, P.; Gardin, C.; Zavan, B. Methods to isolate adipose tissue-derived stem cells. Methods Cell Biol. 2022, 171, 215–228. [Google Scholar] [PubMed]
- Cocci, A.; Ivan, G.; Ignacio, J.; Salamanca, M.; Ralph, D.; Palmieri, A.; Mondaini, N. The End of an Era: Withdrawal of Xiapex (Clostridium histolyticum Collagenase) from the European Market. Eur. Urol. 2019, 77, 660–661. [Google Scholar] [CrossRef]
- Basso, M.A.; Bernasconi, A.; Balato, G.; Cozzolino, A.; Famiglietti, G.; Smeraglia, F. Clinical Results of Collagenase Treatment for Dupuytren’s Disease: A Case Series Study with 2-Years Follow-Up. Acta Ortopédica Bras. 2023, 31, 2021–2024. [Google Scholar] [CrossRef]
- Riccio, M.; Marchesini, A.; Zingaretti, N.; Carella, S.; Senesi, L.; Onesti, M.G.; Parodi, P.C.; Ribuffo, D.; Vaienti, L.; De Francesco, F. A Multicentre Study: The Use of Micrografts in the Reconstruction of Full-Thickness Posttraumatic Skin Defects of the Limbs—A Whole Innovative Concept in Regenerative Surgery. Stem Cells Int. 2019, 2019, 5043518. [Google Scholar] [CrossRef]

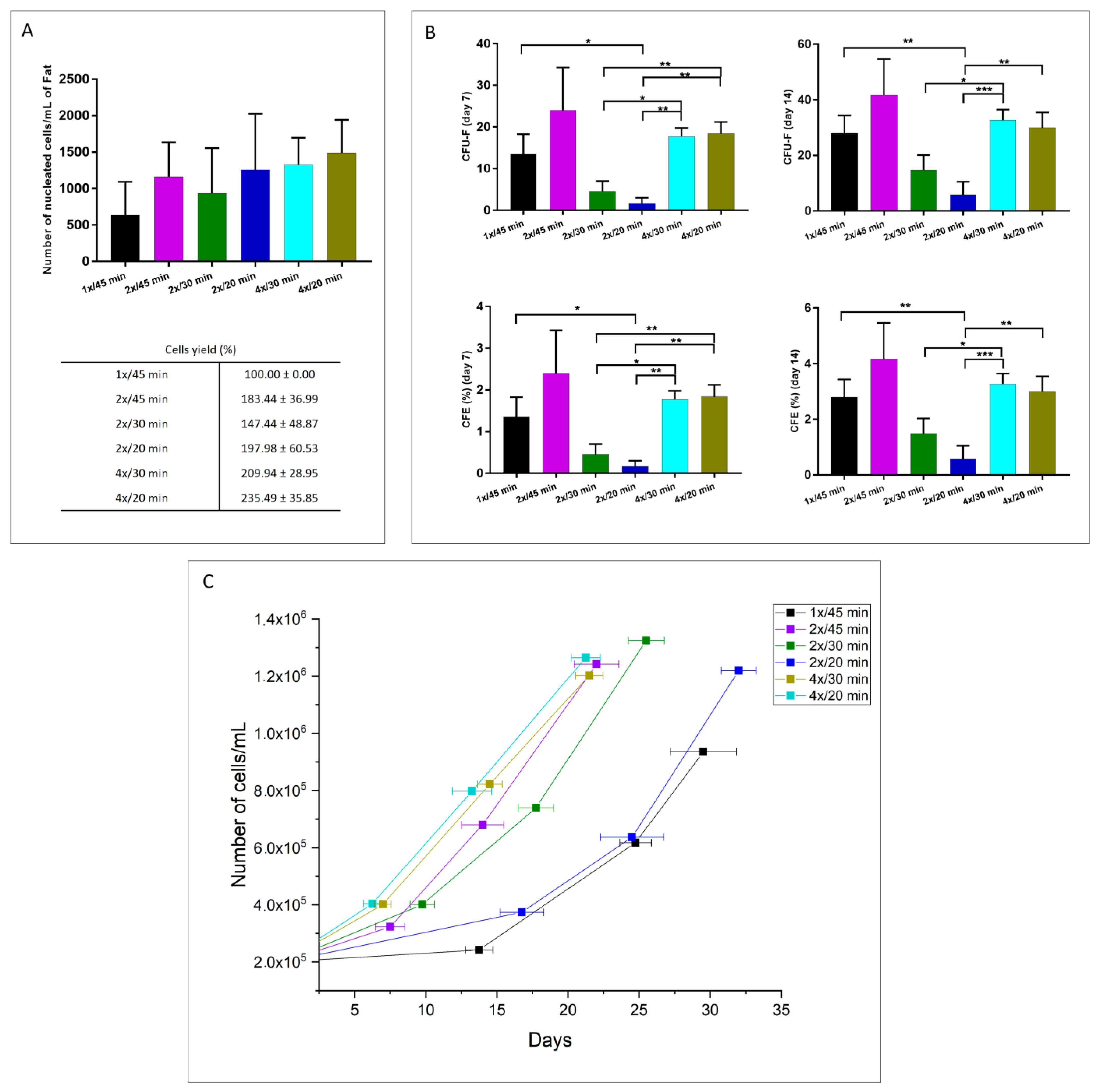


| Name Code | Enzyme Concentration (mg/mL) | Incubation Time (min) |
|---|---|---|
| 1x | 0.9 | 45 |
| 2x | 1.8 | 45, 30, 20 |
| 4x | 3.6 | 30, 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero Sierra, L.A.; Biswas, R.; Busato, A.; Conti, A.; Ossanna, R.; Conti, G.; Zingaretti, N.; Caputo, M.; Cuppari, C.; Parodi, P.C.; et al. In Vitro Study of a Novel Vibrio alginolyticus-Based Collagenase for Future Medical Application. Cells 2023, 12, 2025. https://doi.org/10.3390/cells12162025
Quintero Sierra LA, Biswas R, Busato A, Conti A, Ossanna R, Conti G, Zingaretti N, Caputo M, Cuppari C, Parodi PC, et al. In Vitro Study of a Novel Vibrio alginolyticus-Based Collagenase for Future Medical Application. Cells. 2023; 12(16):2025. https://doi.org/10.3390/cells12162025
Chicago/Turabian StyleQuintero Sierra, Lindsey Alejandra, Reetuparna Biswas, Alice Busato, Anita Conti, Riccardo Ossanna, Giamaica Conti, Nicola Zingaretti, Michele Caputo, Christian Cuppari, Pier Camillo Parodi, and et al. 2023. "In Vitro Study of a Novel Vibrio alginolyticus-Based Collagenase for Future Medical Application" Cells 12, no. 16: 2025. https://doi.org/10.3390/cells12162025










