Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia
Abstract
:1. Introduction
2. Nutritional Approach
2.1. HMB
2.2. Polyphenol
2.2.1. Catechin
2.2.2. Isoflavones
2.3. Ursolic Acid (UA)
2.4. Omega-3 PUFAs
3. Pharmacological Approach
3.1. Vitamin D
3.2. Myostatin Inhibition
3.3. Anabolic Steroids
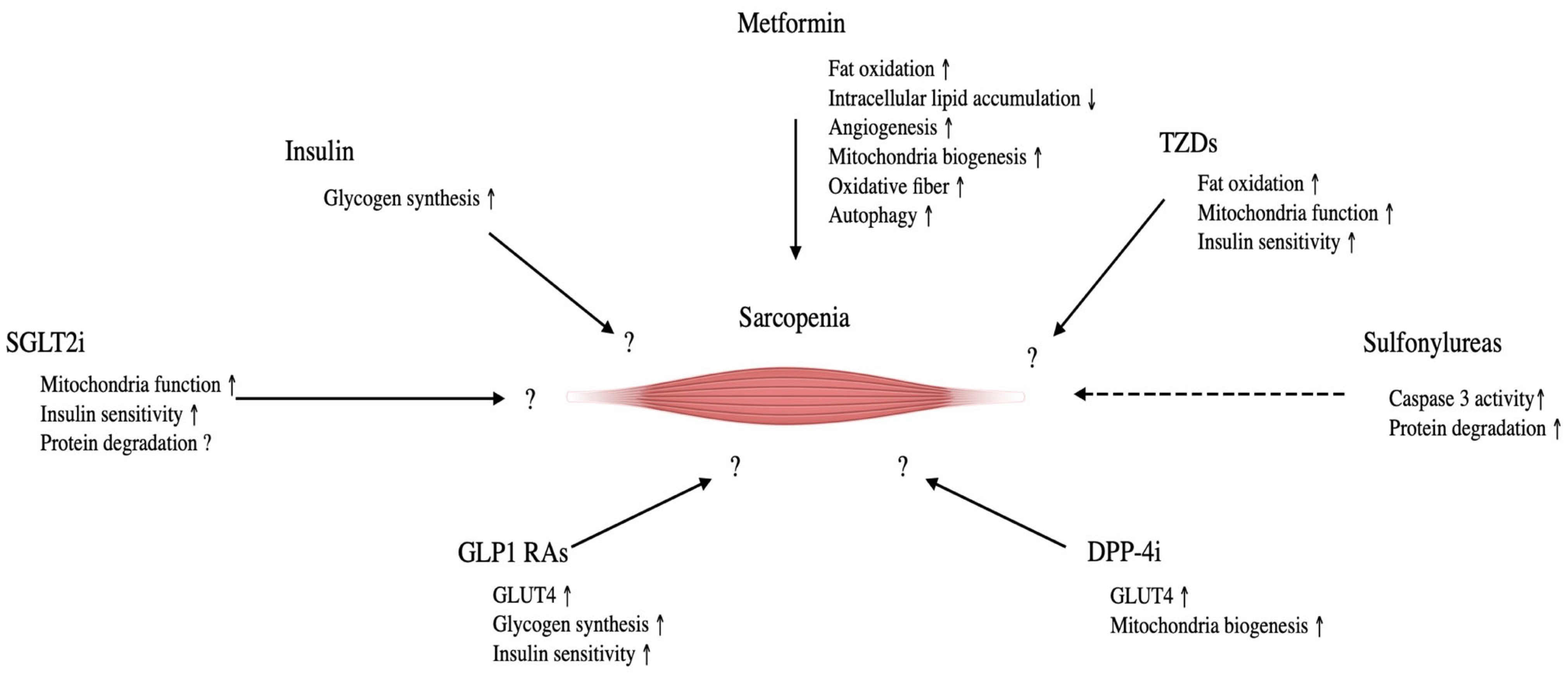
3.4. Glucose-Lowering Drugs
3.4.1. Metformin
3.4.2. Thiazolidinediones (TZDs)
3.4.3. Sulfonylureas
3.4.4. Dipeptidyl Peptidase-4 Inhibitor (DPP-4i)
3.4.5. Glucagon-like Peptide-1 Receptor Agonist (GLP-1 Ras)
3.4.6. Sodium-Glucose Transporter Protein 2 Inhibitors (SGLT2i)
3.4.7. Insulin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Parkington, J.D.; LeBrasseur, N.K.; Siebelt, A.P.; Fielding, R.A. Contraction-mediated mTOR, p70S6K, and ERK1/2 phosphorylation in aged skeletal muscle. J. Appl. Physiol. 2004, 97, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef]
- Sakuma, K.; Kinoshita, M.; Ito, Y.; Aizawa, M.; Aoi, W.; Yamaguchi, A. p62/SQSTM1 but not LC3 is accumulated in sarcopenic muscle of mice. J. Cachexia Sarcopenia Muscle 2016, 7, 204–212. [Google Scholar] [CrossRef]
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: Recent research advances. Pflügers Arch. 2017, 469, 573–591. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signaling pathways regulating muscle mass in ageing skeletal muscle. The role of IGF-1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Nicastro, H.; Artioli, G.G.; Dos Santos Costa, A.; Sollis, M.Y.; Da Luz, C.R.; Blachier, F.; Lancha, A.H., Jr. An overview of the ther apeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids 2011, 40, 287–300. [Google Scholar] [CrossRef]
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitohchondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 88–95. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Reidy, P.T.; Gundermann, D.M.; Borack, M.S.; Walker, D.K.; D’Lugos, A.C.; Volpi, E.; Rasmussen, B.B. The impact of postexercise essential amino acid ingestion on the ubiquitin proteasome and autophagosomal-lysosomal systems in skeletal muscle of older men. J. Appl. Physiol. 2017, 122, 620–630. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Volpi, E. Amino acid metabolism and regulatory effets in aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, S.E.; Seo, A.Y.; Marzetti, E.; Lees, H.A.; Leeuwenburgh, C. Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Exp. Gerontol. 2010, 45, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Recent advances in pharmacological, hormonal, nutritional intervention for sarcopenia. Pflügers Arch. 2018, 470, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A.; Shimizu, M. Sarcopenia: Molecular mechanism and current nutritional approach. In Encyclopedia of Human Nutrition; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2023; Volume 3, pp. 633–644. [Google Scholar]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Chen, L.K.; Arai, H.; Assantachai, P.; Akishita, M.; Chew, S.T.H.; Dumlao, L.C.; Duque, G.; Woo, J. Roles of nutrition in muscle health of community-dwelling older adults: Evidence-based expert consensus from Asian Working for Sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 1653–1672. [Google Scholar] [CrossRef]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-hydroxyl-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Hsieh, L.C.; Chow, C.J.; Chang, W.C.; Liu, T.H.; Chang, C.K. Effect of beta-hydrox-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac. J. Clin. Nutr. 2010, 19, 200–208. [Google Scholar]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of beta-hydroxyl-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxyl-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Peng, N.; Zhou, M.; Liu, P.P.; Qi, X.L.; Wang, N.; Wang, G.; Wu, Z.P. Tai Chi and whole-body vibrating therapy in sarcopenic men in advanced old age: A clinical randomized controlled trial. Eur. J. Ageing 2019, 16, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, C.; Chim, Y.N.; Yao, H.; Shi, L.; Xu, J.; Wang, J.; Wang, R.M.Y.; Leung, K.S.; Chow, S.K.H.; et al. Vibration and beta-hydroxy-beta-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J. Cachexia Sarcopenia Muscle 2020, 11, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Nakae, Y.; Hirasaka, K.; Goto, J.; Nikawa, T.; Shono, M.; Yoshida, M.; Stoward, P.J. Subcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: A quantitative histological, immunohistochemical and electrophysiological study. Histochem. Cell Biol. 2008, 129, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumaran, V.; Arulmathi, K.; Srividhya, R.; Kalaiselvi, P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp. Gerontol. 2008, 43, 176–183. [Google Scholar] [CrossRef]
- Si, H.; Wang, X.; Zhang, L.; Parnell, L.D.; Admed, B.; LeRoith, T.; Ansah, T.A.; Zhang, L.; Li, J.; Ordovás, J.M.; et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 2019, 33, 965–977. [Google Scholar] [CrossRef]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Liu, H.W.; Wei, C.C.; Chen, Y.J.; Chen, Y.A.; Chang, S.J. Flavanol-rich lychee fruit extract alleviates diet-induced insulin resistance via suppressing mTOR/SREBP-1 mediated lipogenesis in liver and restoring insulin signaling in skeletal muscle. Mol. Nutr. Food Res. 2016, 60, 2288–2296. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, Y.T.; Liu, H.W.; Chan, Y.C.; Liu, M.Y.; Hu, S.H.; Tseng, W.T.; Wu, H.L.; Wang, M.F.; Chang, S.J. Oligonol alleviates sarcopenia by regulation of signaling pathways involved in protein turnover and mitochondrial quality. Mol. Nutr. Food Res. 2019, 63, e1801102. [Google Scholar] [CrossRef]
- Beekmann, K.; de Haan, L.H.; Actis-Goretta, L.; Houtman, R.; van Bladeren, P.J.; Rietjens, I.M. The effect of glucuronidation on isoflavone induced estrogen receptor (ER) α and ERβ mediated coregulator interactions. J. Steroid Biochem. Mol. Biol. 2015, 154, 245–253. [Google Scholar] [CrossRef]
- Kurrat, A.; Blei, T.; Kluxen, F.M.; Mueller, D.R.; Piechotta, M.; Soukup, S.T.; Kulling, S.E.; Diel, P. Lifelong exposure to dietary isoflavones reduces risk of obesity in ovariectomized Wistar rats. Mol. Nutr. Food Res. 2015, 59, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kohno, S.; Yama, T.; Ochi, A.; Suto, T.; Hirasaka, K.; Ohno, A.; Teshima-Kondo, S.; Okumira, Y.; Oarada, M.; et al. Soy glycinin contains a functional inhibitory sequence against muscle-atrophy-associated ubiquitin ligase Cbl-b. Int. J. Endocrinol. 2013, 2013, 907565. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Aizawa, M.; Kinoshita, M.; Ito, Y.; Kawamura, Y.; Takebe, M.; Pan, W.; Sakuma, K. The influence of isoflavone for denervation-induced muscle atrophy. Eur. J. Nutr. 2018, 58, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Nakao, R.; Yamamoto, H.; Nikawa, T.; Takeda, E.; Terao, J. Quercetin prevents unloading-derived disused muscle atrophy by attenuating the induction of ubiquitin ligases in tail-suspension mice. J. Nat. Prod. 2010, 73, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, J.A.; Xu, J.; Cao, J.; Wang, Y.; Thomas, S.S.; Hu, Z. Suppression of muscle wasting by the plant-derived compound ursolic acid in a model of chronic kidney disease. J. Cachexia Sarcopenia Muscle 2017, 8, 327–341. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Faramarzi, M.; Hedayati, M.; Ghaffari, M. The effect of resistance and endurance training with ursolic acid on atrophy-related biomarkers in muscle tissue of diabetic male rats induced by streptozotocin and a high-fat diet. J. Food Biochem. 2022, 46, e14202. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, S.Y.; Kim, C.M.; Kim, N.D.; Choe, S.; Lee, C.H.; Shin, J.H. Effect of loquat leaf extract on muscle strength, muscle mass, and muscle function in healthy adults: A randomized, double-blinded, and placebo-controlled trial. Evid.-Based Complement. Altern. Med. 2016, 2016, 4301621. [Google Scholar] [CrossRef]
- Bang, H.S.; Seo, D.Y.; Chung, Y.M.; Oh, K.M.; Park, J.J.; Arturo, F.; Jeong, S.H.; Kim, N.; Han, J. Ursolic Acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J. Physiol. Pharmacol. 2014, 18, 441–446. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef]
- Otsuka, Y.; Iidaka, T.; Horii, C.; Muraki, S.; Oka, H.; Nakamura, K.; Izumo, T.; Rogi, T.; Shibata, H.; Tanaka, S.; et al. Dietary intake of vitamin E and fats associated with sarcopenia in community-dwelling older Japanese people: A cross-sectional study from the fifty survey of the ROAD study. Nutrients 2021, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Hertfordshire Cohort Study Group. Diet and its relationship with grip strength in community-dwelling older men and women: The Hertfordshire cohort study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 23–29. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Annweiler, C.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Vitamin D-related changes in physical performance: A systemic review. J. Nutr. Health Aging 2009, 13, 893–898. [Google Scholar] [CrossRef]
- Wicherts, I.S.; can Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Triantafyllidis, K.K.; Kechagias, K.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopnie Muscle 2022, 13, 1642–1652. [Google Scholar] [CrossRef]
- Lee, S.J. Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 2004, 20, 61–86. [Google Scholar] [CrossRef]
- Wang, D.T.; Yang, Y.J.; Huang, R.H.; Zhang, Z.H.; Lin, X. Myostatin activates the ubiquitin-proteasome and autophagy-lysosome systems contributing to muscle wasting in chronic kidney disease. Oxidative Med. Cell. Longev. 2015, 2015, 684965. [Google Scholar] [CrossRef]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.E.; Hsu, M.; Conboy, I.M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 2008, 454, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.T.; Chee, A.; Gleeson, B.G.; Naim, T.; Swiderski, K.; Koopman, R.; Lynch, G.S. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R716–R726. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; McMillan, H.J.; Mah, J.K.; Tarnopolsky, N.; Selby, K.; McClure, T.; Wilson, D.M.; Sherman, M.L.; Escolar, D.; Attie, K.M. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve 2017, 55, 458–464. [Google Scholar] [CrossRef]
- Woodhouse, L.; Gandhi, R.; Warden, S.J.; Poiraudeau, S.; Myers, S.L.; Benson, C.T.; Hu, L.; Ahmad, Q.I.; Linnemeier, P.; Gomez, E.V.; et al. A phase 2 randomized study investigating the efficacy and safety of myostatin antibody LY2495655 versus placebo in patients undergoing elective total hip arthroplasty. J. Frailty Aging 2016, 5, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
- Lee, S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, T.; et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef]
- Sinha, M.; Jang, Y.C.; Oh, J.; Khong, D.; Wu, E.Y.; Manohar, R.; Miller, C.; Regalado, S.G.; Loffredo, F.S.; Pancoast, J.R.; et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 2014, 344, 649–652. [Google Scholar] [CrossRef]
- Schafer, M.J.; Atkinson, E.J.; Vanderboom, P.M.; Kotajarvi, B.; White, T.A.; Moore, M.M.; Bruce, C.J.; Greason, K.L.; Suri, R.M.; Khosla, S.; et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016, 23, 1207–1215. [Google Scholar] [CrossRef]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longituidinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Lehtihet, M.; Hylander, B. Semen quality in men with chronic kidney disease and its correlation with chronic kidney disease stages. Andrologia 2015, 47, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Calof, O.; Storer, T.W.; Lee, M.L.; Mazer, N.A.; Jasuja, R.; Montori, V.M.; Gao, W.; Dalton, J.T. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for physical dysfunction in chronic illness and ageing. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, V.; Elliott, M.; Gentili, A.; Godschalk, M.; Mulligan, T. Testosterone improves rehabilitation outcomes in ill older men. J. Am. Geriatr. Soc. 2000, 48, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E601–E607. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef]
- Crawford, J.; Prado, C.M.M.; Ann Johnston, M.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER Trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef]
- Dalton, J.T.; Barnette, K.G.; Bohl, C.E.; Hancock, M.L.; Rodriguez, D.; Dodson, S.T.; Morton, R.A.; Steiner, M.S. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2011, 2, 153–161. [Google Scholar] [CrossRef]
- Neil, D.; Clark, R.V.; Magee, M.; Billiard, J.; Chan, A.; Xue, Z.; Russell, A. GSK2881078, a SARM, produces dose-dependent increases in lean mass in healthy older men and women. J. Clin. Endocrinol. Metab. 2018, 103, 3215–3224. [Google Scholar] [CrossRef]
- Supasyndh, O.; Satirapoj, B.; Aramwit, P.; Viroonudomphol, D.; Chaiprasert, A.; Thanachatwej, V.; Vanichakarn, S.; Kopple, J.D. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 271–279. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ku, S.K.; Lee, K.W.; Song, C.H.; An, W.G. Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018, 212, 175–187. [Google Scholar] [CrossRef]
- Kristensen, J.M.; Treebak, J.T.; Schjerling, P.; Goodyear, L.; Wojtaszewski, J.F. Two weeks of metformin treatment induces AMPK-dependent enhancement of insulin-stimulated glucose uptake in mouse soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1099–E1109. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Suwa, M.; Egashira, T.; Nakano, H.; Sasaki, H.; Kumagai, S. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J. Appl. Physiol. 2006, 101, 1685–1692. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Brutsaert, E.F.; Anghel, V.; Zhang, K.; Bloomgarden, N.; Pollak, M.; Mar, J.C.; Hawkins, M.; Crandall, J.P.; Barzilai, N. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 2018, 17, e12723. [Google Scholar] [CrossRef]
- Laksmi, P.W.; Setiati, S.; Tamin, T.Z.; Soewondo, P.; Rochmah, W.; Nafrialdi, N.; Prihartono, J. Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: A double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Medica Indones. 2017, 49, 118–127. [Google Scholar]
- Rabøl, R.; Boushel, R.; Almdal, T.; Hansen, C.N.; Ploug, T.; Haugaard, S.B.; Prats, C.; Madsbad, S.; Dela, F. Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes. Metab. 2010, 12, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Coletta, D.K.; Sriwijitkamol, A.; Wajcberg, E.; Tantiwong, P.; Li, M.; Prentki, M.; Madiraju, M.; Jenkinson, C.P.; Cersosimo, E.; Musi, N.; et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: A randomised trial. Diabetologia 2009, 52, 723–732. [Google Scholar] [CrossRef]
- Shea, M.K.; Nicklas, B.J.; Marsh, A.P.; Houston, D.K.; Miller, G.D.; Isom, S.; Miller, M.E.; Carr, J.J.; Lyles, M.F.; Harris, T.B.; et al. The effect of pioglitazone and resistance training on body composition in older men and women undergoing hypocaloric weight loss. Obesity 2011, 19, 1636–1646. [Google Scholar] [CrossRef]
- Mele, A.; Calzolaro, S.; Cannone, G.; Cetrone, M.; Conte, D.; Tricarico, D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol. Res. Perspect. 2014, 2, e00028. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Barbieri, M.; Fava, I.; Desiderio, M.; Coppola, C.; Marfella, R.; Paolisso, G. Sarcopenia in elderly diabetic patients: Role of dipeptidyl peptidase 4 inhibitors. J. Am. Med. Dir. Assoc. 2016, 17, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Nagai, Y.; Kato, H.; Fukuda, H.; Tanaka, Y. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin on muscle mass and the muscle/fat ratio in patients with type 2 diabetes. J. Clin. Med. Res. 2020, 12, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Giannocco, G.; Oliveira, K.C.; Crajoinas, R.O.; Venturini, G.; Salles, T.A.; Fonseca-Alaniz, M.H.; Maciel, R.M.; Girardi, A.C. Dipeptidyl peptidase IV inhibition upregulates GLUT4 translocation and expression in heart and skeletal muscle of spontaneously hypertensive rats. Eur. J. Pharmacol. 2013, 698, 74–86. [Google Scholar] [CrossRef]
- Sato, H.; Kubota, N.; Kubota, T.; Takamoto, I.; Iwayama, K.; Tokuyama, K.; Moroi, M.; Sugi, K.; Nakaya, K.; Goto, M.; et al. Anagliptin increases insulin-induced skeletal muscle glucose uptake via an NO-dependent mechanism in mice. Diabetologia 2016, 59, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Masaki, Y.; Kinugawa, S.; Matsumoto, J.; Furihata, T.; Mizushima, W.; Kadoguchi, T.; Fukushima, A.; Homma, T.; Takahashi, M.; et al. Dipeptidyl peptidase-4 inhibitor improved exercise capacity and mitochondrial biogenesis in mice with heart failure via activation of glucagon-like peptide-1 receptor signalling. Cardiovasc. Res. 2016, 111, 338–347. [Google Scholar] [CrossRef]
- Green, C.J.; Henriksen, T.I.; Pedersen, B.K.; Solomon, T.P. Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS ONE 2012, 7, e44284. [Google Scholar] [CrossRef] [PubMed]
- Choung, J.S.; Lee, Y.S.; Jun, H.S. Exendin-4 increases oxygen consumption and thermogenic gene expression in muscle cells. J. Mol. Endocrinol. 2017, 58, 79–90. [Google Scholar] [CrossRef]
- Yajima, T.; Yajima, K.; Takahashi, H.; Yasuda, K. The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis. J. Diabetes Complicat. 2018, 32, 759–763. [Google Scholar] [CrossRef]
- Perna, S.; Guido, D.; Bologna, C.; Solerte, S.B.; Guerriero, F.; Isu, A.; Rondanelli, M. Liraglutide and obesity in elderly: Efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin. Exp. Res. 2016, 28, 1251–1257. [Google Scholar] [CrossRef]
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Investig. 2014, 124, 509–514. [Google Scholar] [CrossRef]
- Esterline, R.L.; Vaag, A.; Oscarsson, J.; Vora, J. Mechanisms in endocrinology: SGLT2 inhibitors: Clinical benefits by restoration of normal diurnal metabolism? Eur. J. Endocrinol. 2018, 178, R113–R125. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J. Diabetes Investig. 2019, 10, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Sakai, K.; Saito, K.; Tsutsui, K.; Yamashita, S.; Kato, N. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes receiving conventional therapy: Clinical implication of the importance of exercise habits during treatment with ipragliflozin. Diabetol. Int. 2017, 8, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, R.; Matsuba, I.; Shimokawa, M.; Nagai, Y.; Tanaka, Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes. Metab. 2018, 20, 1311–1315. [Google Scholar] [CrossRef]
- Sano, M.; Meguro, S.; Kawai, T.; Suzuki, Y. Increased grip strength with sodium-glucose cotransporter 2. J. Diabetes 2016, 8, 736–737. [Google Scholar] [CrossRef]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif. Tissue Int. 2017, 101, 1–8. [Google Scholar] [CrossRef]
- Ferrari, U.; Then, C.; Rottenkolber, M.; Selte, C.; Seissler, J.; Conzade, R.; Linkohr, B.; Peters, A.; Drey, M.; Thorand, B. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-Age study. Acta Diabetol. 2020, 57, 1057–1063. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fykuda, M. Sodium–glucose cotransporter 2 inhibitor induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Madushika Abeywickrama, H.; Uchiyama, M.; Sumiyoshi, T.; Okuda, A.; Koyama, Y. The role of zinc on nutritional status, sarcopenia, and frailty in older adults: A scoping review. Nutr. Rev. 2023; in press. [Google Scholar] [CrossRef]

| Title 1 | Duration, Intervention Pattern | Species | Outcome | Authors |
|---|---|---|---|---|
| HMB | 3 g, 10 days | Human, unloading patients | Muscle strength ↑ | Deutz et al. [19] |
| 2 to 4 weeks | Human | Anthropometric parameters → | Hsieh et al. [20] | |
| RCT, HMB | Human, elderly | LBM ↑, fat mass → Muscle strength → | Flakoll et al. [21] | |
| HMB | RCT, HMB + Arg + Lys (2/5/1.5 g per day) | Human, elderly | LBM ↑, fat mass → Whole body protein synthesis↑ Leg and grip strength ↑ Limb circumference ↑ Muscle strength → | Flakoll et al. [21] |
| HMB + vibration | RCT, 12–16 Hz, 3–5 mm, 8 weeks | Human, sarcopenia | Muscle strength ↑, Physical performance (walking speed, 5-stance test, timed stand test) ↑ | Zhu et al. [23] |
| LMHFV | Ageing mice (SAMP8) | Myostatin expression ↓ Intramuscular fat mass ↓ | Wang et al. [24] | |
| Catechin | EGCG, 5 mg/kg, 4 times/week, 8 weeks | Muscular dystrophic mice | fibrosis ↓, necrotic fibers ↓ | Nakae et al. [25] |
| EGCG, 100 mg/kg, 30 days | Aged male rats | Oxidative stress marker ↓ | Senthil Kumaran et al. [26] | |
| EC or EGCG (0.25% in drinking water), 37 weeks | 20-month old male mice | Survival rate: EC ↑, EGCG → Muscle degeneration ↓: EC >> EGCG Physical activity ↑: EC >> EGCG | Si et al. [27] | |
| Oligonol, 200 mg/kg, 8 weeks | SAMP8 mice, 32-weeks old | PGC-1α and Mfn2 ↓ LC3-II, p62, and ATG13 ↑ Autophagosome number ↑ | Chang et al. [30] | |
| Catechin + resistance training | RCT, tea catechin (350 mL/day), 3 month | Human, sarcopenia, women | Leg muscle mass ↑ Normal walking speed ↑ | Mafi et al. [28] |
| 120 days | Male mice | Fat accumulation ↓ | Kurrat et al. [32] | |
| 20% of the diet, 4 days | Mouse, denervated | IRS-1 and p-Akt protein ↑ | Abe et al. [33] | |
| Isoflavones | 0.6% of the diet, 2 weeks daidzein/genistein/ glycitein (7:1:2) | Mouse, denervated | Apoptosis-dependent signaling ↓ | Tabata et al. [34] |
| Muscular injection (50 μL; 2.5 pmol of quercetin) | Hindlimb-unloaded mice | Skeletal muscle mass ↑ Atrogin-1 and MuRF1 ↓ | Mukai et al. [35] | |
| Ursolic acid | Orally treatment (100 mg/kg), 3 weeks | Mice models of CKD | Muscle mass ↑ Inflammatory cytokines (IL-6 and TNF-α) ↓ Ubiquitin E3 ligases (MuRF1, atrogin-1, MUSA1) ↓ | Yu et al. [37] |
| 50 mg/kg, loquat leaf extract intake | Human (healthy adults) | Muscle mass and muscle strength → | Cho et al. [39] | |
| Ursolic acid + endurance training | 500 mg/kg, 8 weeks | 21-month old male rats with diabetes by high fat diet | Body weight ↓ Insulin resistance ↓ Insulin and glucose concentration ↓ p53, ATF4, and p21 protein level ↓ | Zolfaghari et al. [38] |
| Ursolic acid + resistance training | 1 capsule (450 mg), 3 times/day, 8 weeks | Healthy male human (age: 29.4 ± 5.1 years) | Muscle strength ↑ Lean body mass → | Bang et al. [40] |
| 1.86 g EPA and 1.5 g DHA/day, 6 months | Human (60-85 year men and women, elderly) | Muscle protein synthesis ↑ | Smith et al. [43] | |
| Omega-3 PUFAs | RCT, EPA and DHA, 8 weeks | Middle-aged and older adults | TNF-α, IL-6, and IL-1β ↓ | Tan et al. [45] |
| RCT, 8 weeks | Healthy older men and women (>65 years) | Muscle protein synthesis → | Smith et al. [46] |
| Title 1 | Duration, Intervention Pattern | Species | Outcome | Authors |
|---|---|---|---|---|
| Vitamin D | Systematic review | Nursing home residents and older adults living in areas with low vitamin D | Risk of falls ↓ Muscle performance ↑ Muscle strength ↑ | Annweiler et al. [47] |
| Subcutaneous injection | CKD model mice | Body weight ↑ Muscle mass ↑ Inflammation marker ↓ | Zhang et al. [52] | |
| Myostatin inhibition | RCT, ACE-031, Cohort 1: 0.5 mg/kg every 4 weeks, Cohort 2: 1 mg/kg every 2 weeks | DMD patients | Muscle mass ↑ Performance (6 min walk test) ↑ Non-muscular side effects (nosebleeds, capillary dilation) ↑ | Campbell et al. [55] |
| RCT, LY2495655, 8 and 16 weeks | Patients undergoing elective total hip arthroplasty | LBM ↑ | Woodhouse et al. [56] | |
| Phase 2 trial, LY2495655, 24 weeks | Frail elderly (75 years and older) subjects worldwide (e.g., US, France, Australia) | LBM ↑ Functional characteristics (stair climbing time and chair standing with arms) ↑ | Becker et al. [57] | |
| GDF11 | IP injection, 0.1 mg/kg, 30 days | Aged (22–24 months) mice | Satellite cells ↑ Skeletal muscle regeneration ↑ Muscle physiological parameters (Run time and grip strength) ↑ PGC-1α ↑ | Sinha et al. [60] |
| Anabolic steroids | Testosterone, high dose | Community-dwelling older men, 6 months | LBM ↑ Leg and arm muscle strength ↑ | Sinha-Hikim et al. [67] |
| Double-blind study, SARMs (enobosarm) | Healthy postmenopausal elderly men | LBM ↑ Physical function (stair climbing speed) ↑ | Neil et al. [70] | |
| SARMs (GSK2881078), Once daily, 50 days | Healthy elderly men and women | LBM ↑ | Neil et al. [70] | |
| RCT, oxymetholone, 24-weeks | Hemodyalysis patients | LBM ↑ Physical function and grip strength ↑ CSA of Type I fibers ↑ | Supasyndh et al. [71] | |
| Oxymetholone, 50 mg/kg | 10-month-old mice | Myostatin, sirtuin1, Fiber size (soleus and gastrocnemius muscles) ↑ | Kim et al. [72] | |
| Metformin, 3 × 500 mg, 16 weeks | Elderly patients with diabetes | Walking speed ↑ | Laksmi et al. [77] | |
| Thiazolidinediones (pioglitazone) | Diabetic patients | Thigh muscle mass ↓ | Shea et al. [80] | |
| Sulfonylurea, 24 month | Elderly patients with diabetes | Skeletal muscle mass → Muscle strength → Gait speed → | Rizzo et al. [82] | |
| Glucose-lowering drugs | DPP-4i, 24 month | Elderly patients with diabetes | Skeletal muscle mass ↑ Muscle strength ↑ Gait speed ↑ | Rizzo et al. [82] |
| DPP-4i (tenelingliptin), 20 mg/day, 6 months | Diabetic patients undergoing hemodyalysis | Skeletal muscle mass → Fat mass → | Yajima et al. [89] | |
| GLP-1 RAs (dulaglutide), 0.75 mg/week, 6 months | Diabetic patients undergoing hemodyalysis | Skeletal muscle mass ↓ Fat mass ↓ | Yajima et al. [89] | |
| GLP-1 RAs (dulaglutide), 3 g/day, 6 months | Obese elderly patients with diabetes | Sarcopenic parameters → | Perna et al. [90] | |
| SGLT2i (depaglifrozin), 10 mg/day, 2 weeks | Diabetic patients | Insulin sensitivity ↑ Muscle catabolism ↓ | Merovci et al. [91] | |
| SGLT2i (luseoglifrozin), 2.5–5 mg/day, 1 year | Diabetic patients | Body weight ↓ Body mass index ↓ Waist circumference ↓ | Sasaki et al. [100] | |
| Insulin, 3 years | Diabetic patients | Skeletal muscle mass ↑ | Ferrari et al. [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, K.; Hamada, K.; Yamaguchi, A.; Aoi, W. Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia. Cells 2023, 12, 2422. https://doi.org/10.3390/cells12192422
Sakuma K, Hamada K, Yamaguchi A, Aoi W. Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia. Cells. 2023; 12(19):2422. https://doi.org/10.3390/cells12192422
Chicago/Turabian StyleSakuma, Kunihiro, Kento Hamada, Akihiko Yamaguchi, and Wataru Aoi. 2023. "Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia" Cells 12, no. 19: 2422. https://doi.org/10.3390/cells12192422






