Voclosporin: Unique Chemistry, Pharmacology and Toxicity Profile, and Possible Options for Implementation into the Management of Lupus Nephritis
Abstract
:1. Introduction
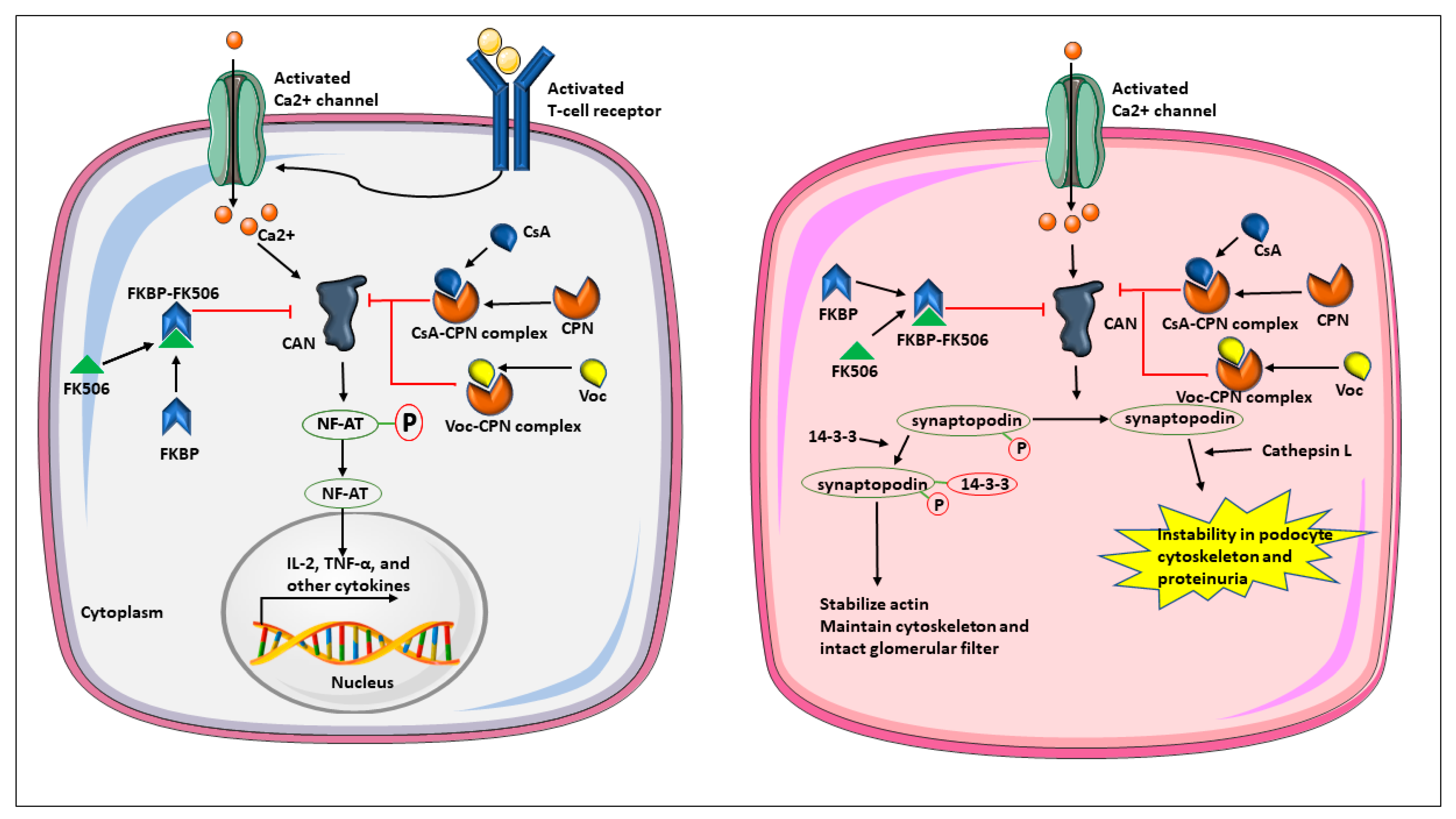
2. Calcineurin as a Molecular Drug Target
3. Chemistry of the Calcineurin Inhibitors
3.1. Cyclosporin
3.2. Tacrolimus
3.3. Voclosporin or ISA247
4. Pharmacology of the Calcineurin Inhibitors
4.1. Cyclosporine A (Sandimmune®, Neoral®)
4.2. Tacrolimus (Astagraf XL, Envarsus XR, Prograf, and Protopic)
4.3. Voclosporin (Lupkynis™)
5. Experience with Calcineurin Inhibitors in Autoimmune Glomerulonephritis and Podocytopathies
5.1. Preclinical Data of Voclosporin and the Rationale to Develop Voclosporin for Lupus Nephritis
5.2. Clinical Efficacy Data of Voclosporin in Lupus Nephritis
5.3. Clinical Safety Data of Voclosporin in Lupus Nephritis
5.4. How to Integrate Voclosporin into the Treatment Landscape of Lupus Nephritis
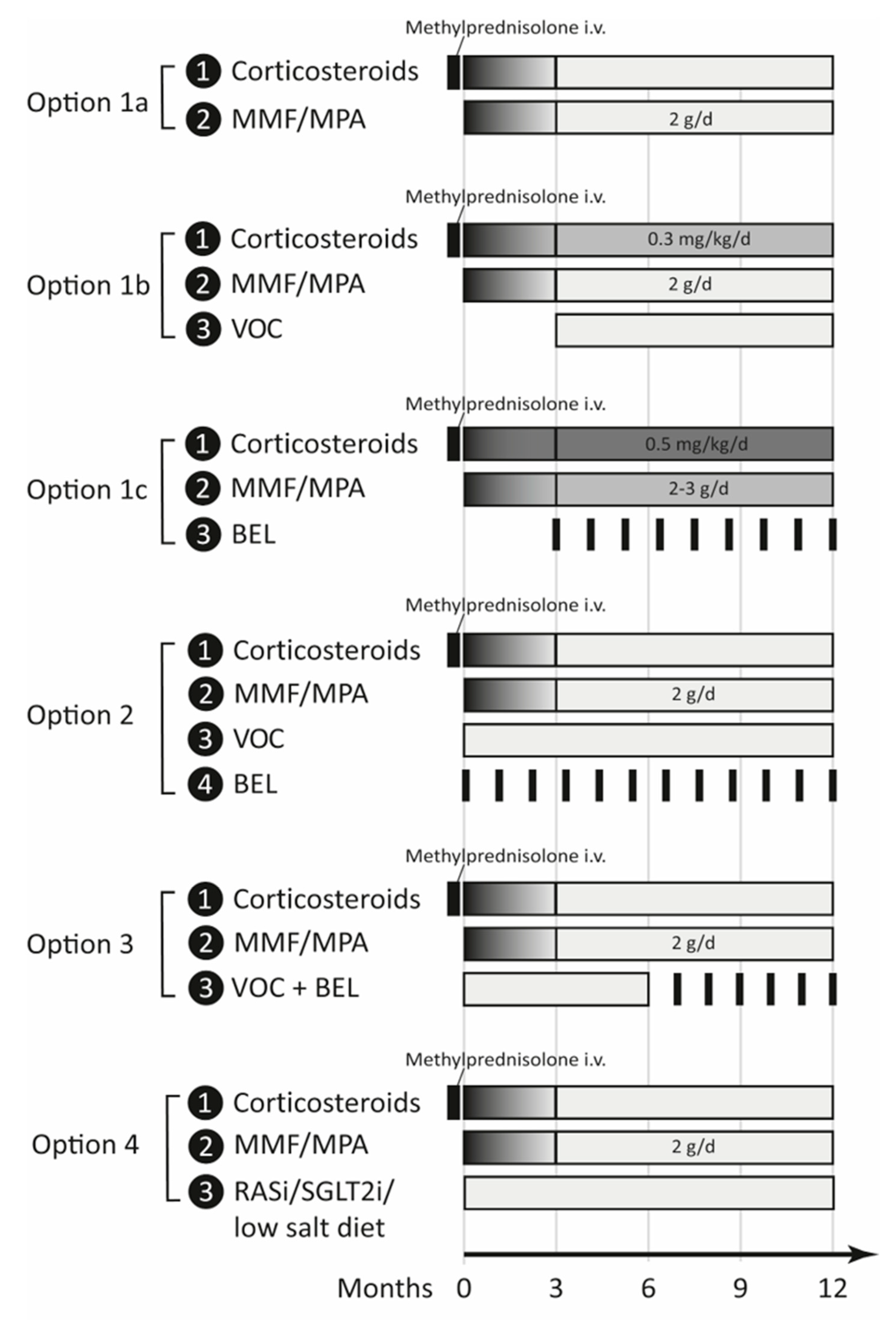
5.4.1. Option 1—Patient Selection
5.4.2. Option 2—Combination Therapy
5.4.3. Option 3—Sequential Therapy
5.4.4. Option 4—Other Antiproteinuric Drugs
5.5. Cost of Voclosporin Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engwenyu, L.R.; Anderson, A.S. A Comprehensive Review of Calcineurin Inhibitors Used for Immunosuppression in Cardiac Transplantation. Handb. Exp. Pharmacol. 2022, 272, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Safarini, O.A.; Keshavamurthy, C.; Patel, P. Calcineurin Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Peleg, Y.; Bomback, A.S.; Radhakrishnan, J. The Evolving Role of Calcineurin Inhibitors in Treating Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2020, 15, 1066–1072. [Google Scholar] [CrossRef]
- Malakasioti, G.; Iancu, D.; Tullus, K. Calcineurin inhibitors in nephrotic syndrome secondary to podocyte gene mutations: A systematic review. Pediatr. Nephrol. 2021, 36, 1353–1364. [Google Scholar] [CrossRef]
- Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef]
- Wu, Q.; Kuca, K. Metabolic Pathway of Cyclosporine A and Its Correlation with Nephrotoxicity. Curr. Drug Metab. 2019, 20, 84–90. [Google Scholar] [CrossRef]
- Ong, S.C.; Gaston, R.S. Thirty Years of Tacrolimus in Clinical Practice. Transplantation 2021, 105, 484–495. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, T.; Lerma, E. Voclosporin: A novel calcineurin inhibitor for the treatment of lupus nephritis. Expert Rev. Clin. Pharmacol. 2022, 15, 515–529. [Google Scholar] [CrossRef]
- Heo, Y.A. Voclosporin: First Approval. Drugs 2021, 81, 605–610. [Google Scholar] [CrossRef]
- Li, W.; Shrivastava, M.; Lu, H.; Jiang, Y. Calcium-calcineurin signaling pathway in Candida albicans: A potential drug target. Microbiol. Res. 2021, 249, 126786. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.P. Calcineurin. Cell Commun. Signal. 2020, 18, 137. [Google Scholar] [CrossRef]
- Ronan, P.J.; Flynn, S.A.; Beresford, T.P. Calcineurin signaling as a target for the treatment of alcohol abuse and neuroinflammatory disorders. Prog. Mol. Biol. Transl. Sci. 2019, 167, 125–142. [Google Scholar] [CrossRef]
- Ponticelli, C.; Reggiani, F.; Moroni, G. Old and New Calcineurin Inhibitors in Lupus Nephritis. J. Clin. Med. 2021, 10, 4832. [Google Scholar] [CrossRef]
- Reiser, J.; Sever, S. Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med. 2013, 64, 357–366. [Google Scholar] [CrossRef]
- Garg, P. A Review of Podocyte Biology. Am. J. Nephrol. 2018, 47 (Suppl. S1), 3–13. [Google Scholar] [CrossRef]
- Kopp, J.B.; Anders, H.J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, A.; Fukusumi, Y.; Hasegawa, E.; Tomita, M.; Watanabe, T.; Narita, I.; Kawachi, H. Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm. Physiol. Rep. 2016, 4, 12679. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, H.; Ying, M.; Xie, Z.; Li, X.; Wang, H.; Zhao, J.; Lin, C.; Wang, Y.; Feng, S.; et al. Calcineurin inhibitors cyclosporin A and tacrolimus protect against podocyte injury induced by puromycin aminonucleoside in rodent models. Sci. Rep. 2016, 6, 32087. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Lin, C.; Weng, C.; Wang, Y.; Feng, S.; Wang, C.; Shao, X.; Lin, W.; Li, B.; et al. Calcineurin inhibitors ameliorate PAN-induced podocyte injury through the NFAT-Angptl4 pathway. J. Pathol. 2020, 252, 227–238. [Google Scholar] [CrossRef]
- Ahlbach, C.L.; Lexa, K.W.; Bockus, A.T.; Chen, V.; Crews, P.; Jacobson, M.P.; Lokey, R.S. Beyond cyclosporine A: Conformation-dependent passive membrane permeabilities of cyclic peptide natural products. Future Med. Chem. 2015, 7, 2121–2130. [Google Scholar] [CrossRef]
- Kazmi, S.; Mujeeb, A.A.; Owais, M. Cyclic undecapeptide Cyclosporin A mediated inhibition of amyloid synthesis: Implications in alleviation of amyloid induced neurotoxicity. Sci. Rep. 2018, 8, 17283. [Google Scholar] [CrossRef]
- Wenger, R.M. Synthesis of ciclosporin and analogues: Structural and conformational requirements for immunosuppressive activity. Prog. Allergy 1986, 38, 46–64. [Google Scholar]
- Corbett, K.M.; Ford, L.; Warren, D.B.; Pouton, C.W.; Chalmers, D.K. Cyclosporin Structure and Permeability: From A to Z and Beyond. J. Med. Chem. 2021, 64, 13131–13151. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Swedberg, J.E.; Harvey, P.J.; Kaas, Q.; Craik, D.J. Conformational Flexibility Is a Determinant of Permeability for Cyclosporin. J. Phys. Chem. B 2018, 122, 2261–2276. [Google Scholar] [CrossRef] [PubMed]
- Kailasam, V.; Cheruvu, S.S.; Malani, M.; Kameswari, S.M.S.; Kesharwani, P.; Nirmal, J. Recent advances in novel formulation approaches for tacrolimus delivery in treatment of various ocular diseases. J. Drug Deliv. Sci. Technol. 2022, 78, 103945. [Google Scholar] [CrossRef]
- Solionov, D.S.; Poshekhontseva, V.Y.; Fokina, V.V.; Shutov, A.A.; Nokilaeva, V.M.; Vasiarov, G.G.; Titova, E.V.; Karasev, V.S.; Staroverov, S.M.; Donova, M.V. Biosynthesis of Tacrolimus by the Streptomyces tsukubensis VKM Ac-2618D Strain in the Presence of Polymeric Sorbents and Development of a Method for Its Isolation and Purification. Appl. Biochem. Microbiol. 2020, 56, 699–707. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Colombo, D.; De Mieri, M.; Grisenti, P. Evaluation, synthesis and characterization of tacrolimus impurities. J. Antibiot. 2012, 65, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Skytte, D.M.; Frydenvang, K.; Hansen, L.; Nielsen, P.G.; Jaroszewski, J.W. Synthesis and characterization of an epimer of tacrolimus, an immunosuppressive drug. J. Nat. Prod. 2010, 73, 776–779. [Google Scholar] [CrossRef]
- Kuglstatter, A.; Mueller, F.; Kusznir, E.; Gsell, B.; Stihle, M.; Thoma, R.; Benz, J.; Aspeslet, L.; Freitag, D.; Hennig, M. Structural basis for the cyclophilin A binding affinity and immunosuppressive potency of E-ISA247 (voclosporin). Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 119–123. [Google Scholar] [CrossRef]
- Roesel, M.; Tappeiner, C.; Heiligenhaus, A.; Heinz, C. Oral voclosporin: Novel calcineurin inhibitor for treatment of noninfectious uveitis. Clin. Ophthalmol. 2011, 5, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.J. ISAtx-247 (Isotechnika/Roche). Curr. Opin. Investig. Drugs 2004, 5, 542–550. [Google Scholar]
- Wang, C.P.; Burckart, G.J.; Venkataramanan, R.; Ptachcinski, R.J.; Cuellar, R.E.; Makowka, L.; Van Thiel, D.H.; Starzl, T.E. Cyclosporine Metabolite Profiles in the Blood of Liver Transplant Patients. Transplant. Proc. 1988, 20, 173–175. [Google Scholar]
- Kempkes-Koch, M.; Fobker, M.; Erren, M.; August, C.; Gerhardt, U.; Suwelack, B.; Hohage, H. Cyclosporine A metabolite AM19 as a potential biomarker in urine for CSA nephropathy. Transplant. Proc. 2001, 33, 2167–2169. [Google Scholar] [CrossRef]
- Ling, S.Y.; Huizinga, R.B.; Mayo, P.R.; Freitag, D.G.; Aspeslet, L.J.; Foster, R.T. Pharmacokinetics of voclosporin in renal impairment and hepatic impairment. J. Clin. Pharmacol. 2013, 53, 1303–1312. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Kuca, K.; Wu, W. Cyclosporine A: Chemistry and Toxicity—A Review. Curr. Med. Chem. 2021, 28, 3925–3934. [Google Scholar] [CrossRef]
- Morris, R.G. Cyclosporin therapeutic drug monitoring—An established service revisited. Clin. Biochem. Rev. 2003, 24, 33–46. [Google Scholar] [PubMed]
- Han, K.; Pillai, V.C.; Venkataramanan, R. Population pharmacokinetics of cyclosporine in transplant recipients. AAPS J. 2013, 15, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, P.; Zhang, Z.; Wang, Z.; Liu, L.; Liu, X. Prediction of Cyclosporin-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Model Characterizing Interplay of Drug Transporters and Enzymes. Int. J. Mol. Sci. 2020, 21, 7023. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Wang, Y.; Yang, H.; Kuča, K. Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophagy, and signalings. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 118, 889–907. [Google Scholar] [CrossRef]
- Flores, C.; Fouquet, G.; Moura, I.C.; Maciel, T.T.; Hermine, O. Lessons to Learn From Low-Dose Cyclosporin-A: A New Approach for Unexpected Clinical Applications. Front. Immunol. 2019, 10, 588. [Google Scholar] [CrossRef]
- Webb, N.J.A.; Baumann, U.; Camino, M.; Frauca, E.; Undre, N. Pharmacokinetics of tacrolimus granules in pediatric de novo liver, kidney, and heart transplantation: The OPTION study. Pediatr. Transplant. 2019, 23, e13328. [Google Scholar] [CrossRef] [PubMed]
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 2015, Cd009864. [Google Scholar] [CrossRef]
- Schutte-Nutgen, K.; Tholking, G.; Suwelack, B.; Reuter, S. Tacrolimus—Pharmacokinetic Considerations for Clinicians. Curr. Drug Metab. 2018, 19, 342–350. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Francke, M.I.; Hesselink, D.A.; Li, Y.; Koch, B.C.P.; de Wit, L.E.A.; van Schaik, R.H.N.; Yang, L.; Baan, C.C.; van Gelder, T.; de Winter, B.C.M. Monitoring the tacrolimus concentration in peripheral blood mononuclear cells of kidney transplant recipients. Br. J. Clin. Pharmacol. 2021, 87, 1918–1929. [Google Scholar] [CrossRef]
- Van Zyl, J.S.; Sam, T. De novo tacrolimus extended-release tablets (LCPT) versus twice-daily tacrolimus in adult heart transplantation: Results of a single-center non-inferiority matched control trial. Clin. Transplant. 2021, 35, e14487. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, N.; Kim, M.G.; Yun, H.-Y.; Lee, S.; Bae, E.; Kim, Y.S.; Kim, I.-W.; Oh, J.M. Increased Exposure of Tacrolimus by Co-administered Mycophenolate Mofetil: Population Pharmacokinetic Analysis in Healthy Volunteers. Sci. Rep. 2018, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Mendez, R.; Gonwa, T.; Yang, H.C.; Weinstein, S.; Jensik, S.; Steinberg, S. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: Results at 1 year. Transplantation 2005, 80, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, G.; Tan, L.; Li, J. Current progress of tacrolimus dosing in solid organ transplant recipients: Pharmacogenetic considerations. Biomed. Pharmacother. 2018, 102, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, T. Drug interactions with tacrolimus. Drug Saf. 2002, 25, 707–712. [Google Scholar] [CrossRef]
- Shrestha, B.M. Two Decades of Tacrolimus in Renal Transplant: Basic Science and Clinical Evidences. Exp. Clin. Transplant. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S.; et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef]
- Sin, F.E.; Isenberg, D. An evaluation of voclosporin for the treatment of lupus nephritis. Expert Opin. Pharmacother. 2018, 19, 1613–1621. [Google Scholar] [CrossRef]
- van Gelder, T.; Huizinga, R.B.; Lisk, L.; Solomons, N. Voclosporin: A novel calcineurin inhibitor with no impact on mycophenolic acid levels in patients with SLE. Nephrol. Dial. Transplant. 2022, 37, 917–922. [Google Scholar] [CrossRef]
- Bissonnette, R.; Papp, K.; Poulin, Y.; Lauzon, G.; Aspeslet, L.; Huizinga, R.; Mayo, P.; Foster, R.T.; Yatscoff, R.W.; Maksymowych, W.P. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J. Am. Acad. Dermatol. 2006, 54, 472–478. [Google Scholar] [CrossRef]
- Busque, S.; Cantarovich, M.; Mulgaonkar, S.; Gaston, R.; Gaber, A.O.; Mayo, P.R.; Ling, S.; Huizinga, R.B.; Meier-Kriesche, H.U. The PROMISE study: A phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am. J. Transplant. 2011, 11, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. Suppl. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Anders, H.J.; Kitching, A.R.; Leung, N.; Romagnani, P. Glomerulonephritis: Immunopathogenesis and immunotherapy. Nat. Rev. Immunol. 2023, 23, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Ronco, P.; Beck, L.; Debiec, H.; Fervenza, F.C.; Hou, F.F.; Jha, V.; Sethi, S.; Tong, A.; Vivarelli, M.; Wetzels, J. Membranous nephropathy. Nat. Rev. Dis. Primers 2021, 7, 69. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Jefferson, J.A.; Gipson, P.E.; Rizk, D.V.; Sedor, J.R.; et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 36–46. [Google Scholar] [CrossRef]
- Fernández-Juárez, G.; Rojas-Rivera, J.; Logt, A.V.; Justino, J.; Sevillano, A.; Caravaca-Fontán, F.; Ávila, A.; Rabasco, C.; Cabello, V.; Varela, A.; et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021, 99, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Ho, L.Y.; Ying, S.K.Y.; Leung, M.C.; To, C.H.; Ng, W.L. Long-term outcome of a randomised controlled trial comparing tacrolimus with mycophenolate mofetil as induction therapy for active lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Ying, K.Y.; Yim, C.W.; Siu, Y.P.; Tong, K.H.; To, C.H.; Ng, W.L. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: A randomised controlled trial and long-term follow-up. Ann. Rheum. Dis. 2016, 75, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; de Souza, R.G.; Yu, Z.; Stern, M.E.; de Paiva, C.S.; Pflugfelder, S.C. Calcineurin Inhibitor Voclosporin Preserves Corneal Barrier and Conjunctival Goblet Cells in Experimental Dry Eye. J. Ocul. Pharmacol. Ther. 2020, 36, 679–685. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Austin, B.A.; Li, Z.; Liu, B.; Yeh, S.; Chan, C.C.; Anglade, E.; Velagaleti, P.; Nussenblatt, R.B. LX211 (voclosporin) suppresses experimental uveitis and inhibits human T cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Liu, Q.; Zheng, Z.; Fan, J.; Peng, W.; Kong, Q.; He, H.; Yang, S.; Chen, W.; Tang, X.; et al. Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS ONE 2015, 10, e0132724. [Google Scholar] [CrossRef]
- Yamamoto, K.; Mori, A.; Nakahama, T.; Ito, M.; Okudaira, H.; Miyamoto, T. Experimental treatment of autoimmune MRL-lpr/lpr mice with immunosuppressive compound FK506. Immunology 1990, 69, 222–227. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J.; et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 713–723. [Google Scholar] [CrossRef]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Solomons, N.; Pendergraft, W.F., 3rd; Dooley, M.A.; Tumlin, J.; Romero-Diaz, J.; Lysenko, L.; Navarra, S.V.; Huizinga, R.B. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019, 95, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Federico, R.; Birardi, V.; Leher, H. Voclosporin Is Effective in Achieving Proteinuria Treatment Targets in Lupus Nephritis Defined by EULAR/ERA Recommendations. Arthritis Rheumatol. 2022, 74, 708–710. [Google Scholar] [CrossRef]
- Anders, H.J.; Loutan, J.; Bruchfeld, A.; Juarez, G.M.F.; Floege, J.; Goumenos, D.; Turkmen, K.; van Kooten, C.; Frangou, E.; Stevens, K.; et al. The management of lupus nephritis as proposed by EULAR/ERA 2019 versus KDIGO 2021. Nephrol. Dial. Transplant. 2021, 38, 551–561. [Google Scholar] [CrossRef]
- Saxena, A.; Teng, Y.K.O.; Collins, C.; England, N.; Leher, H. Voclosporin for lupus nephritis: Results of the two year AURORA 2 continuation study. Ann. Rheum. Dis. 2022, 81, POSO186. [Google Scholar] [CrossRef]
- Steiger, S.; Ehreiser, L.; Anders, J.; Anders, H.J. Biological drugs for systemic lupus erythematosus or active lupus nephritis and rates of infectious complications. Evidence from large clinical trials. Front. Immunol. 2022, 13, 999704. [Google Scholar] [CrossRef]
- Parikh, S.V.; Arriens, C.; Yap, E.; Piper, K.; Huizinga, R.; Leher, H. Follow-up Kidney Biopsies from the AURORA 2 Clinical Trial Evaluating Voclosporin for the Treatment of Lupus Nephritis. Clin. Congr. Rheumatol. 2023. [Google Scholar]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Wallace, D.J.; Atsumi, T.; Daniels, M.; Hammer, A.; Meizlik, P.; Quasny, H.; Schwarting, A.; Zhang, F.; Roth, D.A. Safety of belimumab in adult patients with systemic lupus erythematosus: Results of a large integrated analysis of controlled clinical trial data. Lupus 2022, 31, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Malvar, A.; Pirruccio, P.; Alberton, V.; Lococo, B.; Recalde, C.; Fazini, B.; Nagaraja, H.; Indrakanti, D.; Rovin, B.H. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol. Dial. Transplant. 2017, 32, 1338–1344. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Malvar, A.; Arazi, A.; Rovin, B.H. The lupus nephritis management renaissance. Kidney Int. 2022, 101, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kirou, K.A.; Dall Era, M.; Aranow, C.; Anders, H.J. Belimumab or anifrolumab for systemic lupus erythematosus? A risk-benefit assessment. Front. Immunol. 2022, 13, 980079. [Google Scholar] [CrossRef]
- Rovin, B.H.; Furie, R.; Teng, Y.K.O.; Contreras, G.; Malvar, A.; Yu, X.; Ji, B.; Green, Y.; Gonzalez-Rivera, T.; Bass, D.; et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2022, 101, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Peired, A.J.; Romagnani, P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, ‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol. Dial. Transplant. 2022, 37, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Mandrik, O.; Fotheringham, J.; Ren, S.; Tice, J.A.; Chapman, R.H.; Stevenson, M.D.; Pearson, S.D.; Herron-Smith, S.; Agboola, F.; Thokala, P. The Cost-Effectiveness of Belimumab and Voclosporin for Patients with Lupus Nephritis in the United States. Clin. J. Am. Soc. Nephrol. 2022, 17, 385–394. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Clinical Use | Contraindications/ Adverse Effects |
|---|---|---|
| Cyclosporine | Solid organ transplantation (liver, kidney, and heart), rheumatoid arthritis, psoriasis, amyotrophic lateral sclerosis, nephrotic syndrome, graft vs. host disease (GVHD), refractory posterior uveitis, and Behcet disease. Off-label: Allergic conjunctivitis, autoimmune hepatitis, keratoconjunctivitis, Langerhans cells histiocytosis, Duchenne muscular dystrophy, ocular graft vs. host disease, ulcerative colitis, pure red cell aplasia, Henoch Schönlein purpura nephritis, proteinuric forms of glomerulonephritis and podocytopathies. | Contraindications: Amphotericin B, neomycin, atorvastatin, cidofovir, elbasvir/grazoprevir, flibanserin, lomitapide, mifepristone, tacrolimus, life vaccines, etc. Adverse effects: Dyslipidemia, hyperkalemia, gynecomastia, hypertension, arrhythmia, decrease in eGFR and creatinine clearance, convulsions, bleeding gums, GIT upset, infectious complications. |
| Tacrolimus | Solid organ transplantation (liver, kidney, and heart. Off-label: Crohn’s disease, Graft-versus-host disease (GVHD), Myasthenia gravis, and Rheumatoid arthritis, Ulcerative colitis, proteinuric forms of glomerulonephritis and podocytopathies. | Contraindications: some antifungal agents, polyoxyl 60 hydrogenated castor oil (HCO-60), and derivatives. Adverse effects: Nephrotoxicity, neurotoxicity, post-transplant diabetes mellitus, hypertension, dyslipidemia, angina pectoris, cardiac arrhythmias, urinary tract infections, cosmetic and electrolyte disturbances, infectious complications. |
| Voclosporin | Active lupus nephritis. Off-label: Plaque psoriasis, prevention of organ rejection after transplantation, uveitis, arthritis, and Crohn’s disease. | Contraindications: Phenylalanine, flunisolide, bortezomib, cladribine, and in patients with renal and hepatic impairments. Adverse effects: Reduced eGFR, increased blood pressure, diarrhea, headache, anemia, cough, UTI, upper abdominal pain, dyspepsia, alopecia, renal dysfunction, abdominal pain, mouth ulceration, nausea, tremor, acute kidney injury and decreased appetite, infectious complications. |
| Adverse Effects | Cyclosporin | Tacrolimus | Voclosporin |
|---|---|---|---|
| Hypertension | ++ | + | + |
| Nephrotoxicity | ++ | ++ | + |
| Decrease in eGFR | + | + | + |
| Arrhythmia/cardiovascular risk | + | + | − |
| Anemia | + | + | + |
| Neurotoxicity/convulsions | + | ++ | + |
| Gastrointestinal upset | + | ++ | + |
| Gum hyperplasia | ++ | − | + |
| Dyslipidemia | ++ | + | − |
| Gynecomastia | + | − | − |
| Alopecia | − | ++ | + |
| Post-transplant diabetes mellitus | + | ++ | − |
| Urinary tract infection | + | + | |
| Hyperkalemia | ++ | + | + |
| Cosmetic and electrolyte disturbances | ++ | + | − |
| Hyperuricemia | ++ | + | − |
| Hirsutism/hypertrichosis | ++ | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kale, A.; Shelke, V.; Lei, Y.; Gaikwad, A.B.; Anders, H.-J. Voclosporin: Unique Chemistry, Pharmacology and Toxicity Profile, and Possible Options for Implementation into the Management of Lupus Nephritis. Cells 2023, 12, 2440. https://doi.org/10.3390/cells12202440
Kale A, Shelke V, Lei Y, Gaikwad AB, Anders H-J. Voclosporin: Unique Chemistry, Pharmacology and Toxicity Profile, and Possible Options for Implementation into the Management of Lupus Nephritis. Cells. 2023; 12(20):2440. https://doi.org/10.3390/cells12202440
Chicago/Turabian StyleKale, Ajinath, Vishwadeep Shelke, Yutian Lei, Anil Bhanudas Gaikwad, and Hans-Joachim Anders. 2023. "Voclosporin: Unique Chemistry, Pharmacology and Toxicity Profile, and Possible Options for Implementation into the Management of Lupus Nephritis" Cells 12, no. 20: 2440. https://doi.org/10.3390/cells12202440






