Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications
Abstract
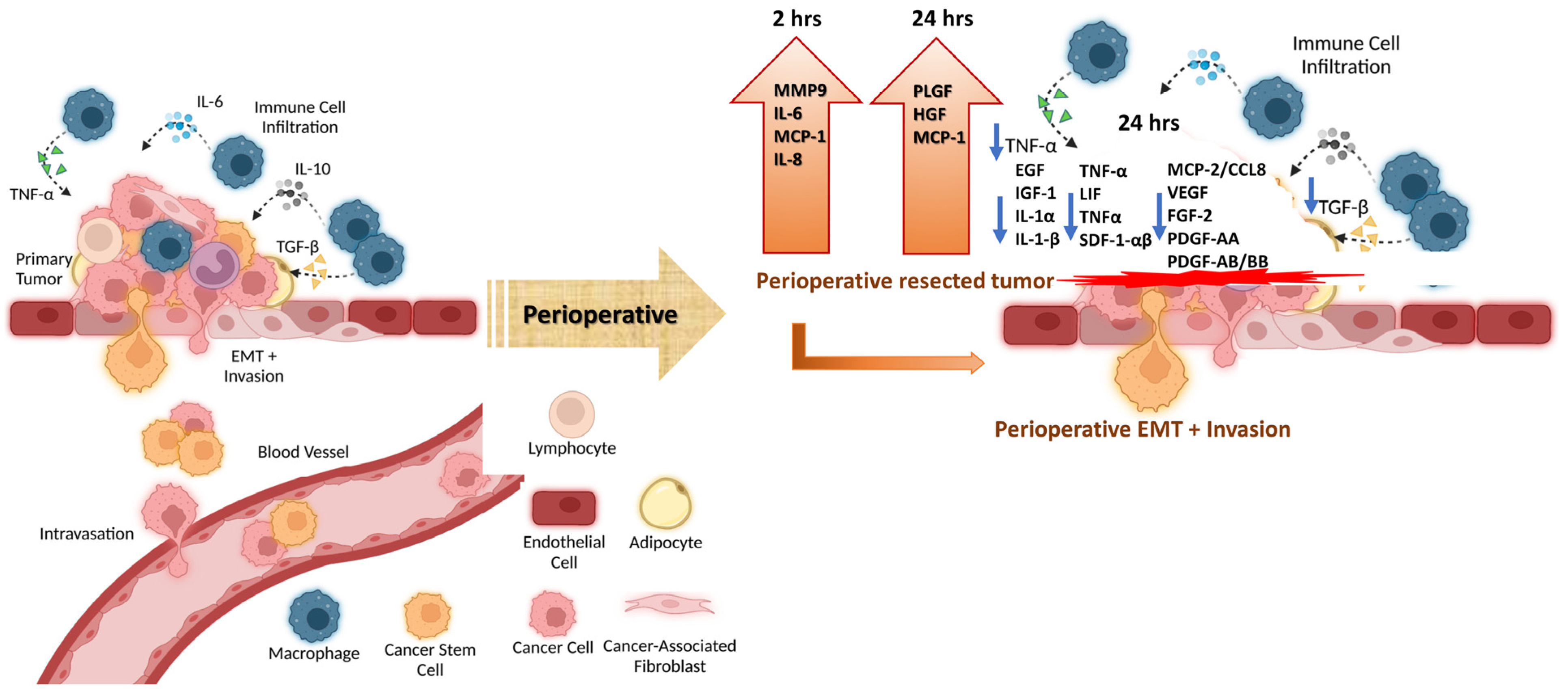
:1. Introduction
2. Materials and Methods
2.1. Patient Study
2.2. Multi-Plex Magnetic Bead Panel Kits
2.3. Preparation of Plasma Samples and Test Reagents
2.4. Immunoassay Procedures
2.5. Statistical Analysis
3. Results
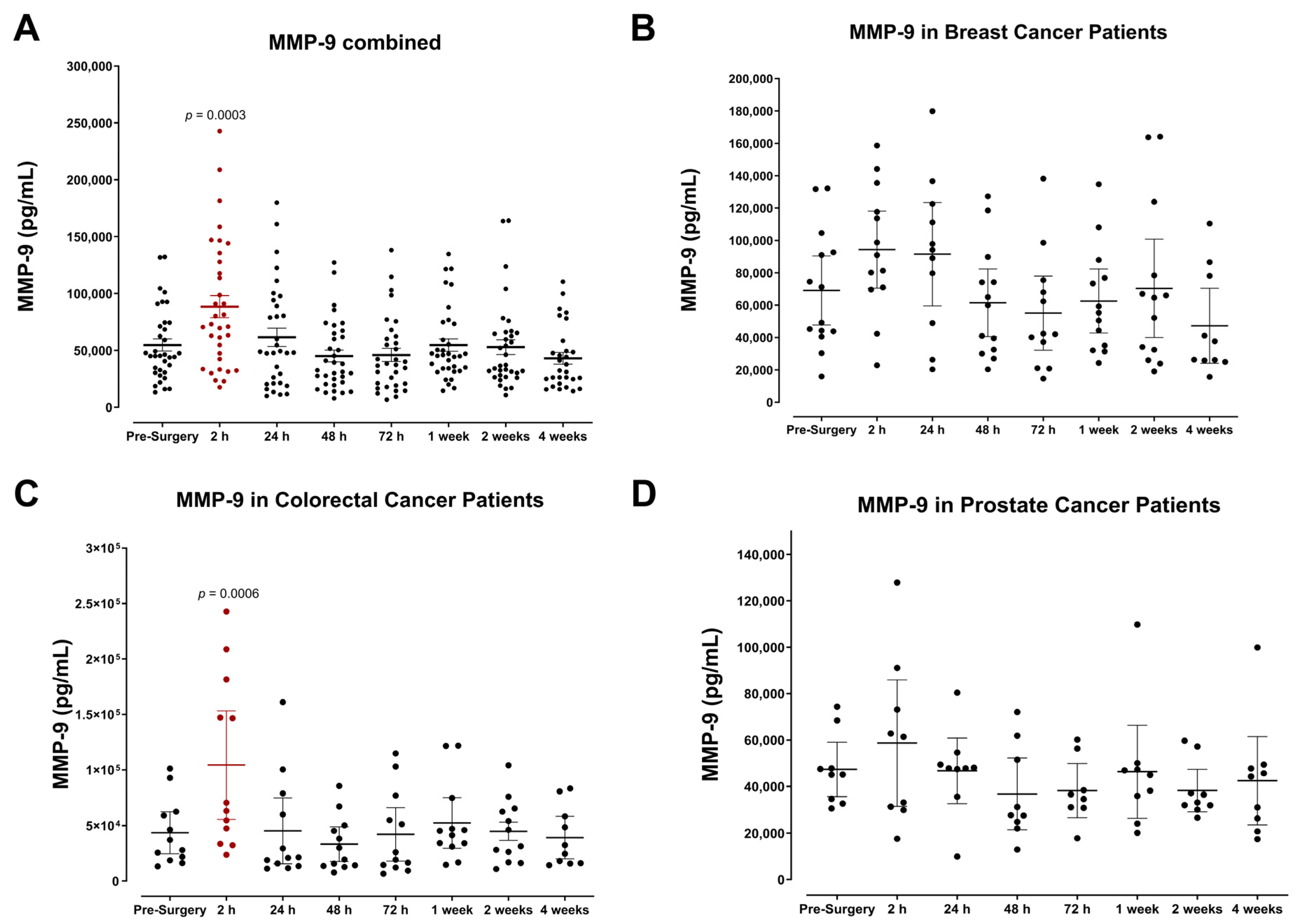
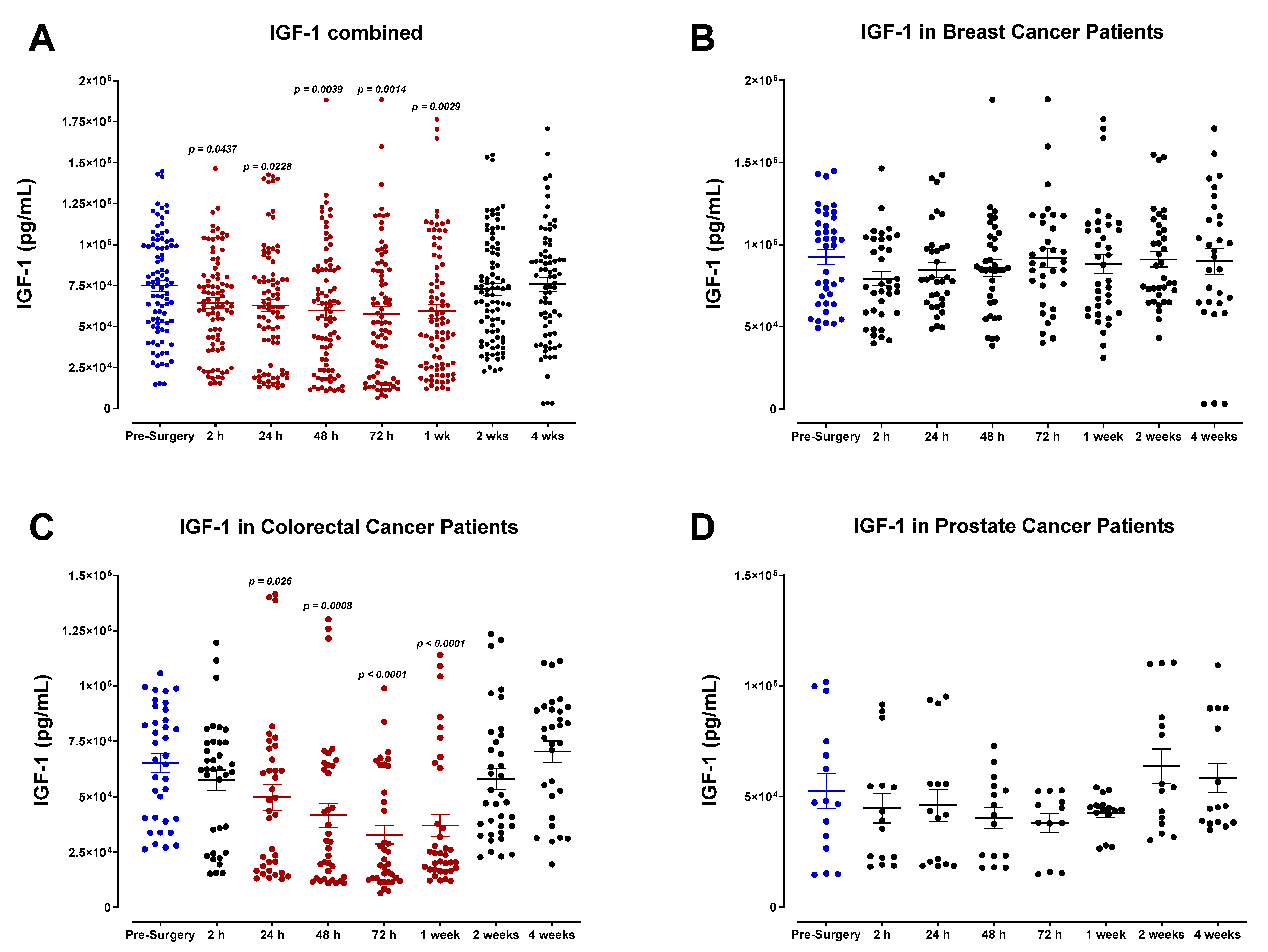
3.1. Plasma Expression of MMP-9 and Growth Factors Levels during the Perioperative Period
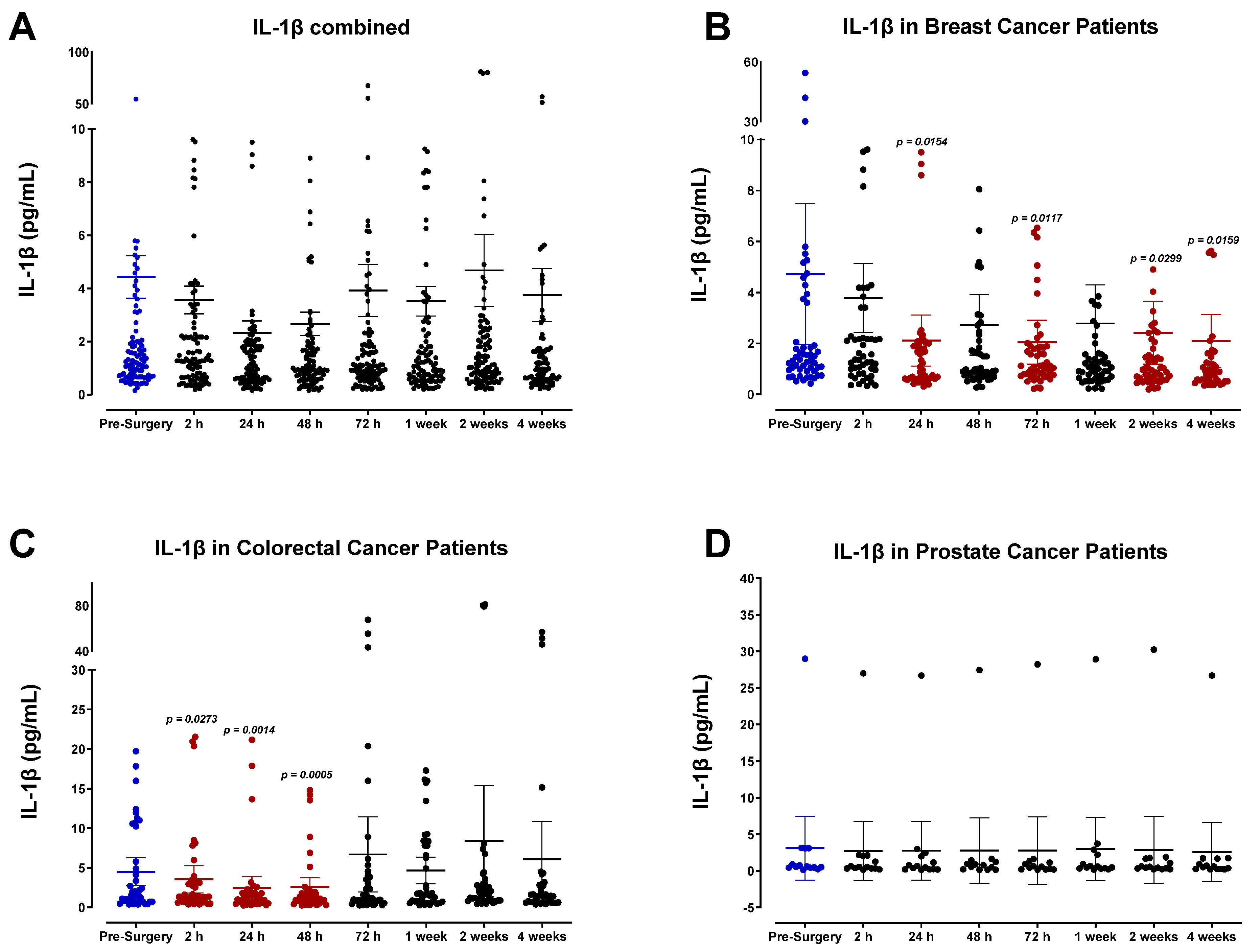
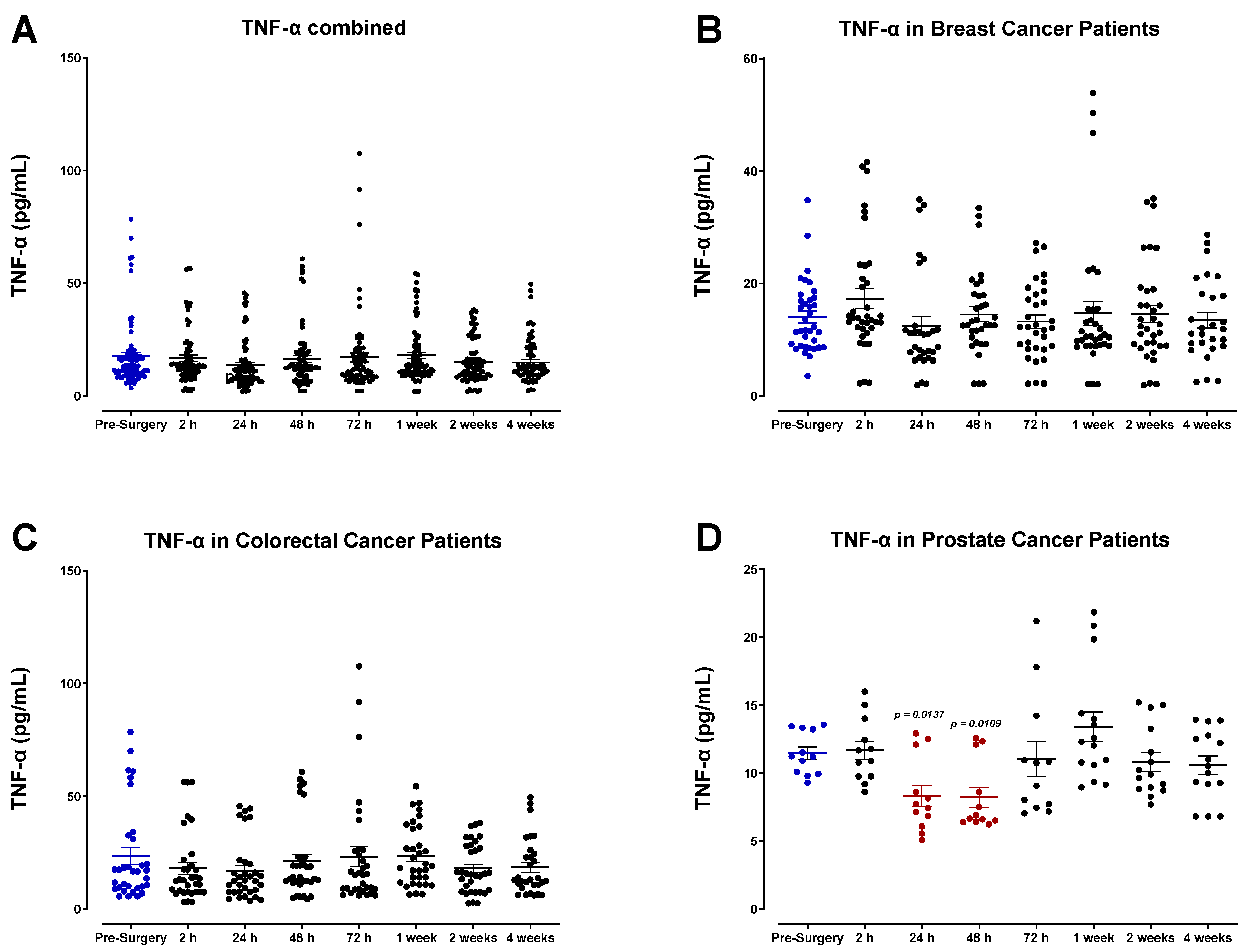
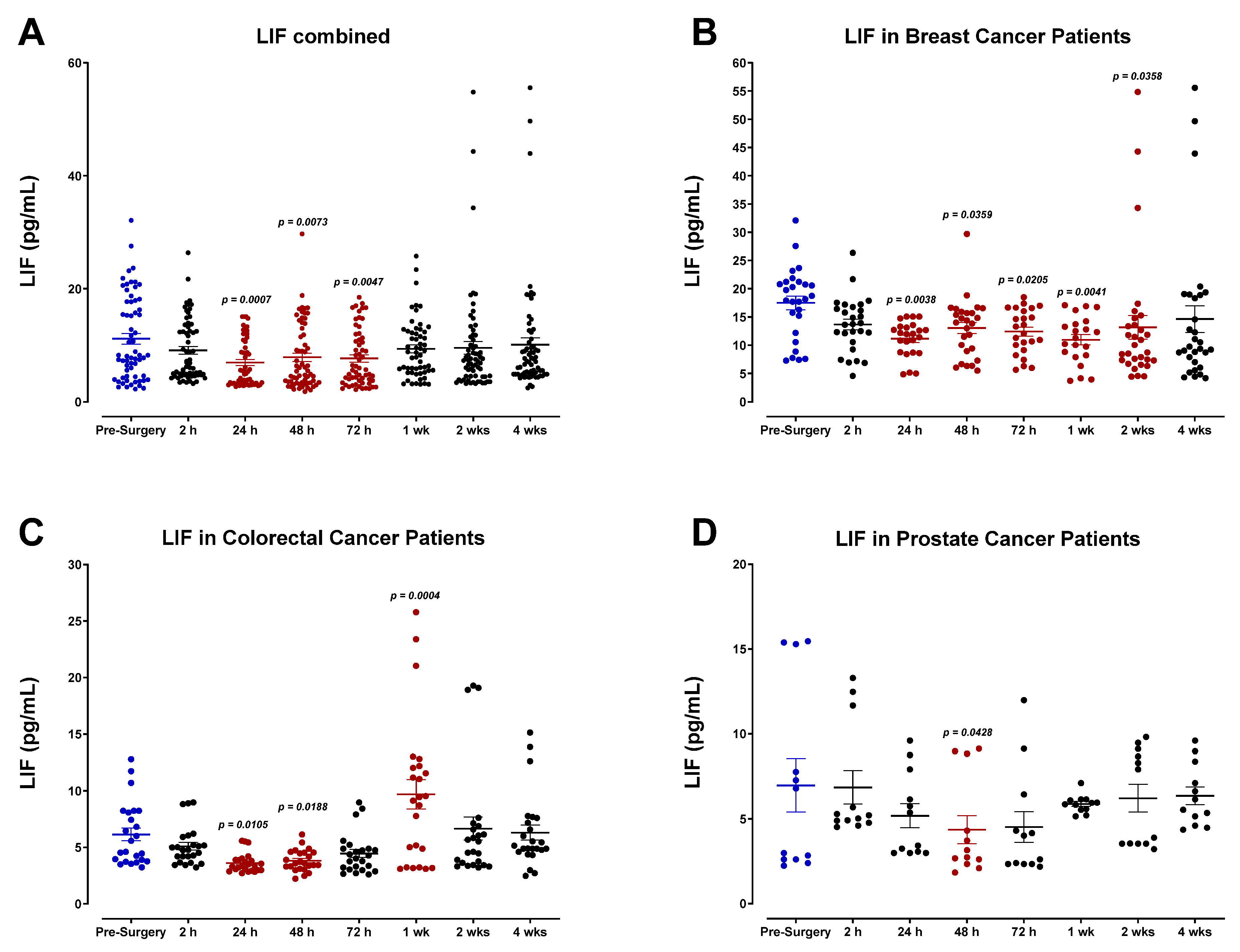
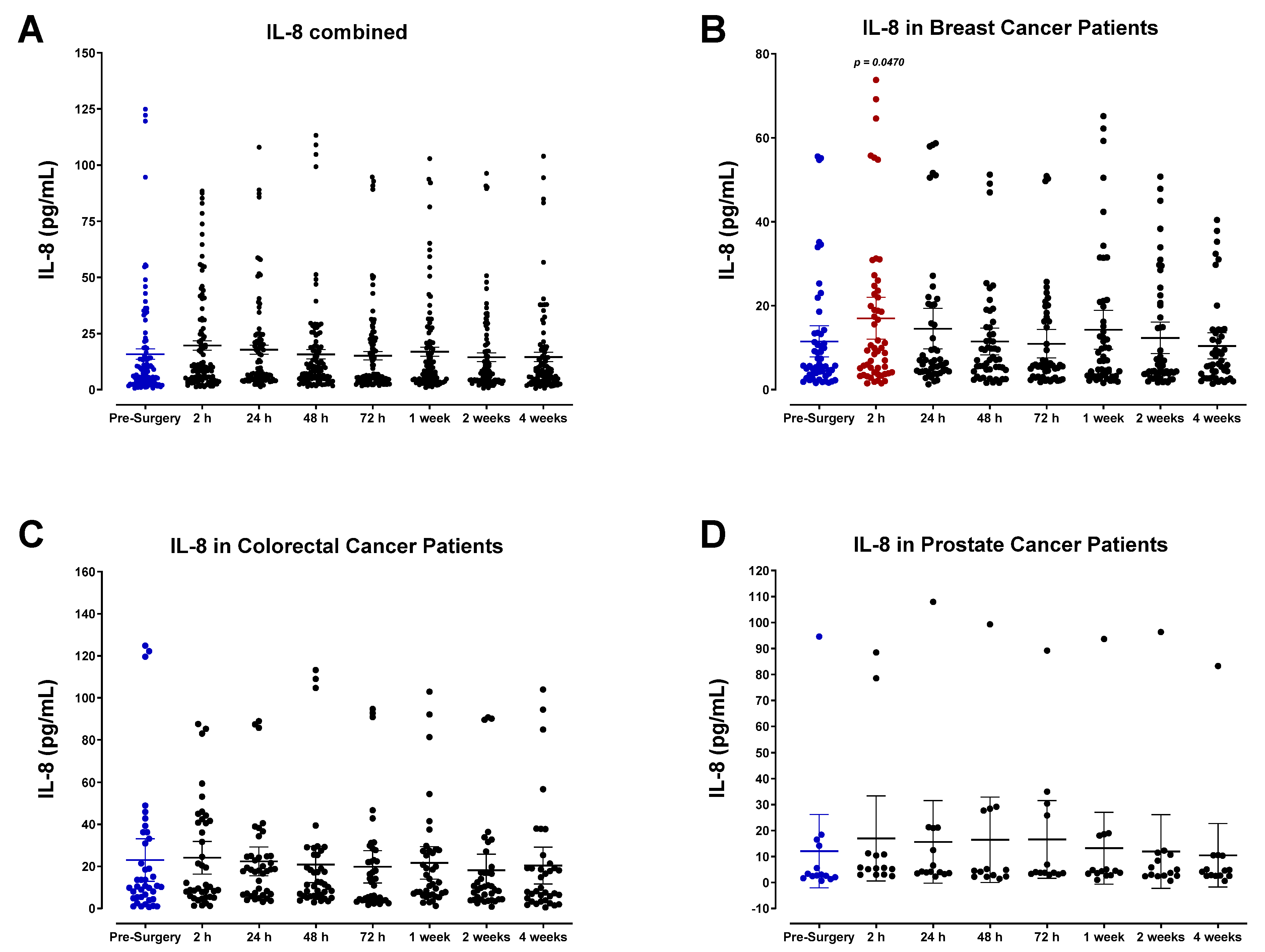
3.2. The Pro-Inflammatory Plasma Cytokine Profile following Surgical Tumor Removal
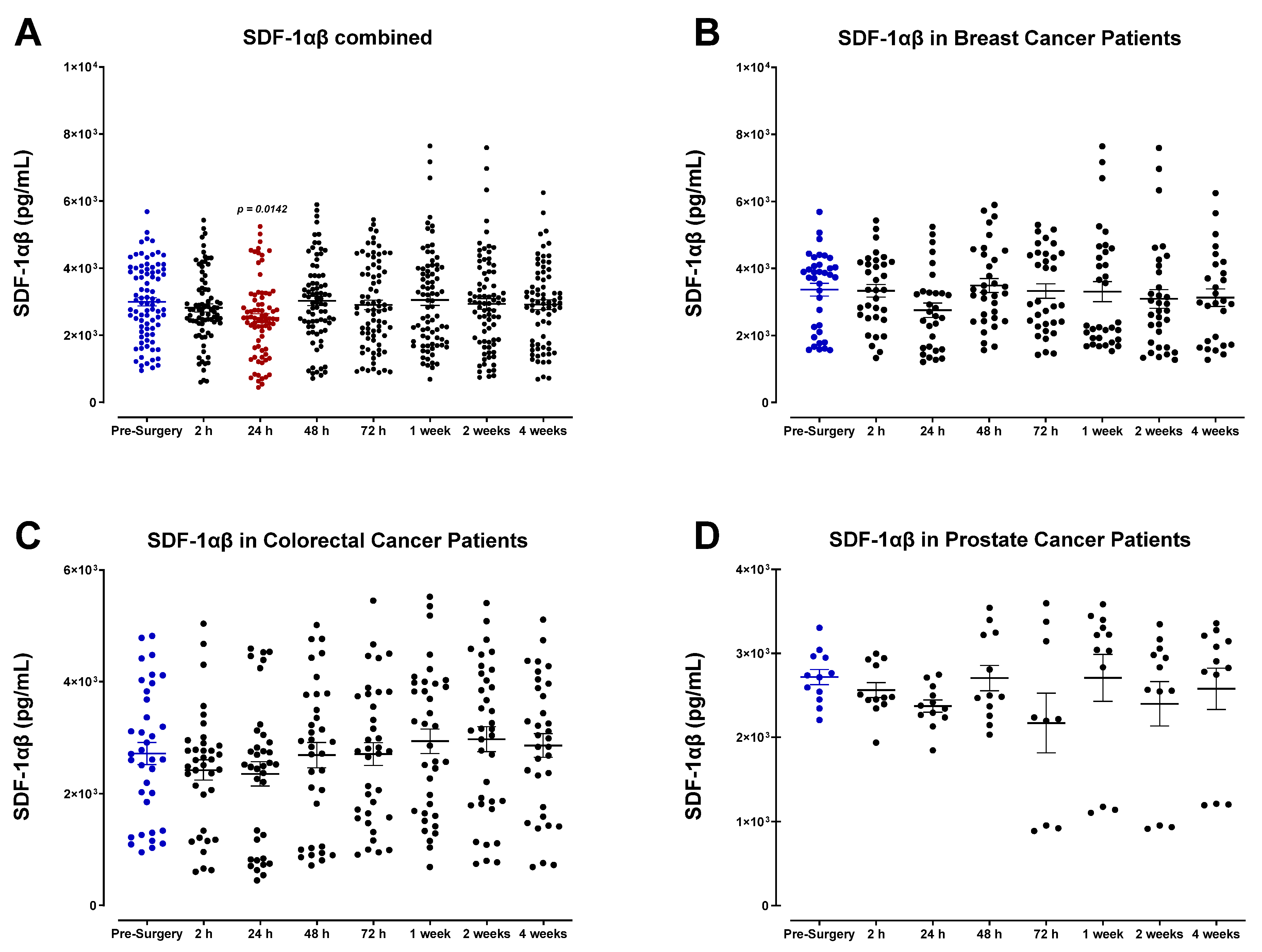
3.3. Surgery-Induced Wound Healing and Angiogenesis Are Mediated by Plasma Chemokines
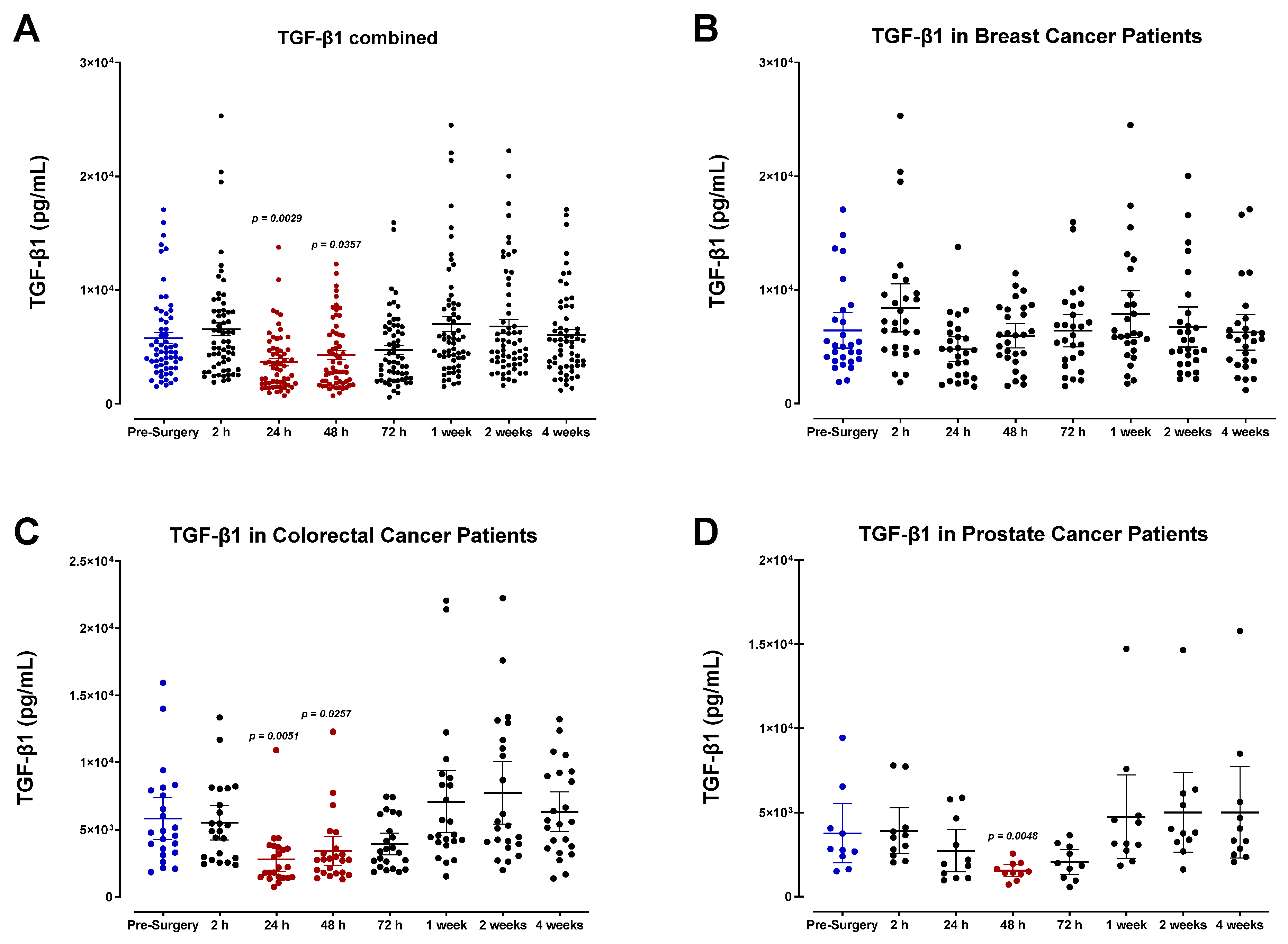
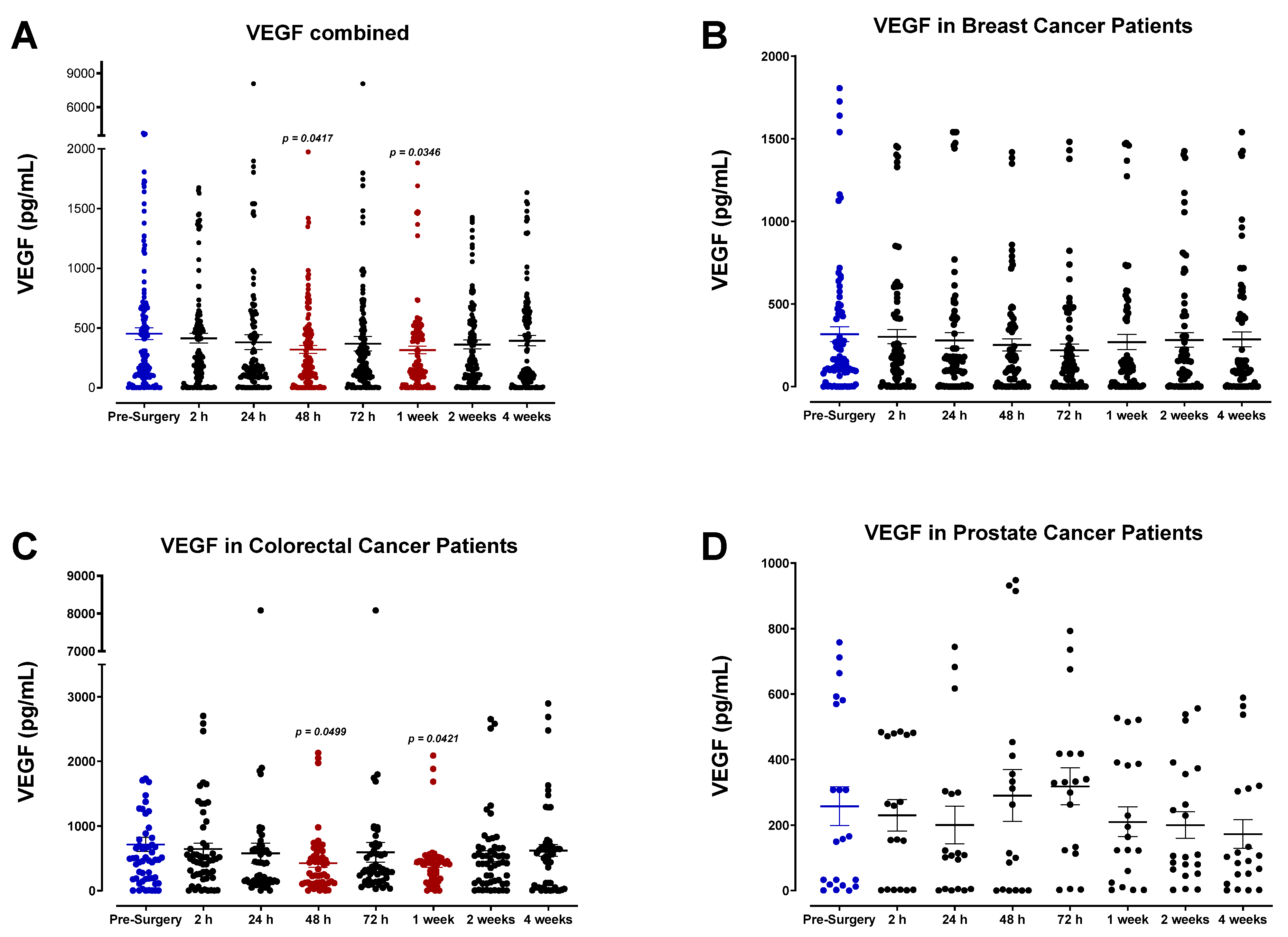
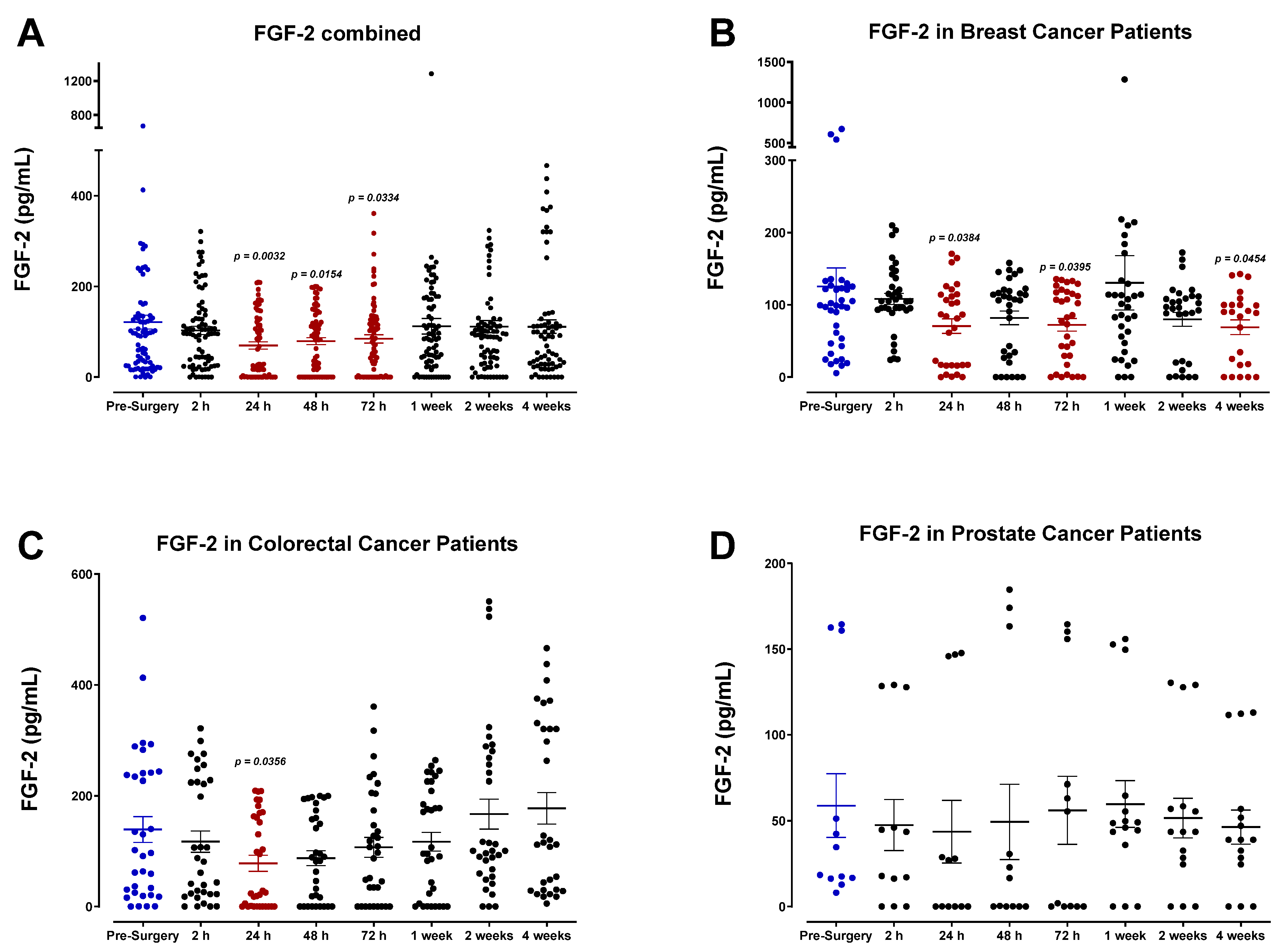
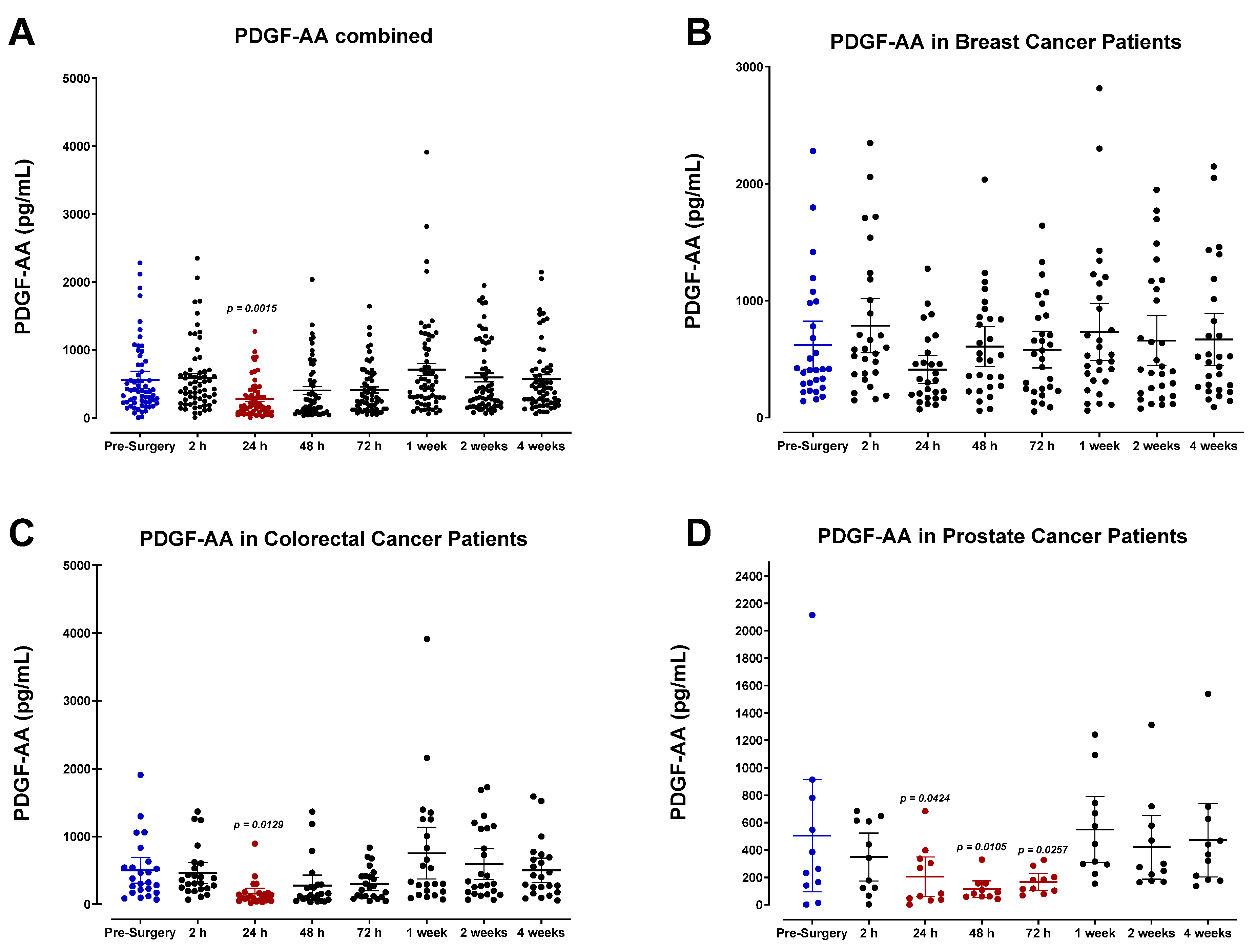
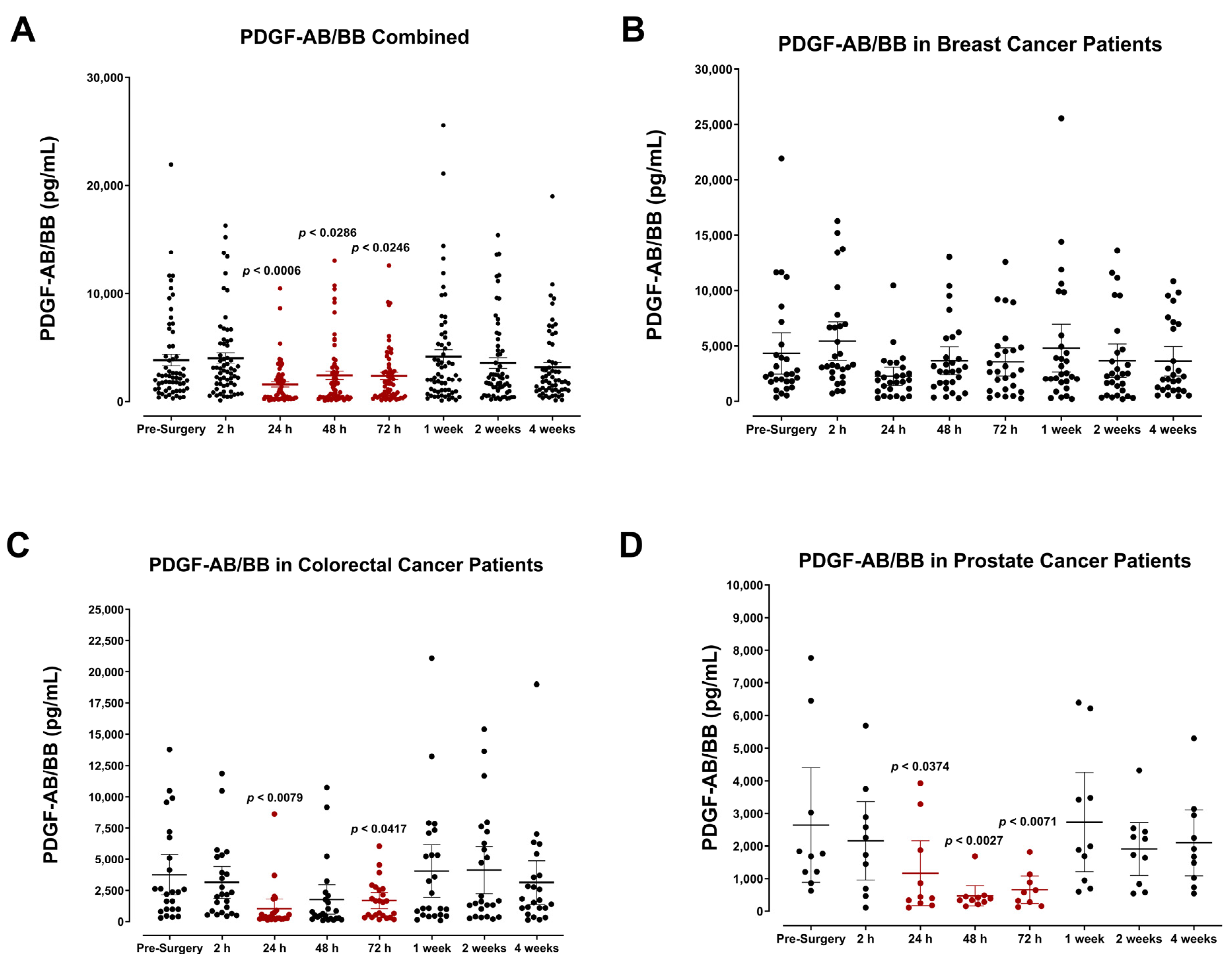
3.4. The Perioperative Period Is Characterized by a Drop in Plasma Pro-Angiogenic Growth Factors
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann. Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol./Hematol. 2014, 89, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.I.; Kurusu, Y.; Fujino, N.; Saisyoji, T.; Ogawa, M. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: A potential hazard for intraoperative hematogenous tumor cell dissemination. J. Thorac. Cardiovasc. Surg. 2000, 119, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.J.; Shi, D.; Wu, Q.C.; Wang, M.; Li, L.B. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J. Cancer Res. Clin. Oncol. 2006, 132, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takagi, Y.; Aoki, S.; Futamura, M.; Saji, S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000, 232, 58–65. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Old and New Blood Markers in Human Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 12968. [Google Scholar] [CrossRef]
- Brown, D.C.; Purushotham, A.D.; Birnie, G.D.; George, W.D. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery 1995, 117, 95–101. [Google Scholar] [CrossRef]
- Camara, O.; Kavallaris, A.; Noschel, H.; Rengsberger, M.; Jorke, C.; Pachmann, K. Seeding of epithelial cells into circulation during surgery for breast cancer: The fate of malignant and benign mobilized cells. World J. Surg. Oncol. 2006, 4, 67. [Google Scholar] [CrossRef]
- Xia, S.H.; Zhou, D.; Ge, F.; Sun, M.; Chen, X.; Zhang, H.; Miao, C. Influence of Perioperative Anesthesia on Cancer Recurrence: From Basic Science to Clinical Practice. Curr. Oncol. Rep. 2023, 25, 63–81. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Zhao, J.; Ju, H. Circulating tumor cells in perioperative esophageal cancer patients: Quantitative assay system and potential clinical utility. Clin. Cancer Res. 2007, 13, 2992–2997. [Google Scholar] [CrossRef]
- Eschwege, P.; Dumas, F.; Blanchet, P.; Le Maire, V.; Benoit, G.; Jardin, A.; Lacour, B.; Loric, S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995, 346, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, F.; Natsugoe, S.; Takao, S.; Tokuda, K.; Kijima, F.; Aridome, K.; Hokita, S.; Baba, M.; Eizuru, Y.; Aikou, T. Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann. Surg. 2001, 233, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Uchikura, K.; Takao, S.; Nakajo, A.; Miyazono, F.; Nakashima, S.; Tokuda, K.; Matsumoto, M.; Shinchi, H.; Natsugoe, S.; Aikou, T. Intraoperative molecular detection of circulating tumor cells by reverse transcription-polymerase chain reaction in patients with biliary-pancreatic cancer is associated with hematogenous metastasis. Ann. Surg. Oncol. 2002, 9, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, T.; Matsumata, T.; Kanematsu, T.; Yasunaga, C.; Sugimachi, K. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J. Surg. Res. 1990, 49, 92–97. [Google Scholar] [CrossRef]
- Romsdahl, M.M.; McGrath, R.G.; Hoppe, E.; McGrew, E.A. Experimental Model for the Study of Tumor Cells in the Blood. Acta Cytol. 1965, 9, 141–145. [Google Scholar]
- Li, S.; Yan, W.; Yang, X.; Chen, L.; Fan, L.; Liu, H.; Liu, K.; Zhang, Y.; Jiang, J. Less micrometastatic risk related to circulating tumor cells after endoscopic breast cancer surgery compared to open surgery. BMC Cancer 2019, 19, 1070. [Google Scholar] [CrossRef]
- Nierodzik, M.L.; Klepfish, A.; Karpatkin, S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb. Haemost. 1995, 74, 282–290. [Google Scholar]
- Honn, K.V.; Tang, D.G.; Crissman, J.D. Platelets and cancer metastasis: A causal relationship? Cancer Metastasis Rev. 1992, 11, 325–351. [Google Scholar] [CrossRef]
- Li, F.; Xu, T.; Chen, P.; Sun, R.; Li, C.; Zhao, X.; Ou, J.; Li, J.; Liu, T.; Zeng, M.; et al. Platelet-derived extracellular vesicles inhibit ferroptosis and promote distant metastasis of nasopharyngeal carcinoma by upregulating ITGB3. Int. J. Biol. Sci. 2022, 18, 5858–5872. [Google Scholar] [CrossRef]
- Dardik, R.; Kaufmann, Y.; Savion, N.; Rosenberg, N.; Shenkman, B.; Varon, D. Platelets mediate tumor cell adhesion to the subendothelium under flow conditions: Involvement of platelet GPIIb-IIIa and tumor cell alpha(v) integrins. Int. J. Cancer 1997, 70, 201–207. [Google Scholar] [CrossRef]
- Ahmad, A.; Nawaz, M.I. Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell. Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.B. Cancer and thrombosis: From Phlegmasia alba dolens to transgenic mice. Thromb. Haemost. 1995, 74, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Jirouskova, M.; Degen, J.L. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005, 105, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, P.G.; Sloane, B.F.; Honn, K.V. Role of the coagulation system in tumor-cell-induced platelet aggregation and metastasis. Pathophysiol. Haemost. Thromb. 1988, 18, 37–46. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Nagy, J.A.; Berse, B.; Brown, L.F.; Yeo, K.T.; Yeo, T.K.; Dvorak, A.M.; van de Water, L.; Sioussat, T.M.; Senger, D.R. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann. N. Y. Acad. Sci. 1992, 667, 101–111. [Google Scholar] [CrossRef]
- Dupuy, E.; Habib, A.; Lebret, M.; Yang, R.; Levy-Toledano, S.; Tobelem, G. Thrombin induces angiogenesis and vascular endothelial growth factor expression in human endothelial cells: Possible relevance to HIF-1alpha. J. Thromb. Haemost. 2003, 1, 1096–1102. [Google Scholar] [CrossRef]
- Massberg, S.; Konrad, I.; Schurzinger, K.; Lorenz, M.; Schneider, S.; Zohlnhoefer, D.; Hoppe, K.; Schiemann, M.; Kennerknecht, E.; Sauer, S.; et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J. Exp. Med. 2006, 203, 1221–1233. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Balkwill, F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin. Cancer Biol. 2004, 14, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pearlstein, E.; Ambrogio, C.; Karpatkin, S. Effect of antiplatelet antibody on the development of pulmonary metastases following injection of CT26 colon adenocarcinoma, Lewis lung carcinoma, and B16 amelanotic melanoma tumor cells into mice. Cancer Res. 1984, 44, 3884–3887. [Google Scholar] [PubMed]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, F.; Amano, H.; Ito, Y.; Matsui, Y.; Hosono, K.; Kitasato, H.; Satoh, Y.; Majima, M. Aspirin reduces lung cancer metastasis to regional lymph nodes. Biomed. Pharmacother. 2014, 68, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. The Multifaceted Clinical Readouts of Platelet Inhibition by Low-Dose Aspirin. J. Am. Coll. Cardiol. 2015, 66, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Gunduz, N.; Coyle, J.; Rudock, C.; Saffer, E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989, 49, 1996–2001. [Google Scholar]
- Gunduz, N.; Fisher, B.; Saffer, E.A. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979, 39, 3861–3865. [Google Scholar]
- Fisher, B.; Gunduz, N.; Saffer, E.A. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983, 43, 1488–1492. [Google Scholar]
- Yang, Y.; Lu, Y.; Tan, H.; Bai, M.; Wang, X.; Ge, S.; Ning, T.; Zhang, L.; Duan, J.; Sun, Y.; et al. The optimal time of starting adjuvant chemotherapy after curative surgery in patients with colorectal cancer. BMC Cancer 2023, 23, 422. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.H.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Cao, Y.; Moses, M.; Lane, W.S.; Sage, E.H.; Folkman, J. Angiostatin: A circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb. Symp. Quant. Biol. 1994, 59, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.L.; Kenan, D.J.; Gonzalez-Gronow, M.; Pizzo, S.V. Angiostatin’s molecular mechanism: Aspects of specificity and regulation elucidated. J. Cell. Biochem. 2005, 96, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Kaneda, Y.; Ueda, K.; Hamano, K.; Zempo, N.; Esato, K. The influence of tumour resection on angiostatin levels and tumour growth—An experimental study in tumour-bearing mice. Eur. J. Cancer 2001, 37, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Preuss, S.F.; Grieshober, D.; Augustin, H.G. Systemic Reprogramming of Endothelial Cell Signaling in Metastasis and Cachexia. Physiology 2023, 38, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Boyer, C.; Liu, X.; Wen, F.Q.; Kobayashi, T.; Fang, Q.; Wang, X.; Hashimoto, M.; Sharp, J.G.; Rennard, S.I. Cells derived from the circulation contribute to the repair of lung injury. Am. J. Respir. Crit. Care Med. 2004, 170, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Kollet, O.; Shivtiel, S.; Chen, Y.Q.; Suriawinata, J.; Thung, S.N.; Dabeva, M.D.; Kahn, J.; Spiegel, A.; Dar, A.; Samira, S.; et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Investig. 2003, 112, 160–169. [Google Scholar] [CrossRef]
- Tian, W.; Tong, H.; Sun, Y.; Wu, Q.; Ma, C.; Jiao, P. [Changes in serum vascular endothelial growth factor and matrix metalloproteinase-9 after video-assisted thoracoscopic surgery and thoracotomic Lobectomy in the treatment of patients with non-small cell lung cancer]. Chin. J. Lung Cancer 2014, 17, 24–29. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Song, C. [Clinical research of perioperative serum VEGF and MMP-9 levels in patients with non-small cell lung cancer]. Chin. J. Lung Cancer 2008, 11, 734–738. [Google Scholar] [CrossRef]
- Deegan, C.A.; Murray, D.; Doran, P.; Moriarty, D.C.; Sessler, D.I.; Mascha, E.; Kavanagh, B.P.; Buggy, D.J. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg. Anesth. Pain Med. 2010, 35, 490–495. [Google Scholar] [CrossRef]
- Shantha Kumara, H.M.; Cabot, J.C.; Yan, X.; Herath, S.A.; Luchtefeld, M.; Kalady, M.F.; Feingold, D.L.; Baxter, R.; Whelan, R.L. Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg. Endosc. 2011, 25, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Maae, E.; Olsen, D.A.; Steffensen, K.D.; Jakobsen, E.H.; Brandslund, I.; Sorensen, F.B.; Jakobsen, A. Prognostic impact of placenta growth factor and vascular endothelial growth factor A in patients with breast cancer. Breast Cancer Res. Treat. 2012, 133, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.J.; Lee, J.J.; Cheng, S.L.; Chen, H.M.; Chang, H.H.; Wang, Y.P.; Kok, S.H.; Kuo, M.Y.; Chiang, C.P. Increased serum placenta growth factor level is significantly associated with progression, recurrence and poor prognosis of oral squamous cell carcinoma. Oral Oncol. 2012, 48, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Galustian, C.; Kumar, D.; Hagger, R.; Melville, D.M.; Bodman-Smith, M.; Jourdan, I.; Gudgeon, A.M.; Dalgleish, A.G. Impact of surgery on immunologic function: Comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am. J. Surg. 2009, 197, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Bobocea, A.C.; Trandafir, B.; Bolca, C.; Cordos, I. Minimally invasive surgery in cancer. Immunological response. Chirurgia 2012, 107, 154–157. [Google Scholar] [PubMed]
- Verhage, R.J.; Hazebroek, E.J.; Boone, J.; Van Hillegersberg, R. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: A systematic review of the literature. Minerva Chir. 2009, 64, 135–146. [Google Scholar] [PubMed]
- Parisi, A.; Reim, D.; Borghi, F.; Nguyen, N.T.; Qi, F.; Coratti, A.; Cianchi, F.; Cesari, M.; Bazzocchi, F.; Alimoglu, O.; et al. Minimally invasive surgery for gastric cancer: A comparison between robotic, laparoscopic and open surgery. World J. Gastroenterol. 2017, 23, 2376–2384. [Google Scholar] [CrossRef]
- Mouraviev, V.; Nosnik, I.; Sun, L.; Robertson, C.N.; Walther, P.; Albala, D.; Moul, J.W.; Polascik, T.J. Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: A single-institution experience. Urology 2007, 69, 311–314. [Google Scholar] [CrossRef]
- Cathcart, P.; Murphy, D.G.; Moon, D.; Costello, A.J.; Frydenberg, M. Perioperative, functional and oncological outcomes after open and minimally invasive prostate cancer surgery: Experience from Australasia. BJU Int. 2011, 107 (Suppl. 3), 11–19. [Google Scholar] [CrossRef]
- Laparoscopic surgery is safe and effective for rectal cancer. Ongoing studies may confirm that the results of minimally invasive surgery are just as good as with open surgery. DukeMed. Healthnews 2010, 16, 6.
- Bodnar, R.J. Chemokine Regulation of Angiogenesis During Wound Healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef]
- Ruiter, D.J.; Schlingemann, R.O.; Westphal, J.R.; Denijn, M.; Rietveld, F.J.; De Waal, R.M. Angiogenesis in wound healing and tumor metastasis. Behring Inst. Mitt. 1993, 92, 258–272. [Google Scholar]
- Kaminska, J.; Kowalska, M.M.; Nowacki, M.P.; Chwalinski, M.G.; Rysinska, A.; Fuksiewicz, M. CRP, TNF-alpha, IL-1ra, IL-6, IL-8 and IL-10 in blood serum of colorectal cancer patients. Pathol. Oncol. Res. 2000, 6, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Neagu, S.; Lerescu, L.; Costea, R.; Tucureanu, C.; Caras, I.; Gangura, G.; Pitica, R.; Salageanu, A. [Perioperative immunologic changes in colorectal cancer patients]. Chirurgia 2012, 107, 59–65. [Google Scholar] [PubMed]
- Balasoiu, M.; Balasoiu, A.T.; Mogoanta, S.S.; Barbalan, A.; Stepan, A.E.; Ciurea, R.N.; Alexandru, D.O.; Enescu, A.; Mogoanta, L. Serum and tumor microenvironment IL-8 values in different stages of colorectal cancer. Rom. J. Morphol. Embryol. 2014, 55, 575–578. [Google Scholar] [PubMed]
- Feng, S.; Li, Z.; Liu, M.; Ye, Q.; Xue, T.; Yan, B. Postoperative serum interleukin-6 levels correlate with survival in stage I-III colorectal cancer. BMC Gastroenterol. 2023, 23, 156. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Akerstrom, V.; Hackler, L.; Pan, W. Different mechanisms influencing permeation of PDGF-AA and PDGF-BB across the blood-brain barrier. J. Neurochem. 2003, 87, 7–12. [Google Scholar] [CrossRef]
- Paulsson, J.; Ryden, L.; Strell, C.; Frings, O.; Tobin, N.P.; Fornander, T.; Bergh, J.; Landberg, G.; Stal, O.; Ostman, A. High expression of stromal PDGFRbeta is associated with reduced benefit of tamoxifen in breast cancer. J. Pathol. Clin. Res. 2017, 3, 38–43. [Google Scholar] [CrossRef]
- Frings, O.; Augsten, M.; Tobin, N.P.; Carlson, J.; Paulsson, J.; Pena, C.; Olsson, E.; Veerla, S.; Bergh, J.; Ostman, A.; et al. Prognostic significance in breast cancer of a gene signature capturing stromal PDGF signaling. Am. J. Pathol. 2013, 182, 2037–2047. [Google Scholar] [CrossRef]
- Paulsson, J.; Ehnman, M.; Ostman, A. PDGF receptors in tumor biology: Prognostic and predictive potential. Future Oncol. 2014, 10, 1695–1708. [Google Scholar] [CrossRef]
- Wong, H.P.; Ho, J.W.; Koo, M.W.; Yu, L.; Wu, W.K.; Lam, E.K.; Tai, E.K.; Ko, J.K.; Shin, V.Y.; Chu, K.M.; et al. Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci. 2011, 88, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.V.; Kim, S.J.; Donovan, E.L.; Chen, M.; Gross, A.C.; Webster Marketon, J.I.; Barsky, S.H.; Glaser, R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav. Immun. 2009, 23, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.K.; Bhatty, R.; Kamat, A.A.; Landen, C.N.; Han, L.; Thaker, P.H.; Li, Y.; Gershenson, D.M.; Lutgendorf, S.; Cole, S.W. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006, 12, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, D.G.; Tamae, A.C.; Biasoli, E.R.; Oliveira, S.H. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav. Immun. 2011, 25, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Kirman, I.; Jain, S.; Cekic, V.; Belizon, A.; Balik, E.; Sylla, P.; Arnell, T.; Forde, K.A.; Whelan, R.L. Altered plasma matrix metalloproteinase-9/tissue inhibitor of matrix [corrected] metalloproteinase-1 concentration during the early postoperative period in patients with colorectal cancer. Surg. Endosc. 2006, 20, 482–486. [Google Scholar] [CrossRef]
- Curigliano, G.; Petit, J.Y.; Bertolini, F.; Colleoni, M.; Peruzzotti, G.; de Braud, F.; Gandini, S.; Giraldo, A.; Martella, S.; Orlando, L.; et al. Systemic effects of surgery: Quantitative analysis of circulating basic fibroblast growth factor (bFGF), Vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-beta) in patients with breast cancer who underwent limited or extended surgery. Breast Cancer Res. Treat. 2005, 93, 35–40. [Google Scholar] [CrossRef]
- Hormbrey, E.; Han, C.; Roberts, A.; McGrouther, D.A.; Harris, A.L. The relationship of human wound vascular endothelial growth factor (VEGF) after breast cancer surgery to circulating VEGF and angiogenesis. Clin. Cancer Res. 2003, 9, 4332–4339. [Google Scholar]
- De Vita, F.; Orditura, M.; Lieto, E.; Infusino, S.; Morgillo, F.; Martinelli, E.; Castellano, P.; Romano, C.; Ciardiello, F.; Catalano, G.; et al. Elevated perioperative serum vascular endothelial growth factor levels in patients with colon carcinoma. Cancer 2004, 100, 270–278. [Google Scholar] [CrossRef]
- Mantur, M.; Snarska, J.; Sidorska, A.; Ostrowska, H.; Kruszewska-Wnorowska, K.; Wojszel, J. Changes in PDGF concentration in surgically treated colorectal carcinoma. Adv. Med. Sci. 2008, 53, 37–41. [Google Scholar] [CrossRef]
- Mantur, M.; Koper, O.; Snarska, J.; Sidorska, A.; Kruszewska-Wnorowska, K. Evaluation of PDGF-AB and sP-selectin concentrations in relation to platelet count in patients with colorectal cancer before and after surgical treatment. Pol. Arch. Med. Wewn. 2008, 118, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Bohm, M.; Nelius, T.; Filleur, S.; Reiher, F.; Allhoff, E.P. Evaluation of peri-operative peripheral and renal venous levels of pro- and anti-angiogenic factors and their relevance in patients with renal cell carcinoma. BJU Int. 2007, 100, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.T.; McGovern, F.J. Renal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell. Mol. Med. 2005, 9, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Lau, C.P.; Cheung, S.T.; Yu, W.C.; Fan, S.T. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003, 63, 3121–3126. [Google Scholar] [PubMed]
- Wu, F.P.; Hoekman, K.; Meijer, S.; Cuesta, M.A. VEGF and endostatin levels in wound fluid and plasma after breast surgery. Angiogenesis 2003, 6, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Sako, T.; Shibao, K.; Okazaki, K.; Rempo, N.; Onitsuka, K.; Minagawa, N.; Akahane, K.; Nagashima, N.; Nagata, N.; et al. Prognostic value of plasma vascular endothelial growth factor in patients with colorectal cancer. Anticancer Res. 2002, 22, 2437–2442. [Google Scholar]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef]
- Nafissi, N.; Mohammadlou, M.; Akbari, M.E.; Mahdavi, S.R.; Sheikh, M.; Borji, M.; Babaee, E.; Baharlou, R. The impact of intraoperative radiotherapy on breast cancer: Focus on the levels of angiogenic factors. World J. Surg. Oncol. 2022, 20, 191. [Google Scholar] [CrossRef]
- Neeman, E.; Zmora, O.; Ben-Eliyahu, S. A new approach to reducing postsurgical cancer recurrence: Perioperative targeting of catecholamines and prostaglandins. Clin. Cancer Res. 2012, 18, 4895–4902. [Google Scholar] [CrossRef]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Penn, I. The effect of immunosuppression on pre-existing cancers. Transplantation 1993, 55, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Detry, O.; Honore, P.; Meurisse, M.; Jacquet, N. Cancer in transplant recipients. Transplant. Proc. 2000, 32, 127. [Google Scholar] [CrossRef] [PubMed]
- Poullenot, F.; Laharie, D. Management of Inflammatory Bowel Disease in Patients with Current or Past Malignancy. Cancers 2023, 15, 1083. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, K.; Tamiya, T.; Hasegawa, E.; Fukaya, T.; Hashimoto, M.; Kakoi, K.; Kashiwagi, I.; Kimura, A.; Inoue, N.; Morita, R.; et al. Suppression of SOCS3 in macrophages prevents cancer metastasis by modifying macrophage phase and MCP2/CCL8 induction. Cancer Lett. 2011, 308, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Struyf, S.; Proost, P.; Vandercappellen, J.; Dempe, S.; Noyens, B.; Nelissen, S.; Gouwy, M.; Locati, M.; Opdenakker, G.; Dinsart, C.; et al. Synergistic up-regulation of MCP-2/CCL8 activity is counteracted by chemokine cleavage, limiting its inflammatory and anti-tumoral effects. Eur. J. Immunol. 2009, 39, 843–857. [Google Scholar] [CrossRef]
- Rupertus, K.; Sinistra, J.; Scheuer, C.; Nickels, R.M.; Schilling, M.K.; Menger, M.D.; Kollmar, O. Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer. Clin. Exp. Metastasis 2014, 31, 447–459. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Kucia, M.; Reca, R.; Miekus, K.; Wanzeck, J.; Wojakowski, W.; Janowska-Wieczorek, A.; Ratajczak, J.; Ratajczak, M.Z. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: Pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005, 23, 879–894. [Google Scholar] [CrossRef]
- Welford, A.F.; Biziato, D.; Coffelt, S.B.; Nucera, S.; Fisher, M.; Pucci, F.; Di Serio, C.; Naldini, L.; De Palma, M.; Tozer, G.M.; et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J. Clin. Investig. 2011, 121, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, M.; Schaider, H.; Miller, T.H.; Herlyn, M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J. Immunol. 2001, 166, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Melinceanu, L.; Lerescu, L.; Tucureanu, C.; Caras, I.; Pitica, R.; Sarafoleanu, C.; Salageanu, A. Serum perioperative profile of cytokines in patients with squamous cell carcinoma of the larynx. J. Otolaryngol. Head Neck Surg. 2011, 40, 143–150. [Google Scholar] [PubMed]
- Purdy, M.; Kokki, M.; Anttila, M.; Aspinen, S.; Juvonen, P.; Korhonen, R.; Selander, T.; Kokki, H.; Eskelinen, M. Does the Rectus Sheath Block Analgesia Reduce the Inflammatory Response Biomarkers’ IL-1ra, IL-6, IL-8, IL-10 and IL-1beta Concentrations Following Surgery? A Randomized Clinical Trial of Patients with Cancer and Benign Disease. Anticancer Res. 2016, 36, 3005–3011. [Google Scholar] [PubMed]
- Rennekampff, H.O.; Hansbrough, J.F.; Kiessig, V.; Dore, C.; Sticherling, M.; Schroder, J.M. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res. 2000, 93, 41–54. [Google Scholar] [CrossRef]
- Low, Q.E.; Drugea, I.A.; Duffner, L.A.; Quinn, D.G.; Cook, D.N.; Rollins, B.J.; Kovacs, E.J.; DiPietro, L.A. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am. J. Pathol. 2001, 159, 457–463. [Google Scholar] [CrossRef]
- Hyllner, M.; Avall, A.; Bengtson, J.P.; Bengtsson, A. IL-6 and IL-8 response to erythropoietin therapy in radical hysterectomy. Acta Anaesthesiol. Scand. 2005, 49, 47–51. [Google Scholar] [CrossRef]
- Mehigan, B.J.; Hartley, J.E.; Drew, P.J.; Saleh, A.; Dore, P.C.; Lee, P.W.; Monson, J.R. Changes in T cell subsets, interleukin-6 and C-reactive protein after laparoscopic and open colorectal resection for malignancy. Surg. Endosc. 2001, 15, 1289–1293. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Mandal, M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016, 375, 51–61. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Howley, P.M.; Israel, M.A.; Gray, J.W.; Thompson, C. The Molecular Basis of Cancer, 4th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2015; p. xxiii, 863p. [Google Scholar]
- Pencik, J.; Wiebringhaus, R.; Susani, M.; Culig, Z.; Kenner, L. IL-6/STAT3/ARF: The guardians of senescence, cancer progression and metastasis in prostate cancer. Swiss Med. Wkly. 2015, 145, w14215. [Google Scholar] [CrossRef]
- Mauer, J.; Denson, J.L.; Bruning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015, 36, 92–101. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Morris, D.L. Inhibition of the IL-6 signaling pathway: A strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev. 2013, 24, 163–173. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Huerta-Yepez, S.; Law, I.K.; Baay-Guzman, G.J.; Tirado-Rodriguez, B.; Hoffman, J.M.; Iliopoulos, D.; Hommes, D.W.; Verspaget, H.W.; Chang, L.; et al. Diminished expression of CRHR2 in human colon cancer promotes tumor growth and EMT via persistent IL-6/Stat3 signaling. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 610–630. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, G.; Jiang, T.; Zhu, G.; Li, H.; Qiu, Z. The effects and mechanisms of blockage of STAT3 signaling pathway on IL-6 inducing EMT in human pancreatic cancer cells in vitro. Neoplasma 2011, 58, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tang, N.; Wang, C.; Xiao, L.; Yu, M.; Zhao, L.; Cai, H.; Han, L.; Xie, C.; Zhang, Y. TNF-alpha-inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 484, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shim, J.W.; Eum, D.Y.; Kim, S.D.; Choi, S.H.; Yang, K.; Heo, K.; Park, M.T. Downregulation of UHRF1 increases tumor malignancy by activating the CXCR4/AKT-JNK/IL-6/Snail signaling axis in hepatocellular carcinoma cells. Sci. Rep. 2017, 7, 2798. [Google Scholar] [CrossRef]
- Zang, C.; Liu, X.; Li, B.; He, Y.; Jing, S.; He, Y.; Wu, W.; Zhang, B.; Ma, S.; Dai, W.; et al. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget 2017, 8, 11228–11238. [Google Scholar] [CrossRef]
- Su, Y.W.; Xie, T.X.; Sano, D.; Myers, J.N. IL-6 stabilizes Twist and enhances tumor cell motility in head and neck cancer cells through activation of casein kinase 2. PLoS ONE 2011, 6, e19412. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Matsumoto, M.; Baba, T.; Nakamura, T.; Kawamoto, T. Elevation of serum human hepatocyte growth factor (HGF) level in patients with pneumonectomy during a perioperative period. Intensive Care Med. 1998, 24, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Namekata, K.; Takamori, S.; Kojima, K.; Beppu, T.; Futagawa, S. Significant changes in the serum levels of IL-6, h-HGF, and type IV collagen 7S during the perioperative period of a hepatectomy: Relevance to SIRS. Surg. Today 2000, 30, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, T.; Takenaka, K.; Yoshizumi, T.; Yanaga, K.; Soejima, Y.; Shirabe, K.; Sugimachi, K. Alteration in levels of human hepatocyte growth factor following hepatectomy. J. Am. Coll. Surg. 1995, 181, 6–10. [Google Scholar] [PubMed]
- Matsumoto, K.; Miyake, Y.; Umeda, Y.; Matsushita, H.; Matsuda, H.; Takaki, A.; Sadamori, H.; Nouso, K.; Yagi, T.; Fujiwara, T.; et al. Serial changes of serum growth factor levels and liver regeneration after partial hepatectomy in healthy humans. Int. J. Mol. Sci. 2013, 14, 20877–20889. [Google Scholar] [CrossRef] [PubMed]
- Hoot, K.E.; Oka, M.; Han, G.; Bottinger, E.; Zhang, Q.; Wang, X.J. HGF upregulation contributes to angiogenesis in mice with keratinocyte-specific Smad2 deletion. J. Clin. Investig. 2010, 120, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- Kirchhofer, D.; Yao, X.; Peek, M.; Eigenbrot, C.; Lipari, M.T.; Billeci, K.L.; Maun, H.R.; Moran, P.; Santell, L.; Wiesmann, C.; et al. Structural and functional basis of the serine protease-like hepatocyte growth factor beta-chain in Met binding and signaling. J. Biol. Chem. 2004, 279, 39915–39924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am. J. Physiol. 1999, 277, F624–F633. [Google Scholar] [CrossRef]
- Mungunsukh, O.; McCart, E.A.; Day, R.M. Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling. Biomedicines 2014, 2, 301–326. [Google Scholar] [CrossRef]
- Ishikawa, D.; Takeuchi, S.; Nakagawa, T.; Sano, T.; Nakade, J.; Nanjo, S.; Yamada, T.; Ebi, H.; Zhao, L.; Yasumoto, K.; et al. mTOR inhibitors control the growth of EGFR mutant lung cancer even after acquiring resistance by HGF. PLoS ONE 2013, 8, e62104. [Google Scholar] [CrossRef]
- Kang, X.H.; Xu, Z.Y.; Gong, Y.B.; Wang, L.F.; Wang, Z.Q.; Xu, L.; Cao, F.; Liao, M.J. Bufalin Reverses HGF-Induced Resistance to EGFR-TKIs in EGFR Mutant Lung Cancer Cells via Blockage of Met/PI3k/Akt Pathway and Induction of Apoptosis. Evid.-Based Complement. Altern. Med. 2013, 2013, 243859. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Yamada, T.; Takeuchi, S.; Nakagawa, T.; Kita, K.; Nakamura, T.; Matsumoto, K.; Suda, K.; Mitsudomi, T.; Yano, S. Hsp90 inhibition overcomes HGF-triggering resistance to EGFR-TKIs in EGFR-mutant lung cancer by decreasing client protein expression and angiogenesis. J. Thorac. Oncol. 2012, 7, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Bi, M. [Research progress of HGF/MET signaling pathway in EGFR-TKI resistance in non-small cell lung cancer]. Chin. J. Lung Cancer 2014, 17, 755–759. [Google Scholar] [CrossRef]
- Wu, H.; Fan, F.; Liu, Z.; Shen, C.; Wang, A.; Lu, Y. Norcantharidin combined with EGFR-TKIs overcomes HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via inhibition of Met/PI3k/Akt pathway. Cancer Chemother. Pharmacol. 2015, 76, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Takeuchi, S.; Kita, K.; Bando, H.; Nakamura, T.; Matsumoto, K.; Yano, S. Hepatocyte growth factor induces resistance to anti-epidermal growth factor receptor antibody in lung cancer. J. Thorac. Oncol. 2012, 7, 272–280. [Google Scholar] [CrossRef]
- Yano, S.; Wang, W.; Li, Q.; Matsumoto, K.; Sakurama, H.; Nakamura, T.; Ogino, H.; Kakiuchi, S.; Hanibuchi, M.; Nishioka, Y.; et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008, 68, 9479–9487. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Il’yasova, D.; Colbert, L.H.; Harris, T.B.; Newman, A.B.; Bauer, D.C.; Satterfield, S.; Kritchevsky, S.B. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2413–2418. [Google Scholar] [CrossRef]
- Van Hilst, J.; Brinkman, D.J.; de Rooij, T.; van Dieren, S.; Gerhards, M.F.; de Hingh, I.H.; Luyer, M.D.; Marsman, H.A.; Karsten, T.M.; Busch, O.R. The inflammatory response after laparoscopic and open pancreatoduodenectomy and the association with complications in a multicenter randomized controlled trial. HPB 2019, 21, 1453–1461. [Google Scholar] [CrossRef]
- Bartın, M.K.; Kemik, Ö.; Çaparlar, M.A.; Bostancı, M.T.; Öner, M.Ö. Evaluation of the open and laparoscopic appendectomy operations with respect to their effect on serum IL-6 levels. Turk. J. Trauma Emerg. Surg. 2016, 22, 466–470. [Google Scholar]
- Pascual, M.; Alonso, S.; Parés, D.; Courtier, R.; Gil, M.; Grande, L.; Pera, M. Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer. Br. J. Surg. 2011, 98, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Harless, W.W. Cancer treatments transform residual cancer cell phenotype. Cancer Cell Int. 2011, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Harless, W.W. Revisiting perioperative chemotherapy: The critical importance of targeting residual cancer prior to wound healing. BMC Cancer 2009, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Harless, W.; Qiu, Y. Cancer: A medical emergency. Med. Hypotheses 2006, 67, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.; Nicholson, G.; Hall, G. Endocrine and metabolic response to surgery. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 144–147. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Cent. Eur. J. Immunol. 2020, 45, 469–475. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, E.; Hilsden, R.; Hawel, J.D.; Elnahas, A.I.; Schlachta, C.M.; Alkhamesi, N.A. Preoperative malnutrition in patients with colorectal cancer. Can. J. Surg. 2021, 64, E621–E629. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; D’Angelo, C.; Coppeta, L.; Mangifesta, R.; Jagarlapoodi, S.; Di Nicola, M.; Di Giampaolo, L. Network between cytokines, cortisol and occupational stress in gas and oilfield workers. Int. J. Mol. Sci. 2020, 21, 1118. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghaie, L.; Haxho, F.; Leroy, F.; Lewis, B.; Wawer, A.; Minhas, S.; Harless, W.W.; Szewczuk, M.R. Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications. Cells 2023, 12, 2767. https://doi.org/10.3390/cells12232767
Baghaie L, Haxho F, Leroy F, Lewis B, Wawer A, Minhas S, Harless WW, Szewczuk MR. Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications. Cells. 2023; 12(23):2767. https://doi.org/10.3390/cells12232767
Chicago/Turabian StyleBaghaie, Leili, Fiona Haxho, Fleur Leroy, Beth Lewis, Alexander Wawer, Shamano Minhas, William W. Harless, and Myron R. Szewczuk. 2023. "Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications" Cells 12, no. 23: 2767. https://doi.org/10.3390/cells12232767




