Abstract
Breast cancer (BC) can be classified into various histological subtypes, each associated with different prognoses and treatment options, including surgery, radiation, chemotherapy, and endocrine therapy. Despite advances in this area, many patients still face treatment failure, the risk of metastasis, and disease recurrence, which can ultimately lead to death. Mammary tumors, like other solid tumors, contain a population of small cells known as cancer stem-like cells (CSCs) that have high tumorigenic potential and are involved in cancer initiation, progression, metastasis, tumor recurrence, and resistance to therapy. Therefore, designing therapies specifically targeting at CSCs could help to control the growth of this cell population, leading to increased survival rates for BC patients. In this review, we discuss the characteristics of CSCs, their surface biomarkers, and the active signaling pathways associated with the acquisition of stemness in BC. We also cover preclinical and clinical studies that focus on evaluating new therapy systems targeted at CSCs in BC through various combinations of treatments, targeted delivery systems, and potential new drugs that inhibit the properties that allow these cells to survive and proliferate.
1. Introduction
1.1. Breast Cancer: General Aspects
Breast cancer (BC) is the most diagnosed cancer and the leading cause of cancer death in women worldwide [1]. BC has a worldwide incidence of 2,261,419 new cases per year (11.7% of all cancers) and 684,996 deaths (6.9% of all cancer-related deaths) in 2020 [2]. Rates of BC incidence and mortality rates in women are primarily influenced by geographic location and socioeconomic status. High-income North America, Oceania, and Western Europe countries have higher BC incidence rates, while lower-middle-income countries such as South America, East Africa, and Central Asia report fewer women diagnosed with BC but higher mortality, mainly due to late diagnosis and lack of health care resources [3].
BC is considered a complex and multifactorial disease, that arises from the accumulation of multiple genetic alterations and environmental factors. Six of the most relevant risk factors that can increase the possibility of developing BC are: (i) gender, most BC occur in women, only 1% of all BC cases occur in men [4]; (ii) age, the incidence rises sharply as people aged; (iii) reproductive factors such as early menarche, late menopause, late age at first pregnancy and low parity [5,6]; (iv) estrogen, both endogenous and exogenous are associated with the risk of this cancer; (v) modern lifestyles including excessive alcohol consumption and excessive fat intake in the diet, can increase the risk of breast cancer [7], and (vi) family history, almost a quarter of all cases of BC are related to family history, where women, whose mother or sister have BC, are prone to this disease [7,8].
The mammary gland is a highly dynamic organ that undergoes multiple phases of remodeling [3]. It is composed of an epithelium containing luminal and basal epithelial cells. The luminal cells form the ducts of the mammary gland, while the basal cells surround the luminal cells, and are in contact with the basal membrane [9]. This apparently hierarchical organization depends on a variety of stem and progenitor cells that populate the mammary gland [3]. The World Health Organization has defined 21 histological types of BC [10]. Breast adenocarcinomas more common are of two types: one that begins in the cells of the ducts (ductal adenocarcinoma) or in the lobules (lobular adenocarcinoma) (Figure 1), which can be in situ, if it has not spread, or invasive (infiltrating) if it has invaded other surrounding breast tissue. In addition, invasive carcinomas are designated by their architecture, secretion (mucinous/colloid), or structural form (medullary, tubular, papillary). Infiltrating ductal carcinoma is the most frequent, representing between 70 to 80% of invasive breast tumors [11]. These subtypes differ in their rate of relapse, metastasis, response to therapies, and composition of cancer cells.
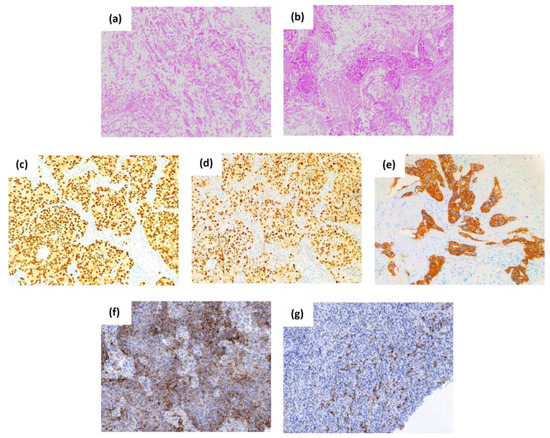
Figure 1.
The histological subtypes, common and stem cell-associated markers of breast cancer. The top panel shows representative histological micrographs stained with hematoxylin-eosin of lobular-type (a) and ductal-type breast carcinoma (b). Representative images of the expression of the first immunohistochemical biomarkers that have laid the foundations for the classification of the BC subtypes, the estrogen receptor (c), the progesterone receptor (d), and the human epidermal growth factor receptor 2 or HER2 (e). The bottom panel shows the expression of the breast cancer stem cell-associated biomarkers CD44 (f), and CD133 (g). Original magnification 100×.
BC is a heterogeneous disease that presents considerable diversity at the molecular, histological, and clinical levels. The first immunohistochemical biomarkers which have laid the foundation for the classification of the BC subtypes mainly include the expression of the estrogen receptor (ER), the progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). The evaluation of the expression of ER, PR, and HER2 is a routine procedure in clinical practice, and it is essential for the determination of these subtypes, and so to guide hormonal and anti-HER2 treatments, additional to predict the prognosis of patients with BC [12]. About 75% of BC tumors are ER and PR positive and are associated with a more favorable prognosis as they respond to endocrine therapies (estrogen receptor modulators, aromatase inhibitors, or estrogen receptor inhibitors) [13]. Tumors are interpreted as ER or PR positive when more than 1% of the tumor cell nuclei are stained (Figure 1) [14]. Approximately 20% of BC tumors have ERBB2 gene amplification and/or HER2 protein overexpression identified by fluorescent in situ hybridization or immunohistochemistry (Figure 1), respectively. These tumors are aggressive and have a poor prognosis and response to chemotherapy [11]. Tumors that do not express ER, PR, and HER2 are referred to as triple-negative (TNBC), and they account for 10-15% of the total [15]. This subtype is a clinical challenge, as it does not respond to standard endocrine therapies, such as tamoxifen (an anti-estrogen agent against the estrogen receptor) and trastuzumab (a monoclonal antibody against HER2), and is, therefore, associated with worse prognosis and survival, increased metastases, and higher risk of recurrence compared to the other BC subtypes [15]. Hence, it is necessary to develop new effective therapies for patients with BC, especially for the subtype TNBC.
More than 15 years ago, the analysis of the gene expression profiling of BC tumors suggested a new molecular classification that divides breast carcinoma into different subtypes according to their expression: (i) luminal (expression of ER, ER regulatory genes, and/or normal luminal epithelial cells), (ii) HER2-positive (amplification and/or overexpression of the ERBB2 gene), (iii) basal (ER, PR, and HER2-negative, but with an expression of genes from normal mammary myoepithelial and basal cells), and (iv) normal-like subtype [16,17,18]. These intrinsic subtypes differ according to the etiology of the cancer, the age of onset, and the predictive prognosis of the patient. Currently, BC characterization includes the recently mentioned immunohistochemical markers (ER, RP, and HER2), proliferation marker proteins such as Ki67, genomic markers (BRCA1, BRCA2, PIK3CA), immunomarkers such as PD-L1, among others [19,20].
These different subtypes and the intratumoral complexity highlight the great heterogeneity of BC, which needs to be understood in order to develop targeted therapies for each subtype. Conventional treatments for BC include surgery, hormone-blocking therapy, radiation therapy, and chemotherapy. However, 30–50% of patients diagnosed with this disease at an early stage will progress to metastatic recurrence, which may occur months or decades after the initial diagnosis despite the treatment administered [21,22].
1.2. Cancer Stem-like Cells in Breast Cancer
Although the largest cell burden of a tumor is formed by the so-called bulk tumor cells, a small subpopulation of cells within the tumor has recently been identified, which presents a stem cell phenotype, due to the similarities to these cells have been called “cancer stem-like cells” (CSCs) or “tumor-initiating cells” (TICs) (Figure 2) [23]. This cell population generally has the characteristic of unlimited self-renew, a hallmark of stem cells. Thus, these cells can divide symmetrically, producing two daughter cells with stem cell properties, or divide asymmetrically, producing a daughter cell with stem cell properties and a second cell that integrates with the tumor mass through differentiation mechanisms [24,25]. The symmetrical division allows excessively increased tumor growth in response to stress conditions, such as cell loss during treatments [26]. The CSCs present high tumorigenicity, several studies have proposed that they participate in all stages of cancer development, and would be responsible for the initiation, maintenance, and progression of tumors. In addition, they would participate in the expansion of the tumor to distant organs during metastasis and recurrence [27].
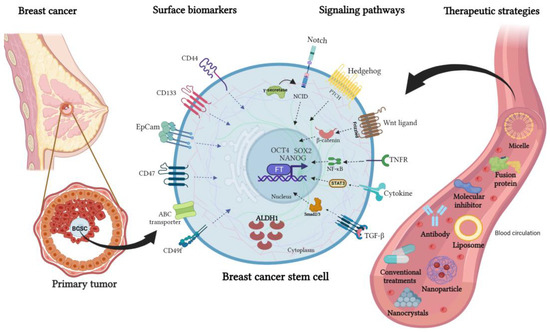
Figure 2.
Surface biomarkers, signaling pathways, and an overview of new therapeutic strategies to target breast cancer stem cells. Mammary CSCs express cell surface proteins that are used as biomarkers such as CD133, CD44, EpCAM, CD47, CD49f, and ATP-binding cassette (ABC) transporters. In addition, this cell subpopulation has active signaling pathways, being the main ones Notch, Hedgehog, TGF-β, Wnt/β-catenin, STAT3, PI3K/AKT, and NF-κB. The expression of transcription factors (Nanog, SOX2, and OCT4) allows them to acquire and maintain the characteristics of self-renewal, pluripotentiality, tumorigenic capacity, resistance to therapies, and high metastatic capacity. Several therapeutic strategies have been developed to deliver BCSC-targeted drugs, such as liposomes, micelles, nanoparticles, nanocrystals, and antibodies directed against membrane proteins or inhibitors of BCSC-associated signaling pathways.
The lethal behavior of CSCs is mainly due to their tumorigenic capacity just described, but these cells have also been shown to be more resistant to chemotherapy, endocrine therapy, and radiotherapy compared to bulk tumor cells [28,29,30]. Accumulated evidence shows that there is an increase in the CSC ratio after conventional treatment [31]. Different mechanisms are involved in resistance to therapies, including the overexpression of membrane transporter genes of the ATP-binding cassette (ABC) family, which encode proteins responsible for pumping drugs out of the cell. These cells are more resistant to chemotherapeutic agents; have increased DNA repair; increased aldehyde dehydrogenase (ALDH) activity, and can reduce intracellular reactive oxygen species levels [32].
Induction of epithelial–mesenchymal transition (EMT) has been shown to result in the acquisition of stem cell-like properties. EMT is characterized by decreased epithelial markers such as E-cadherin and upregulation of mesenchymal proteins (Vimentin, N-cadherin, and Fibronectin). These molecular alterations cause loss of apical polarity, loss of cell-cell epithelial junctions, and promote a reorganization of the cytoskeleton which allows cancer cells to migrate, invade, and metastasize [33].
Cells exhibiting the stemness properties in breast tumors are known as “breast cancer stem-like cells” (BCSCs) [3,27,34,35]. Different BC subtypes exhibit different proportions of BCSCs, subtypes TNBC and BRCA1 hereditary are enriched for cells with the stem cells phenotype (CD44+/CD24−), while HER2+ tumors contain very few cells with this phenotype [36,37]. Similar results have been obtained by evaluating ALDH1, another CSC marker, finding that basal-type breast tumors are enriched for BCSC cells [38]. It has been proposed that CSCs originate from normal adult stem cells or from differentiated progenitor cells undergoing transformation. In BC, this idea is well supported, since the breast, unlike other organs, develops after birth, therefore, it requires a reservoir of adult stem cells that fulfill homeostatic and developmental functions [39].
Tumor cells and the tumor microenvironment are in a dynamic process that plays an imperative role in driving these pathways for BCSC enrichment and maintenance [40]. There are many different non-malignant cell types (such as mesenchymal stem cells, cancer-associated fibroblasts, adipocytes, endothelial cells, and immune cells) that communicate with CSCs. Besides, there is an extracellular matrix, with secreted factors, and other microenvironmental components, that are helping to induce non-CSC cancer cells to acquire a stem cell phenotype and influence the subsequent fate of these cells [40,41,42]. CSCs are plastic and dynamic and can acquire different characteristics after interactions with the tumor microenvironment [43]. In the same way, CSCs can shape the microenvironment to support both primary tumor and metastatic growth [44,45,46].
Different strategies have been used to study, isolate and/or characterize BCSCs, including (i) analysis of cell surface markers by flow cytometry; (ii) in vitro functional detection assays such as side population [47,48], the activity of aldehyde dehydrogenases ALDH1, tumorigenesis assays by soft agar colony formation, evaluation of self-renewal with sphere formation assay (mammosphere in case of breast cancer) [48,49], analysis of the expression of EMT markers and drug resistance markers (mainly ABCG1, ABCC1, and ABCG2); (iii) in vivo tumorigenesis assays by heterotopic and/or orthotopic xenograft models derived from cell lines or from patient tumors, among others [50,51].
1.3. Preclinical Research on Therapeutic Compounds against Biomarkers and Signaling Pathways Associated with Breast Cancer Stem Cells
The identification of biomarkers of BCSCs has had a growing interest in the BC field. Several studies have associated the expression of the biomarkers with diagnosis, therapy, and/or cancer prognosis, however, the clinical use of these CSC-specific biomarkers has been very limited. Thus, these markers could have tremendous potential not only in a better understanding of the clinical diagnosis, and tumor biology understanding but also most importantly, as new therapeutic strategies directed mainly against the BCSC population in tumors.
Currently, conventional therapeutic agents used to treat BC primarily target the tumor bulk but are less effective in killing CSCs. Therefore, it is necessary to develop better therapeutic agents focused on molecular targets expressed in BCSC, and through this strategy, target the cell population that survives conventional treatments, and is the cause of tumor recurrence, promoting the successful BC eradication. In this way, multitarget inhibitors are promising methods to overcome drug resistance and chemoresistance of BCSCs [9]. In this review, we focus on compounds that target biomarker membrane proteins of BCSCs and compounds that regulate CSC-associated signaling pathways in BC. The proteins are listed below and summarized in Table 1.
1.3.1. Compounds Targeting Membrane Proteins
The increased understanding of CSC surface biomarkers has enabled significant progress in the development of CSC-targeted antibodies, and it has become an emerging technology for cancer therapy. This population is characterized by a signature of stem cell gene expression, and cell surface proteins such as CD133, CD44, EpCAM, CD49f, CD47, ABCG2, and CD24 among others that have been associated with the BCSCs phenotype (Figure 2) [52,53].
CD44 is a receptor for hyaluronic acid and participates in the formation of complexes between extracellular components and elements of the cytoskeleton [54,55]. This receptor regulates critical cell processes including cell adhesion, migration, survival, invasion, and epithelial–mesenchymal transition through signaling pathways such as Rho GTPases, Ras-MAPK, and PI3K/AKT [54,56]. Accumulating evidence indicates that CD44 is not only a marker for CSC in BC but also in other types of cancer [57,58,59,60]. BC cells with a CD44+/CD24−/low phenotype have a high tumorigenic capacity compared to those cells with a different phenotype that fail to form tumors [56,61]. CD44 has several isoforms as a result of alternative splicing and N- and O-glycosylation post-translational modifications. The isoform switching from CD44v to CD44s is necessary for cells to undergo EMT. Furthermore, Zhang et al., demonstrated that CD44s activate the PDGFRβ/STAT3 cascade which promotes the acquisition of BCSC characteristics. Additionally, they found that the TNBC subtype has a higher number of CSCs that mainly overexpress the CD44s isoform [58]. CD44 plays a pivotal role in CSCs in communicating with the microenvironment and regulating CSC stemness properties [62].
Hyaluronic acid (HA) has the inherent ability to target CD44 receptors, which is why it has been used to develop CSC-targeted drug delivery systems. Lapatinib (LPT) is a dual EGFR and HER2 inhibitor used as a treatment for advanced BC of the HER2 subtype, however, it has low oral bioavailability and several side effects. Agrawal et al., designed a delivery system for this inhibitor. LPT-HA-NCs are LPT nanocrystals coated with hyaluronic acid to actively target CD44 receptors on BC cells. In vitro and in vivo studies showed that LPT-HA-NCs have several cellular effects including anticancer activity of LPT-HA-NCs, increase of the intracellular concentration of LPT, promote apoptosis, and delay significantly the tumor growth [63]. Han and colleagues, developed a hyaluronan-conjugated liposome delivery system that encapsulates gemcitabine, targeting the BCSC population. This system improves the stability of gemcitabine in the bloodstream, prolonging its half-life. In vitro experiments showed a significant improvement in cytotoxicity compared to free gemcitabine. In addition, gemcitabine induces inhibition of colony-forming and cell migration. In a xenograft mouse model, a significant antitumor effect of the system was found [64]. Mitoxantrone (MTX), is an anthraquinone derivative and is used in the treatment of a wide spectrum of tumors including BC, but it has significant side effects. For this reason, Sargazi et al., developed a more effective form for administration MTX through hyaluronic acid/polyethylene glycol nanoparticles. The authors propose this delivery form as an effective nanomedicine to eliminate CD44-positive breast cancer stem cells [65]. In order to study the efficacy of nanomedicines directed against CSCs, Gener et al., developed a new model of the fluorescent label for CSCs that allows efficient detection of this subpopulation. Using this CSC reporter system, the efficacy of polymeric micelles functionalized with anti-CD44 antibodies and loaded with paclitaxel (PTX) was evaluated. The data showed that antibody-mediated targeting increases the efficacy of PTX, as well as that the mammary CSC population was sensitized to PTX treatment only when these specific micelles were used. The experiments were replicated in colon cancer cell lines, obtaining similar results [66].
CD133 is a transmembrane glycoprotein also known as Prominin 1. It is often used as a biomarker for CSC enrichment in cancer, as it targets a subset of cancer cells that show a drug-resistant phenotype and an increased tumor-initiating ability in xenotransplantation assays [67]. In BC, high levels of CD133 mRNA are associated with basal, estrogen receptor-negative tumors, and higher-grade tumors (grade 3 versus 1–2) [68]. In addition, it is considered a factor of poor prognosis for metastasis-free survival in all the histological subtypes of BC analyzed to date [69]. The precise molecular mechanisms by which CD133 acts in cancer remain unclear [70].
CD133 is critical for the survival and growth of BCSCs, and antibodies against CD133 can reduce BCSC growth. Ohlfest et al., developed an immunotoxin against CD133 by fusing a gene fragment encoding the scFv portion of an anti-CD133 antibody to a gene fragment encoding deimmunized PE38KDEL. This fusion protein dCD133KDEL was shown to represent a new biological assessment tool that can be used to determine the clinical importance of eradicating CD133-positive cells since selectively inhibited CD133+ ductal breast carcinoma cells, and cause regression of tumor growth in mice [71]. Swaminathan et al. designed polymeric nanoparticles conjugated with an anti-CD133 monoclonal antibody (CD133NP) which were loaded with PTX (microtubule-stabilizing anticancer agent). The effect of CD133NPs on tumor-initiating cells was measured in vitro, finding that the treatment with CD133NPs significantly reduces the number of mammospheres. The anticancer effectiveness of CD133NPs in vivo in an orthotopic mouse model of BC was also investigated. This treatment effectively reduced tumor volume compared to treatment with free PTX [72]. Yin et al., designed a therapy with nanoparticles carrying anti-miR21 and the CD133 aptamer to target the stem cell marker as therapy against TNBC. Treatment with these nanoparticles had high efficacy in inhibiting tumor growth in the MDA-MB-231 xenograft model compared to the control group. Furthermore, it showed high specificity in targeting the TNBC tumor [73]. The compound 3,3′-diindolylmethane (DIM) has been studied as a chemo-preventive agent in BC. However, after the treatment with this compound, the CD133+/NANOG+ subpopulation increased, and the AKT signaling pathway was significantly activated. When using DIM in combination with the AKT inhibitor AZD5363, DIM-induced CD133 expression was decreased, further suppressing stemness and promoting anticancer activity [74].
EpCAM also designated CD326, is a membrane glycoprotein located mainly in the basolateral membrane of the cells, that participates in the adhesion of epithelial cells. EpCAM is recognized as one of the cell surface markers used for the identification and isolation of CSCs from various cancer types [75]. In vitro BC studies reported that the overexpression of EpCAM promotes proliferation, invasion, and metastasis in cancer cells. In addition, it has been associated with clinicopathological characteristics such as poor prognosis, larger tumor size, high histological grade, and bone metastases in BC [76]. Recent studies carried out by Dionisio et al., identified that EpCAM, along with other BCSC markers including CD44, and ALDH1, were significantly enriched in BC brain metastases compared with primary tumors. Furthermore, these patients had a worse prognosis, poor overall survival, and positive lymph nodes [77].
Catumaxomab (anti-EpCAM × anti-CD3) is a bispecific monoclonal antibody that binds to both EpCAM (on tumor cells) and CD3 (on T cells). Kubo’s group conducted an in vitro study testing the combination of catumaxomab and activated T cells in BC cell lines, finding that this combination can eliminate chemoresistant EpCAM-positive TNBC cell lines. The authors proposed that catumaxomab combined with activated T cells might act as a strong treatment to eliminate this type of cells [78]. Another immunotherapy approach developed an EpCAM aptamer-linked to small-interfering RNA chimeras to selectively knock down genes in mouse BC. This therapy was applied subcutaneously in orthotopic mouse models of HER2+ and aggressive TNBC tumors. The results obtained with this therapy showed a notable delay in tumor growth, and consequently, the function of tumor-infiltrating immune cells was enhanced [79].
CD47 is an important protein in immune control as it can bind to its SIRPα ligand on macrophages, and inhibit their phagocytic capacity [80]. CD47 antibody B6H12 inhibits BCSC proliferation, asymmetric division, and expression of the mammary CSCs transcription factor KLF4 [81]. The co-expression of CD47 and HER2 markers are detected in BC patients with a poor prognosis [82]. Therefore, Candas-Green et al., generated a B6H12.2 antibody with a dual activity that blocks CD47 and HER2, this enhancing macrophage-mediated phagocytosis, and also suppressing the aggressive phenotype associated with HER2 by killing radioresistant BCSCs [82].
Although HER2 is not a marker of BCSCs by itself, different strategies directed against HER2-positive BC have been developed, including monoclonal antibodies that bind to the extracellular domain of HER2, EGFR-HER2 small-molecule kinase inhibitors, and antibody-drug conjugates. Yet, most patients with metastatic disease eventually progress to anti-HER2 therapy due to resistance to the traditional treatments [83]. Therefore, developing therapeutic strategies against the population of CSCs in HER2-positive tumors is essential. Metformin (MET) is an anti-type 2 diabetic agent effective against BC, and it has become of high therapeutical interest due to its ability to target BCSC [84]. The anticancer effect of MET in Herceptin-Conjugated Liposome (Her-LP-MET) has been evaluated in vitro and in vivo. This treatment produced greater inhibition of BCSC proliferation in vitro compared to free MET. The anti-migratory effect of Her-LP-MET on BCSC was enhanced when used in conjunction with doxorubicin (DOX). In the same way, in a mouse model, Her-LP-MET combined with free DOX was more effective, reducing tumor mass and prolonging tumor remission. This Her-LP-MET formulation is proposed as a new therapy to efficiently targets BCSCs [85]. Other therapeutic strategies have also been tested for cells that express the HER2 receptor. The combined treatment of a carbon-ion beam and LPT, effectively destroys the proportion of HER2-positive BC stem cells (ESA+/CD24−), decreasing cell viability, the formation of spheroids, and promoting apoptosis [86].
1.3.2. Compounds That Regulate Signaling Pathways
In recent years, various strategies have been proposed to eliminate BCSCs. These include the blockade of signaling pathways associated with the acquisition of stem cell characteristics [87]. BCSCs exhibit dysregulation of critical signaling pathways such as Notch [88], Hedgehog (Hh) [89], transforming growth factor-β (TGF-β) [90], Wnt/β-catenin [27,91], STAT3 [92,93], PI3K/AKT/FOXO [94], and NF-κB [95]. These cellular signalings allow BCSCs to have a greater capacity to proliferate and tolerate hostile environments. BCSCs also express metabolic markers of stem cells, such as aldehyde dehydrogenase (ALDH1) [96], transcription factors such as OCT4 (octamer-binding factor 4), SOX2 (SRY-box transcription factor 2), NANOG, KLF4, and MYC, that act as key regulators of pluripotency and self-renewal [97] (Figure 2). Importantly, these pathways also play a fundamental role in the normal development of the mammary glands.
The Wnt/β-catenin pathway is a critical pathway in embryonic development and tissue homeostasis. In cancer, it plays a fundamental role in the functioning of the CSC, orchestrating self-renewal and cell differentiation [98]. In BC, more than half of the tumors have this active pathway, which is associated with decreased patient survival of these patients. Given the importance of this signaling pathway in cancer, a large number of inhibitory compounds targeting proteins of this pathway have been designed [99]. The group of Jang et al., designed a selective small-molecule inhibitor called CWP232228 (US Patent 8,101,751 B2), which prevents β-catenin from binding to TCF in the nucleus. In vitro and in vivo studies demonstrated that treatment with CWP232228 targets BCSC populations, blocking the formation of secondary spheres in cells resistant to conventional chemotherapy. Besides, the inhibitor suppresses tumor formation in a murine xenograft model and metastasis by inhibiting the growth of both, bulk tumor cells and BCSCs. It is proposed that this inhibitor would have significant therapeutic potential for the treatment of BC [100].
The TGF-β signaling pathway has biological activity capable of inducing the oncogenic transformation of non-cancerous cells. TGF-β promotes cell survival and proliferation, in addition to stimulates the maintenance of stemness in the BCSC population [101]. Several studies suggest that TGF-β-induced CSC accumulation in TNBC is a mechanism of drug resistance in TNBC [37]. Park et al., reported that blockade of TGF-β signaling with the inhibitor EW-7197, in combination with PTX, reduce the EMT process and BCSC population (induced by PTX treatment) by suppressing Snail expression. Besides, a reduction of mammosphere formation efficiency, a decrease of ALDH activity and CD44+/CD24− phenotype, and diminish of pluripotency regulators (OCT4, NANOG, KLF4, MYC, and SOX2) was observed. This combined treatment enhances the therapeutic effect of PTX by decreasing lung metastasis, and increasing survival time in vivo [102]. Another study also shows that PTX increases the BCSC population through TGF-β signaling. The inhibition of this pathway with the inhibitor LY2157299 reduced the population of BCSCs resistant to PTX in vivo, and abolished the tumor-initiating potential of BCSCs after chemotherapy [103].
In the Notch signaling pathway, Notch is a ligand-activated transmembrane receptor (delta-like (DLS) and Jagged), which undergoes serial cleavage by γ-secretases, leading to the intracellular portion of Notch translocating to the nucleus where activates the expression of downstream transcription factors [104]. It has been reported that this pathway is over-activated in BC, and would be promoting chemo/radioresistance, both in BC cells and in BCSC [105]. Several research groups hypothesize that Notch inhibition will allow BCSC to be eliminated, controlling the progression of BC [106]. Currently, there are three main clinical methods used to inhibit Notch signaling. These include γ-secretase inhibition (GSI), antibodies against Notch receptor or ligand antibodies, and a combination therapy with other pathways [107]. A study conducted by Li et al., evaluated the in vitro and in vivo effect of five inhibitors directed to BCSC in TNBC. The tested inhibitors were DAPT, GDC-0449, Salinomycin, Ruxolitinib, and Stattic, that target Notch (γ-secretase), Hedgehog (SMO), Wnt (β-catenin), JAK/STAT, and JAK (STAT3) pathways respectively. The inhibitors were found to have antiproliferative and proapoptotic functions, along with suppressing invasion and decreased self-renewal (markedly diminished size and number of mammospheres) in BCSCs, in TNBC cells. Also, these inhibitors reduced the expression or phosphorylation of their downstream signaling target molecules in a dose-dependent manner. Furthermore, these five inhibitors suppress the tumor-forming ability of the TNBC stem cell line HCC1806 when pretreated with the inhibitors in xenograft mouse models [15]. In another study, mesoporous silica nanoparticles (MSNPs) have been designed as vehicles for the targeted delivery of GSI to block Notch signaling. In vivo analysis, showed that MSNP-GSI nanoparticles enhanced Notch signaling inhibition compared to the free drug. These data suggest that MSNP-GSI is an attractive platform for the targeted delivery of anticancer drugs and, specifically, against the population of CSCs [108].
The FK506 binding protein (FKBPL) is considered a prognostic and predictive biomarker of BC since it has antitumor and antiangiogenic activity. FKBPL has been shown to have the ability to target CD44-positive cells and reducing their population. Also, FK506-derived peptides (ALM201 and AD-01) inhibit the BCSC resistant to endocrine therapy. In addition, downregulation of DLL4 and Notch4 has been reported to reduce migration, invasion, and pulmonary metastasis of BC [109,110].
The Hedgehog (Hh) signaling pathway includes three secreted ligands, of which Sonic Hedgehog (SHH) is the most widely expressed, followed by transmembrane receptor/coreceptor Patched (PTCH) and Smoothened (SMO). Activation of this pathway occurs when the Hh ligands bind to the PTCH transmembrane receptor, which regulates the SMO transmembrane protein The binding induces the activation of the GLI oncogene, which is a transcriptional effector of the Hh pathway [111]. This pathway regulates the maintenance, self-renewal, survival, and proliferation of BCSC [37,112]. In TNBC, Hh signaling has been associated with high-grade, highly proliferative cancer, increased metastasis, and poorer disease-free survival [113]. GANT61 is a non-canonical Hh pathway inhibitor. GANT61 was able to decrease E2-induced GLI1/2 activation accompanied by a decrease in the proportion of CSCs in ER-positive BC cells [89]. Another study evaluated the effect of GANT61 on TNBC cells and found that this inhibitor significantly decreased the proportion of BCSCs in all TNBC cell lines analyzed. The combined treatments of GANT61 and PTX greatly enhanced anti-cell growth and/or anti-CSC activities. These results suggest that GANT61 has the potential as a therapeutic agent in the treatment of patients with TNBC [114].
In the STAT3 signaling pathway, the tyrosine kinase Janus-activated kinase 2 (JAK2) is hyperactive in HER2-positive and TNBC tumors [115]. JAK2 phosphorylates and activates STAT3 to induce its nuclear translocation, increasing the expression of genes that promote CSC turnover [116]. Dysregulation of the JAK2-STAT3 pathway is associated with poor clinical outcomes, and it has been investigated as a possible therapeutic target for BCSC [92,93]. The effect of combined inhibition of the JAK2-STAT3 (by Ruxolitinib and Pacritinib) and SMO-GLI1/tGLI1 (by Vismodegib and Sonidegib) signaling pathways on the BCSC population has been evaluated. The combined treatment with these inhibitors suppressed the high CD44high/CD24low BC stem cell population compared with vehicle or either every agent alone. Also, the simultaneous inhibition of the JAK2-STAT3 and SMO signaling pathways suppresses the orthotopic growth of TNBC tumors and reduces metastasis in vivo [93]. Tamoxifen (TAM) is the first-line treatment for ER+ receptor-positive BC. However, resistance to this drug is the main obstacle in clinical practice. Therefore, new therapies are needed to treat TAM resistance. Liu et al., evaluated the effect of Napabucasin, a small STAT3 inhibitor, and found that it attenuates BC cell resistance to TAM by reducing the population of BCSCs, which was evidenced by the decrease in stem markers (OCT4, NANOG, and SOX2), in combination with a reduction in the ability to form spheroids and a decrease of the ALDH1 activity [91].
Aldehyde dehydrogenase-1 (ALDH1) is a detoxification enzyme that catalyzes the oxidation of intracellular aldehyde substrates. Often, ALDH1 is used to isolate and identify normal cell populations enriched in stem and progenitor cells, as well as BCSCs in cancerous tissues [96]. In BC, ALDH1 positivity has been correlated with high histological-grade tumors, HER2 overexpression, and the absence of the expression of estrogen and progesterone receptors [117,118]. In addition, it was reported that the expression of this marker is related to a poor prognosis [118]. ALDH1+ BCSCs are enriched in basal-like and HER2-overexpressed tumors [38]. Furthermore, the expression of ALDH1 contributes to both chemotherapy and radiation resistance [119].
Analyses of gene expression profiles have shown that there are two different subpopulations of BCSCs, those that display a CD44+/CD24− phenotype, which is a mesenchymal marker, located in the ductal structures frequently associated with ductal branch points [120]. Conversely, ALDH+ BCSCs, are epithelial and highly proliferative cells located in an abluminal location within lobules. These two BCSC subgroups can transit through these two dynamic states [120,121,122]. Indeed, BCSC CD44+/CD24−/low/ALDH1+ phenotype is a very small population within the tumor [38]. This population is more tumorigenic, and it is able to recapitulate the tumor the after reduction of the cell population sensitive to therapy, which leads to relapse of the disease [123]. Disulfiram (DSF) is a drug used as a treatment for alcoholism that produces an irreversible inhibition of ALDH [124]. Its effect on the CSC population in BC was evaluated and it was found that DSF eradicates BCSC, inhibits CSC marker expression such as SOX2 and OCT4, and reverses PTX and cisplatin resistance in MDA-MB-231 PAC10 cells (resistant to cytotoxicity) [125]. A subsequent study investigated the mechanism of action of DSF on BCSCs. They found that DSF eliminated BCSCs and inhibits tumor development in vivo through suppression of HER2/AKT signaling. [126]. Another study showed, for the first time, that DSF suppresses stem properties in TNBC by targeting the STAT3 signaling pathway [127].
1.4. Drug Repurposing to Target Breast Cancer Stem-like Cells
New drug development is a significantly expensive multi-step process, involving drug design and synthesis, as well as re-testing in animal models, to ensure precise safety and efficacy. An alternative strategy to de novo drug development is drug repurposing, which uses molecules already approved by the FDA for new therapeutic indications that can effectively eradicate BCSCs and control metastatic disease in BC. The latter represents an effective strategy to rapidly improve the prognosis of BC patients [128]. Salinomycin and pyrvinium pamoate are 2 FDA-approved drugs that have been redirected to the treatment of BCSC [129].
Salinomycin (SLM) is an antibiotic identified as an effective anti-BCSC compound. SLM reduces the proportion of CSC by >100-fold compared to PTX and inhibits mammary tumor growth in vivo. Besides, it decreases BCSC-associated gene expression through modulation of WNT and Hh signaling pathways [130,131,132]. SLM has also been tested in conjunction with LBH589 (Panobinostat, a histone deacetylase inhibitor) as a new therapy against TNBC. The combined treatment of these drugs showed effective and synergistic inhibition of tumor growth of an ALDH1+ TNBC xenograft mouse model, by inducing apoptosis, cell cycle arrest, and EMT regulation, with no apparent associated severe toxicity. Therefore, this drug combination could offer a novel therapeutic strategy for patients with TNBC [133]. A recent study demonstrated that SLM and its C20-propargylamine derivative (Ironomycin 2) eliminate BCSCs by ROS production, induced after iron accumulation in lysosomes [134]. Another delivery system for SLM was designed by Muntimadugu et al., They prepared nanoparticles loaded with SLM or PTX and coated them with hyaluronic acid to target BCSC cells that overexpress the CD44 receptor. The results obtained in this study showed a co-eradication of CD44+ BCSCs as well as bulk tumor cells. Combination therapy of HA-coated SLM nanoparticles and PTX nanoparticles is a promising approach to overcome cancer recurrence due to resistant BCSCs [135].
Pyrvinium pamoate (PP) is an anthelmintic drug and a suppressor of the WNT pathway. Xu’s group evaluated the effect of the pharmacological blockade of this pathway with PP on the BCSC population as a possible therapy to treat BC. They found that this drug inhibits the in vitro proliferation and self-renewal of BCSCs in BC cell lines. Moreover, PP decreases the content of CD44+/CD24−/low and ALDH+ BCSCs in a panel of BC cell lines. In a xenograft model of BC cells, it was shown that cells pretreated with PP strongly delayed tumor development. Furthermore, an in-depth analysis revealed that PP inhibits the activity of the WNT pathway and the expression of stem regulators (NANOG, SOX2, and OCT4) [136]. A related study identified the metabolic consequences of PP. This drug inhibits the anabolic metabolism of fatty acids and cholesterol, both are essential for the survival of BCSC in TNBC [129].
Cui et al. screened a drug library of FDA-approved compounds (Prestwick Library) to identify inhibitors of mammary CSCs. They found that the treatment with benztropine mesylate decreases the cell subpopulation that shows both ALDH1 activity and CD44+/CD24− phenotype, in addition to the suppression in the formation of mammosphere and the decrease of the self-renewal properties of BCSC. Additionally, in vivo studies showed that benztropine mesylate inhibited the potential for tumor initiation of the 4T1 cell line in a mouse model. Their findings show that benztropine mesylate could be a good BCSC inhibitor both in vitro and in vivo [137].

Table 1.
Preclinical studies of therapeutic compounds directed against breast cancer stem-like cells.
Table 1.
Preclinical studies of therapeutic compounds directed against breast cancer stem-like cells.
| Therapeutic Molecule | Targeting | Effects | Ref |
|---|---|---|---|
| LPT-HA-NCs | CD44 | Anticancer activity and delays tumor growth | [63] |
| Hyaluronan-conjugated liposomes encapsulating gemcitabine | CD44 | Inhibits migration and colony formation ability in vitro assays. Potent antitumor effect in vivo | [64] |
| Polymeric micelles functionalized with anti-CD44 antibodies and loaded with paclitaxel | CD44 | Increases the sensitivity of mammary CSCs to PTX treatment | [66] |
| FKBPL and its peptide derivatives | CD44 | It inhibits tumor growth and CD44-dependent antiangiogenic activity. It also targeting CD44-positive BCSCs, it also inhibits migration, invasion, and formation of mammospheres resistant to endocrine therapy. It reduces lung metastases in an in vivo model by downregulating DLL4 and Notch4 | [109,110] |
| Fusion protein dCD133KDEL | CD133 | Selectively inhibits CD133+ ductal breast carcinoma cells, causing regression of tumor growth in mice | [71] |
| Polymeric nanoparticles conjugated with an anti-CD133 antibody loaded with paclitaxel | CD133 | Reduces the number of mammospheres and cell colonies. In animal models decreases tumor volume | [72] |
| 3WJ/CD133apt/anti-miR21 nanoparticles | CD133 | High specificity in targeting TNBC tumor. High efficacy in tumor growth inhibition | [73] |
| Catumaxomab | EpCAM | Eliminates chemoresistant EpCAM-positive triple-negative cell lines | [78] |
| EpCAM aptamer-linked small-interfering RNA chimeras | EpCAM | Delays tumor growth and improves function of tumor-infiltrating immune cells | [79] |
| B6H12 | CD47 | Inhibits proliferation, asymmetric division, and KLF4 expression in BCSC | [81] |
| B6H12.2 | CD47 and HER2 | Inhibits the phagocytosis capacity of macrophages and abolishes the aggressive BCSCs phenotype of BCSCs | [82] |
| Metformin in Herceptin-Conjugated Liposome | HER2 | Inhibits proliferation and migration of BCSCs in vitro. In an animal model, reduces tumor mass and extends tumor remission | [85] |
| Carbon-ion beam combined with lapatinib | HER2 | Decreases BCSC ratio, cell viability, spheroid formation, and promotes apoptosis | [86] |
| CWP232228 | Wnt/β- catenin pathway | Inhibits sphere formation, decreases the proportion of BCSCs resistant to conventional therapy, and further reduces tumor growth and metastasis in vivo | [100] |
| EW-7197 | TGF-β signaling pathway inhibitor | In combination with PTX reduces the EMT process and proportion of BCSCs | [102] |
| LY2157299 | TGF-β signaling pathway inhibitor | Reduces the population of BCSCs resistant to PTX and abolishes the tumor-initiating potential of BCSCs after chemotherapy | [103] |
| Napabucasin | STAT3 inhibitor | Attenuates the BC cell resistance to tamoxifen by reducing the BCSC population | [91] |
| DAPT, GDC-0449, Salinomycin, Ruxolitinib, and Stattic | Notch, Hedgehog, Wnt/β-catenin, JAK, and JAK/STAT3, respectively | Antiproliferative and proapoptotic activities, suppress invasion and self-renewal of BCSC in vitro. They suppress the ability to form tumors in vivo | [15] |
| GANT61 | Non-canonical hedgehog pathway | Decreases E2-induced GLI1/2 activation and the proportion of BCSCs in ER-positive BC and TNBC cells | [89,114] |
| Disulfiram | STAT3 pathway | Inhibits STAT3 pathway suppressing stem properties in BC | [125,127] |
| Salinomycin | WNT and Hh signaling pathways | Reduces the proportion of BCSC and inhibits mammary tumor growth | [134] |
| Pyrvinium pamoate | Selective WNT pathway inhibitor | Inhibits proliferation and self-renewal of CSCs in BC cell lines. In the xenograft model of BC, strongly delays tumor development | [136] |
| Pranlukast | CD49f | Reduces the CSC population in TNBC cells | [138] |
| Benztropine Mesylate | NS | Decreases cell subpopulation with ALDH1 activity and CD44+/CD24− phenotype, diminishes mammosphere formation, and BCSC self-renewal capacities | [137] |
BC: breast cancer; BCSC: breast cancer stem cell; TNBC: triple-negative breast cancer; GLI: glioma-associated oncogene; PTX: Paclitaxel; HER2: Human epidermal growth factor receptor 2; GSI: γ-secretase inhibitor; NS: not specified.
CD49f (α6 integrin; ITGA6) is expressed on breast CSCs and functions in the maintenance of stemness. Pranlukast, a drug used to treat asthma, works as an antagonist of CD49f. Pranlukast treatment decreased BC cell clonogenicity in the mammosphere formation assay, and also diminished CD44 and SOX2 expression, and tumorigenicity in vivo, showing that this drug reduces the CSC population in TNBC cells [138].
Wedelolactone is a promising anticancer drug [139]. To enhance its biological activity, wedelolactone-encapsulated PLGA nanoparticles (nWdl) were formulated. In vitro assays showed that nWdl delayed migration, invasion, and EMT in MDA-MB-231 cells compared to the free drug. Wedelolactone significantly upregulates mesenchymal markers, such as N-cadherin, Vimentin, Twist, Snail, and Slug. Moreover, it also upregulated epithelial markers E-cadherin and cytokeratin-19. Regarding the population of BCSCs, it was observed that the expression of ALDH+ cells was significantly decreased when treated with nWdl combined with PTX relative to PTX alone. Also, nWdl significantly inhibited the formation of mammospheroids, and reduced the expression of pluripotency and chemoresistance markers such as ABCG2, SOX2, and ALDH1. In vivo studies showed that nWdl effectively reduced tumor volume and BCSC population (CD44+/CD24−/low) in xenograft models [140].
One of the main challenges in BCSC research is at the translational level, moving from laboratory experiments toward appropriate clinical trials to test new compounds targeting BCSCs. The challenge includes to assessing their efficacy in killing the BCSC population of patient tumor cells [141].
1.5. Breast Cancer Stem-like Cell Targeted Therapies in Clinical Trials
In the last decade, a wide variety of treatments have been developed in clinical trials based on the classification of BC molecular subtypes. Current therapies for BC (chemotherapy and hormone therapy) are effective in killing cancer cells and controlling tumor growth. Most anticancer compounds only affect tumor cells that are in a proliferative state, and they leave behind a small population of dormant CSCs. These cells exhibit increased tumorigenic potential, and often acquire an EMT phenotype, leading to subsequent relapse and therapy-resistant metastases [142]. It has been reported that almost all patients with metastatic BC, and a quarter of those with early BC will relapse despite the initial response [106]. Nevertheless, only a few clinical trials evaluate the effectiveness of conventional treatments against this specific population of cancer cells, even when targeting BCSCs seems to lead to promising therapies [107].
Antitumor therapies specifically targeting BCSCs have long been advised to be administered in conjunction with the traditional chemotherapeutic regimen with the goal of preventing relapse. Interestingly, there are several clinical trials being developed to determine the efficacy of specific therapies against the BCSC population [143]. In addition, these clinical trials have helped to understand the biology, and regulatory mechanisms of BCSCs, and how they respond to new therapies.
To identify new compounds or treatments that might be evaluated in a clinical trial setting against the BCSCs population, a search on the website ClinicalTrials.gov was performed using the keywords “breast cancer stem cells”. Almost 150 clinical trials were found to date, but only a small part of them were actually therapies directed toward BCSCs. The vast majority were peripheral stem cell transplants as a treatment for patients with BC. Clinical trials targeting BCSCs will be detailed below and are summarized in Table 2.

Table 2.
Clinical trials of therapeutic agents targeting breast cancer stem-like cells.
1.5.1. Antibodies Targeting Breast Cancer Stem-like Cells
One of the clinical trials with therapeutic agents targeting CSC in BC is the AVASTEM NCT01190345 trial, which evaluated the anticancer capacity of preoperative Bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor (VEGF) receptor, along with conventional therapy in 75 participants with BC. The proportion of BCSCs was measured by the number of cells positive for the ALDH1 marker after 4 cycles of treatment. This trial does not confirm the impact of Bevacizumab on breast CSC cells, as it did not change BCSC rates compared to standard neoadjuvant chemotherapy [144]. Therefore, this antibody still has a controversial role in the treatment of BC [145].
In the clinical trial, NCT01424865, the expression of the cancer stem cell biomarker CD44+/CD24− y ALDH1 was evaluated as a predictor of response to Trastuzumab in 1874 samples from BC patients previously treated in the NSABP-B-31 trial. The results of this clinical trial suggest that the CD44+/CD24− phenotype may be used as a predictor of clinical outcome, and as a predictor of response to Trastuzumab treatment in patients with HER2-positive primary BC [146].
Schmidt et al. conducted a randomized phase II study to investigate the efficacy of adecatumumab (anti-EpCAM) as monotherapy in 117 patients with metastatic BC. They found that the probability of tumor progression was significantly lower in patients who received high doses of adecatumumab, and who expressed high levels of EpCAM. But the use of this antibody in BC requires more research [147]. Subsequently, the same group evaluated the combined effect of adecatumumab with Docetaxel (DTX) in a phase IB trial in 31 women with advanced-stage BC, especially for high EpCAM-expressing tumors. They declare that this combination therapy is safe, tolerable, and potentially active in advanced-stage BC [148]. Another clinical trial directed at EpCAM is NCT02915445, in which the safety of EpCAM CAR-T cells was determined in 30 patients with nasopharyngeal carcinoma or BC expressing high levels of EpCAM. Chimeric antigen receptor-modified T cells (CAR-T) have the ability to target tumor antigens such as EpCAM, and can specifically recognize, bind to, and kill antigen-positive tumor cells [149]. In these patients, the potential to inhibit tumor progression will be measured. The results of this clinical trial are expected to provide a new treatment strategy for patients with BC, but nevertheless, the results of this study are not yet known.
The clinical trial NCT02254005 evaluated the maximum tolerable dose, safety, pharmacokinetics, and efficacy of a single dose of the Bivatuzumab mertansine antibody in female patients with CD44v6-positive metastatic BC. The disease was stabilized in 50% of the treated BC patients regardless of the dose level [150].
The currently running clinical study NCT02776917 is evaluating the combined treatment of Cirmtuzumab, a monoclonal antibody directed against the ROR1 (receptor-tyrosine-kinase like orphan receptor 1), with PTX, in 22 patients with unresectable BC, metastatic or locally advanced. After 4 weeks of treatment, ROR1 expression levels will be measured and the proportion of CSCs will be evaluated through ALDH1 and CD133 markers. Additionally, primary tumor samples will be compared before and after treatment.
1.5.2. Inhibitors of Breast Cancer Stem-like Cells Signaling Pathways
Several research groups have hypothesized that inhibition of the Notch pathway is effective to eliminate BCSCs, and thus, controlling advanced BC [106]. In a first phase I clinical trial (NCT00106145) conducted in 103 patients with metastatic or advanced BC, the safety/tolerability and efficacy of the Notch signaling pathway inhibitor, MK-0752, were determined. The study shows a clinical benefit of the γ-secretase inhibition, which led to further trials in combination with other therapies to maximize the clinical benefit of the strategy [151]. Schott et al., suggest that the combination of cytotoxic chemotherapy together with MK-0752 would give better results to efficiently eliminate BCSC, and control the disease. Therefore, preclinical and clinical studies were carried out in parallel [106]. Using human breast tumor graft studies, they evaluated the impact of this inhibitor on the BCSC population, and the efficacy of a combination treatment of MK-0752 with DTX. The study demonstrates that MK-0752 targets the BCSC population. In parallel, a clinical trial (NCT00645333) was carried out in 30 patients with advanced BC, treated with increasing doses of MK-0752 together with DTX. This work was designed to determine the maximum tolerated dose of the inhibitor, administered sequentially with DTX, and to evaluate BCSC biomarkers in tumor biopsies. A decrease in CD44+/ CD24−, and ALDH+ was demonstrated in tumors from patients with this combination therapy [106]. The effect of MK-0752 in combination with TAM or Letrozole to treat early-stage BC patients was also tested in the clinical trial NCT00756717, but its effect on BCSC markers was not evaluated.
Another γ-secretase inhibitor is RO4929097, in preclinical studies, this inhibitor showed a significant reduction in anchorage-independent growth and sensitized BC cells to ionizing radiation, both characteristics of CSCs [152]. Additionally, its antitumor activity in patients with advanced, metastatic, or recurrent triple-negative invasive BC has been studied in clinical trials (NCT01151449). The effect of RO4929097 in combination with other drugs like PTX and Carboplatin in TNBC [153] or in combination with Exemestane in patients with advanced or metastatic BC (clinical trial NCT01149356 [154], has been also evaluated, but its anti-BCSC effect has not yet been addressed.
PF-03084014 is another selective, reversible, non-competitive γ-secretase inhibitor that blocks the Notch signaling pathway. In the phase I trial NCT01876251, they evaluated the maximum tolerated dose, safety, pharmacokinetics, and antitumor activity of PF-03084014 in combination with DTX, in 30 patients with advanced TNBC [155]. Subsequently, in a phase II trial NCT02299635, the effect of PF-03084014 was evaluated in patients with advanced triple-negative breast cancer with or without genomic alterations in Notch receptors, however, its specific anti-CSC activity was not evaluated. Zhang et al., studied the antitumor efficacy of PF-03084014 alone and in combination with DTX against TNBC in vitro. They found that PF-03084014 enhanced the antitumor efficacy of DTX in TNBC xenograft models, through impairment of the Notch Pathway. Furthermore, PF-03084014 significantly reduced tumor-initiating cells or CSCs in the BC xenograft model [156].
Reparixin is a small-molecule inhibitor of the CXCR1 receptor, identified as an investigational drug targeting CSCs. It has been reported that this inhibitor selectively reduced the BCSC population in BC cell lines, and also was able to specifically target the CSC population in human BC xenografts. The latter delays tumor growth rate, and reduces metastasis [157]. A pilot study (NCT01861054) evaluated the effect of reparixin (administered orally) on BCSCs in primary tumors in a population of 20 patients with early BC. BCSCs were measured in tissue samples using CD44, C24, and ALDH1 biomarkers, as well as EMT markers (Snail, Twist, and Notch), among other proteins. ALDH+ and CD44+/CD24− markers measured by flow cytometry decreased by >20%. However, these results could not be confirmed by immunofluorescence due to the very low number of BCSCs [158]. Subsequently, this same group conducted a phase 2 FRIDA NCT02370238 trial where they evaluated progression-free survival in 123 patients with triple-negative metastatic BC treated with PTX in combination with reparixin. In this study, the BCSCs positive for CD44+/CD24−, and ALDH+ markers were measured. However, the combined treatment did not improve progression-free survival in these patients [159].
LGK974 is an inhibitor of Wnt signaling [160]. The phase I clinical trial (NCT01351103) will evaluate its effectiveness and safe administration as a single agent, and in combination with PDR001 (anti-PD-1 immunotherapy) in adult patients with solid malignancies, including TNBC. The results would support the promising use of Wnt inhibitors as candidates to target BCSCs. Previous studies found that this inhibitor can influence the recruitment of immune cells to tumors and can enhance checkpoint inhibitor activity [161].
Preclinical studies together with a phase I/II clinical trial (NCT01118975) determined the effects of the combination of vorinostat (inhibitor of histone deacetylase, which induces epigenetic changes) and LPT (a dual EGFR and HER2 inhibitor) in BCSCs population. In these studies, the combination of vorinostat and LPT was shown to reduce the BCSC population, measured through the surface markers CD49f and CD44+/CD24−/low. Additionally, the ALDH1 activity was evaluated in three BC cell lines. A reduction in the formation of mammospheres, decreased self-renewal proteins (BMI-1 and β-catenin), diminished EMT markers (Twist1 and Vimentin), and the inhibition of cell migration were also observed. The phase I/II clinical trial conducted in patients with BC positive for advanced HER2 demonstrated that the combined treatment of vorinostat and LPT is feasible and safe, with controllable side effects. Interestingly, patients who continued on vorinostat and LPT did not develop any new metastatic sites, supporting the preclinical results that show that this combination targets the BCSC population and prevents metastases. Additional studies are needed to further validate these results [162]. Another phase II clinical trial with LPT is NCT01868503 in which the combination of LPT ditosylate and radiation therapy work in treating patients with locally advanced or locally recurrent BC was tested. This study evaluated the change in BCSC proportion, viability by flow cytometry, and gene expression profiling after combined therapy. The results of this trial are not yet reported. The clinical trial NCT00524303 conducted in 100 women with invasive BC overexpressing HER2, evaluated the effect of LPT in combination with standard neoadjuvant chemotherapy (5FU, Epirubicin, Cyclophosphamide, and PTX). However, the data obtained from BCSCs were of poor quality, therefore, they could not be analyzed.
Ruxolitinib is a recently discovered drug that has been shown to block the IL6/JAK/STAT3 pathway by targeting JAK1 and JAK2 [163]. This inhibitor is being tested in a phase II clinical trial (NCT02876302) as a possible treatment for inflammatory breast cancer in 23 patients in combination with PTX. The distribution of the CD44+/CD24− stem cell population and the inhibition of JAK and pSTAT3 expression in BC tumors before and after treatment with ruxolotinib will be evaluated. This trial has not yet finished.
The retrospective study S9313B (NCT00949013) included 1600 tumor tissue samples recruited from women with early-stage BC. In this trial, the expression of the BCSC marker, ALDH1, was evaluated as a predictor of response to adjuvant chemotherapy (DOX and Cyclophosphamide), together with other biomarkers such as HER2, ER, and PR, but the data has not been published.
2. Conclusions
The great heterogeneity and complexity of BC represents a challenge in the search for new treatments that are effective in reducing the risk of metastasis and recurrence. The accumulated evidence indicates that the current subtype classification of breast cancer tumors is not sufficient to choose appropriate treatments. Instead, the representation and behavior of existing BCSCs in the tumor mass need to be also considered. This finding suggests the need to include the analysis of a variety of specific markers expressed throughout the natural history of the tumor, and in response to conventional treatments in this cell population. This evaluation will allow the development of treatments that combine targeting the inhibition of the mechanisms associated with the proliferation and survival of BCSCs.
As described here, there are several preclinical studies evaluating new therapeutic alternatives directed toward BCSCs. The strategies that have shown the most promising results not only consider the use of inhibitory compounds directed at the most representative signaling pathways of this type of cell (BCSCs), but also consider the use of innovative nanoparticles, and explore targeted delivery by coating these formulations with molecules that recognize surface markers expressed by BCSCs. These approaches have shown better effectiveness and specificity in the treatment of chemoresistant breast cancer than the use of these compounds as free formulations or standard treatments or conventional therapies alone. In order to obtain more comprehensive evidence to guide the design of clinical studies evaluating the effectiveness of treatments for BC patients, it is essential to use not only mammosphere models, but also patient-derived xenograft (PDX) models that accurately recapitulate the tumor microenvironment, including the presence of BCSCs in the tumor. Many of the clinical studies primarily evaluate the effectiveness of treatments targeting this type of cells alone, or in combination with standard treatments. However, the variability in the results obtained highlights the need to improve the translation of findings from preclinical studies to their evaluation in patients. Therefore, the available evidence suggests that the most promising treatments should consider the different subtypes of BC, the expression of specific BCSC markers, the use of nanoparticles targeted toward these markers, and the combination of these new treatments with the most successful current standard treatments or conventional therapies.
Author Contributions
Conceptualization, N.L.; writing—original draft preparation, N.L.; writing—review and editing, R.P.-C.; supervision, R.P.-C. and I.C.; funding acquisition, R.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The “Fondo de Innovación para la Competitividad FIC-R”, Project code 40.027.611−0, Gobierno Regional del Maule; and by “Convenio de desempeño para la Educación Superior Regional, UCM1995, Ministerio de Educación de Chile.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Universidad Católica del Maule (protocol code 176/2021, date of approval, 26 August 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Ingrid Carvacho, and Cristian Salas for their support, proofreading, and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Taurin, S.; Alkhalifa, H. Breast cancers, mammary stem cells, and cancer stem cells, characteristics, and hypotheses. Neoplasia 2020, 22, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Austin-Datta, R.J.; Oh, H.; Heng, Y.J.; Vellal, A.D.; Sirinukunwattana, K.; Baker, G.M.; Collins, L.C.; Murthy, D.; Rosner, B.; et al. Associations of reproductive breast cancer risk factors with breast tissue composition. Breast Cancer Res. 2021, 23, 70. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Landeros, N.; Gonzalez-Hormazabal, P.; Pérez-Moreno, P.; Tapia, J.C.; Jara, L. A Single Variant in Pri-miRNA-155 Associated with Susceptibility to Hereditary Breast Cancer Promotes Aggressiveness in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15418. [Google Scholar] [CrossRef]
- Quaglino, E.; Conti, L.; Cavallo, F. Breast cancer stem cell antigens as targets for immunotherapy. Semin. Immunol. 2020, 47, 101386. [Google Scholar] [CrossRef]
- Dieci, M.V.; Orvieto, E.; Dominici, M.; Conte, P.; Guarneri, V. Rare breast cancer subtypes: Histological, molecular, and clinical peculiarities. Oncologist 2014, 19, 805–813. [Google Scholar] [CrossRef]
- Mueller, C.; Haymond, A.; Davis, J.B.; Williams, A.; Espina, V. Protein biomarkers for subtyping breast cancer and implications for future research. Expert Rev. Proteom. 2018, 15, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Yin, X.; Zhang, X.; Huang, J.; Wu, Y.; Wang, M.; Yi, Z.; Li, H.; Li, H.; et al. Clinicopathological Characteristics and Breast Cancer-Specific Survival of Patients with Single Hormone Receptor-Positive Breast Cancer. JAMA Netw. Open 2020, 3, e1918160. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-Macgregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, H.; Li, X.; Han, L.; Xu, N.; Shi, A. Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer. Oncol. Rep. 2019, 41, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rije, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Eliyatkın, N.; Yalçın, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef]
- Vuong, D.; Yalçın, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular classification of breast cancer. Virchows Arch. 2014, 465, 1–14. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Lee, E.; Moon, A. Identification of Biomarkers for Breast Cancer Using Databases. J. Cancer Prev. 2016, 21, 235–242. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Nandy, A.; Hor, P.; Mukhopadhyay, A. Breast cancer stem cells: A novel therapeutic target. Clin. Breast Cancer 2013, 13, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Bentires-Alj, M. Breast Tumor Heterogeneity: Source of Fitness, Hurdle for Therapy. Mol. Cell 2015, 60, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Clarke, M.F. Self-renewal and solid tumor stem cells. Oncogene 2004, 23, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.D.; Kwon, Y.T. Molecular mechanisms controlling asymmetric and symmetric self-renewal of cancer stem cells. J. Anal. Sci. Technol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Conde, I.; Ribeiro, A.S.; Paredes, J. Breast Cancer Stem Cell Membrane Biomarkers: Therapy Targeting and Clinical Implications. Cells 2022, 11, 934. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer stem cells behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Hadjimichael, C.; Chanoumidou, K.; Papadopoulou, N.; Arampatzi, P.; Papamatheakis, J.; Kretsovali, A. Common stemness regulators of embryonic and cancer stem cells. World J. Stem Cells 2015, 7, 1150–1184. [Google Scholar] [PubMed]
- Pavlopoulou, A.; Oktay, Y.; Vougas, K.; Louka, M.; Vorgias, C.E.; Georgakilas, A.G. Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Lett. 2016, 380, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, C.M.; Kuperwasser, C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Shima, H.; Yamada, A.; Ishikawa, T.; Endo, I. Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy? Gland Surg. 2017, 6, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Landeros, N.; Santoro, P.M.; Carrasco-Avino, G.; Corvalan, A.H. Competing Endogenous RNA Networks in the Epithelial to Mesenchymal Transition in Diffuse-Type of Gastric Cancer. Cancers 2020, 12, 2741. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E. Breast cancer stem cells revealed. Proc. Natl. Acad. Sci. USA 2003, 100, 3547–3549. [Google Scholar] [CrossRef]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar] [CrossRef]
- Honeth, G.; Bendahl, P.-O.; Ringnér, M.; Saal, L.H.; Gruvberger-Saal, S.K.; Lövgren, K.; Grabau, D.; Fernö, M.; Borg, A.; Hegardt, C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008, 10, R53. [Google Scholar] [CrossRef]
- Fultang, N.; Chakraborty, M.; Peethambaran, B. Regulation of cancer stem cells in triple negative breast cancer. Cancer Drug Resist. 2021, 4, 321–342. [Google Scholar] [CrossRef]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitão, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef]
- Lloyd-Lewis, B.; Harris, O.B.; Watson, C.J.; Davis, F.M. Mammary Stem Cells: Premise, Properties, and Perspectives. Trends Cell Biol. 2017, 27, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Butti, R.; Gunasekaran, V.P.; Kumar, T.V.S.; Banerjee, P.; Kundu, G.C. Breast cancer stem cells: Biology and therapeutic implications. Int. J. Biochem. Cell Biol. 2019, 107, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Martínez, C.A.; Koester, J.; Wickström, S.A. Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 2018, 145, dev165399. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.D.; Burness, M.L.; Wicha, M.S. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell 2015, 17, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Mosafer, J.; Akbari, M.; Hashemzaei, M.; Mokhtarzadeh, A.; Baradaran, B. Colon cancer therapy by focusing on colon cancer stem cells and their tumor microenvironment. J. Cell. Physiol. 2020, 235, 4153–4166. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Ho, C.-C.; Ho, H.; Chen, W.-J.; Lin, C.-H.; Lai, Y.-H.; Juan, Y.-C.; Chu, W.-C.; Lee, J.-H.; Su, S.-F.; et al. Tumor microenvironment-based screening repurposes drugs targeting cancer stem cells and cancer-associated fibroblasts. Theranostics 2021, 11, 9667–9686. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Ota, M.; Okada, Y. Isolation of Cancer Stem Cells by Side Population Method. Methods Mol. Biol. 2018, 1692, 49–59. [Google Scholar]
- Cianciosi, D.; Ansary, J.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Villar, S.G.; Villena, E.G.; Pifarre, K.T.; Alvarez-Suarez, J.M.; Battino, M.; et al. The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture. Molecules 2021, 26, 2615. [Google Scholar] [CrossRef]
- Shaw, F.L.; Harrison, H.; Spence, K.; Ablett, M.P.; Simões, B.M.; Farnie, G.; Clarke, R.B. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J. Mammary Gland Biol. Neoplasia 2012, 17, 111–117. [Google Scholar] [CrossRef]
- Hwang-Verslues, W.W.; Lee, W.H.; Lee, E.Y. Biomarkers to Target Heterogeneous Breast Cancer Stem Cells. J. Mol. Biomark. Diagn. 2012, Suppl. 8, 6. [Google Scholar] [CrossRef]
- Xia, P. Surface markers of cancer stem cells in solid tumors. Curr. Stem Cell Res. Ther. 2014, 9, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Miele, L. Breast Cancer Stem Cells. Biomedicines 2018, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Rathod, M.; De, A. Targeting stem cells in the realm of drug-resistant breast cancer. Breast Cancer 2019, 11, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Othman, N.; Alhendi, A.; Ihbaisha, M.; Barahmeh, M.; Alqaraleh, M.; Al-Momany, B.Z. Role of CD44 in breast cancer. Breast Dis. 2020, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Chekhun, V.F.; Lukianova, N.Y.; Chekhun, S.V.; Bezdienizhnykh, N.O.; Zadvorniy, T.V.; Borikun, T.V.; Polishchuk, L.Z.; Klyusov, O.M. Association of CD44. Exp. Oncol. 2017, 39, 203–211. [Google Scholar] [CrossRef]
- Ma, L.; Dong, L.; Chang, P. CD44v6 engages in colorectal cancer progression. Cell Death Dis. 2019, 10, 30. [Google Scholar] [CrossRef]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef]
- Zavros, Y. Initiation and Maintenance of Gastric Cancer: A Focus on CD44 Variant Isoforms and Cancer Stem Cells. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Emich, H.; Chapireau, D.; Hutchinson, I.; Mackenzie, I. The potential of CD44 as a diagnostic and prognostic tool in oral cancer. J. Oral Pathol. Med. 2015, 44, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Dwivedi, M.; Ahmad, H.; Chadchan, S.B.; Arya, A.; Sikandar, R.; Kaushik, S.; Mitra, K.; Jha, R.K.; Dwivedi, A.K. CD44 targeting hyaluronic acid coated lapatinib nanocrystals foster the efficacy against triple-negative breast cancer. Nanomedicine 2018, 14, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Han, N.K.; Shin, D.H.; Kim, J.S.; Weon, K.Y.; Jang, C.-Y.; Kim, J.-S. Hyaluronan-conjugated liposomes encapsulating gemcitabine for breast cancer stem cells. Int. J. Nanomed. 2016, 11, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, A.; Kamali, N.; Shiri, F.; Majd, M.H. Hyaluronic acid/polyethylene glycol nanoparticles for controlled delivery of mitoxantrone. Artif. Cells Nanomed. Biotechnol. 2018, 46, 500–509. [Google Scholar] [CrossRef]
- Gener, P.; Gouveira, L.P.; Sabat, G.R.; de Sousa Rafael, D.F.; Fort, N.B.; Arranja, A.; Fernández, Y.; Prieto, R.M.; Ortega, J.S.; Arango, D.; et al. Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine 2015, 11, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Gehling, P.; Fargaes, C.A.; Dittfeld, C.; Garbe, Y.; Alison, M.R.; Corbeil, D.; Kunz-Schughart, L.A. CD133 as a biomarker for putative cancer stem cells in solid tumours: Limitations, problems and challenges. J. Pathol. 2013, 229, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, F.; Grassilli, S.; Al-Qassab, Y.; Capitani, S.; Bertagnolo, V. CD133 in Breast Cancer Cells: More than a Stem Cell Marker. J. Oncol. 2019, 2019, 7512632. [Google Scholar] [CrossRef]
- Xia, P. mRNA may be a suitable prognostic marker for human breast cancer. Stem Cell Investig. 2017, 4, 87. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Ohlfest, J.R.; Zellmer, D.M.; Panyam, J.; Swaminathan, S.K.; Oh, S.; Waldron, N.N.; Toma, S.; Vallera, D.A. Immunotoxin targeting CD133(+) breast carcinoma cells. Drug Deliv. Transl. Res. 2013, 3, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.K.; Roger, E.; Toti, U.; Niu, L.; Ohlfest, J.R.; Panyam, J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J. Control Release 2013, 171, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xiong, G.; Guo, S.; Xu, C.; Xu, R.; Guo, P.; Shu, D. Delivery of Anti-miRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles Targeting Stem Cell Marker CD133. Mol. Ther. 2019, 27, 1252–1261. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, X.; Liu, C.; Xu, Y.; Fu, Y.; Dong, W.; Yan, Y.; Wang, W.; Qian, C. AKT inhibitor AZD5363 suppresses stemness and promotes anti-cancer activity of 3,3’-diindolylmethane in human breast cancer cells. Toxicol. Appl. Pharmacol. 2021, 429, 115700. [Google Scholar] [CrossRef] [PubMed]
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and function of epithelial cell adhesion molecule EpCAM: Where are we after 40 years? Cancer Metastasis Rev. 2020, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T.; Ito, S.; Nakamura, H. EpCAM expression in breast cancer cells is associated with enhanced bone metastasis formation. Int. J. Cancer 2016, 138, 1698–1708. [Google Scholar] [CrossRef]
- Dionísio, M.R.; Vieira, A.F.; Carvalho, R.; Conde, I.; Oliveira, M.; Gomes, M.; Pinto, M.T.; Pereira, P.; Pimentel, J.; Souza, C.; et al. BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases that Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer. Cells 2020, 9, 2442. [Google Scholar] [CrossRef]
- Kubo, M.; Umebayashi, M.; Kurata, K.; Mori, H.; Kai, M.; Onishi, H.; Katano, M.; Nakamura, M.; Morisaki, T. Catumaxomab with Activated T-cells Efficiently Lyses Chemoresistant EpCAM-positive Triple-negative Breast Cancer Cell Lines. Anticancer Res. 2018, 38, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, X.; Yaganeh, P.N.; Lee, D.-J.; Valle-Garcia, D.; Meza-Sosa, K.F.; Junqueira, C.; Su, J.; Luo, H.R.; Hide, W.; et al. Immunotherapy for breast cancer using EpCAM aptamer tumor-targeted gene knockdown. Proc. Natl. Acad. Sci. USA 2021, 118, e2022830118. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Truijillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Kaur, S.; Elkahloun, A.G.; Singh, S.P.; Chen, Q.-R.; Meerzaman, D.M.; Song, T.; Manu, N.; Wu, W.; Mannan, P.; Garfield, S.H.; et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 2016, 7, 10133–10152. [Google Scholar] [CrossRef] [PubMed]
- Candas-Green, D.; Xie, B.; Huang, J.; Fan, M.; Wang, A.; Menaa, C.; Zhang, Y.; Zhang, L.; Jing, D.; Azghadi, S.; et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat. Commun. 2020, 11, 4591. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, D.H.; Kim, J.S. Anticancer Effect of Metformin in Herceptin-Conjugated Liposome for Breast Cancer. Pharmaceutics 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Sai, S.; Kim, E.H.; Vares, G.; Suzuki, M.; Yu, D.; Horimoto, Y.; Hayashi, M. Combination of carbon-ion beam and dual tyrosine kinase inhibitor, lapatinib, effectively destroys HER2 positive breast cancer stem-like cells. Am. J. Cancer Res. 2020, 10, 2371–2386. [Google Scholar] [PubMed]
- Lv, L.; Shi, Y.; Wu, J.; Li, G. Nanosized Drug Delivery Systems for Breast Cancer Stem Cell Targeting. Int. J. Nanomed. 2021, 16, 1487–1508. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, H.-Y.; Cha, Y.-N.; Surh, Y.-J. Role of heme oxygenase-1 and its reaction product, carbon monoxide, in manifestation of breast cancer stem cell-like properties: Notch-1 as a putative target. Free Radic. Res. 2018, 52, 1336–1347. [Google Scholar] [CrossRef]
- Kurebayashi, J.; Koike, Y.; Ohta, Y.; Saitoh, W.; Yamashita, T.; Kanomata, N.; Moriya, T. Anti-cancer stem cell activity of a hedgehog inhibitor GANT61 in estrogen receptor-positive breast cancer cells. Cancer Sci. 2017, 108, 918–930. [Google Scholar] [CrossRef]
- Woosley, A.N.; Dalton, A.C.; Hussey, G.S.; Howley, B.V.; Mohanty, B.K.; Grelet, S.; Dincman, T.; Bloos, S.; Olsen, S.K.; Howe, P.H. TGFβ promotes breast cancer stem cell self-renewal through an ILEI/LIFR signaling axis. Oncogene 2019, 38, 3794–3811. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Xie, Y.; Zhou, Y.; Wang, R.; Lou, J. Napabucasin Attenuates Resistance of Breast Cancer Cells to Tamoxifen by Reducing Stem Cell-like Properties. Med. Sci. Monit. 2019, 25, 8905–8912. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.-J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 136–150.e5. [Google Scholar] [CrossRef] [PubMed]
- Doheny, D.; Srikisoon, S.; Carpenter, R.L.; Aguayo, N.R.; Regua, A.T.; Anguelov, M.; Manore, S.G.; Arrigo, A.; Jalboush, S.A.; Wong, G.L.; et al. Combined inhibition of JAK2-STAT3 and SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor growth, and metastasis. Oncogene 2020, 39, 6589–6605. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.; Berns, K.; Spence, K.; Ryder, W.D.; Zeps, N.; Madiredjo, M.; Beijersbergen, R.; Bernards, R.; Clarke, R.B. An integrated genomic approach identifies that the PI3K/AKT/FOXO pathway is involved in breast cancer tumor initiation. Oncotarget 2016, 7, 2596–2610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nandi, A.; Singh, S.; Regulapati, R.; Li, N.; Tobias, J.; Siebel, C.; Blanco, M.; Klein-Szanto, A.; Lengner, C.; et al. Dll1+ Quiescent Tumor Stem Cells Drive Chemoresistance in Breast Cancer through NF-κB Survival Pathway. Nat. Commun. 2021, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 3904645. [Google Scholar] [CrossRef]
- Hepburn, A.C.; Steele, R.E.; Veeratterapillay, R.; Wilson, L.; Kounatidou, E.E.; Baranard, A.; Berry, P.; Cassidy, J.R.; Moad, M.; El-Sherif, A.; et al. The induction of core pluripotency master regulators in cancers defines poor clinical outcomes and treatment resistance. Oncogene 2019, 38, 4412–4424. [Google Scholar] [CrossRef]
- Jang, G.B.; Kim, J.-Y.; Cho, S.-D.; Park, K.-S.; Jung, J.-Y.; Lee, H.-Y.; Hong, I.-S.; Nam, J.-S. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci. Rep. 2015, 5, 12465. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Jang, G.B.; Hong, I.-S.; Kim, R.-J.; Lee, S.-Y.; Park, S.-J.; Lee, E.-S.; Park, J.H.; Yun, C.-H.; Chung, J.U.; Lee, K.J.; et al. Wnt/β-Catenin Small-Molecule Inhibitor CWP232228 Preferentially Inhibits the Growth of Breast Cancer Stem-like Cells. Cancer Res. 2015, 75, 1691–1702. [Google Scholar] [CrossRef]
- Bellomo, C.; Caja, L.; Moustakas, A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer 2016, 115, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, M.-J.; Park, S.-A.; Kim, J.-S.; Min, K.-N.; Kim, D.-K.; Lim, W.; Nam, J.-S.; Sheen, Y.Y. Combinatorial TGF-β attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget 2015, 6, 37526–37543. [Google Scholar] [CrossRef] [PubMed]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sánchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Kalagasidou, S.; Pouloliou, S.; Anthoulaki, X.; Georgiou, N.; Papamanolis, V.; Fasoulakis, Z.N. The Notch Pathway in Breast Cancer Progression. Sci. World J. 2018, 2018, 2415489. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef] [PubMed]
- Schott, A.F.; Landis, M.D.; Dontu, G.; Griffith, K.A.; Layman, R.M.; Krop, I.; Paskett, L.A.; Wong, H.; Dobrolecki, L.E.; Lewis, M.T.; et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin. Cancer Res. 2013, 19, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Lindén, M.; et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef]
- McClements, L.; Annett, S.; Yakkundi, A.; O’Rourke, M.; Valentine, A.; Moustafa, N.; Alqudeh, A.; Simões, B.M.; Furlong, F.; Short, A.; et al. FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4. BMC Cancer 2019, 19, 351. [Google Scholar] [CrossRef]
- McClements, L.; Papaspyropoulos, A.Y.A.; Harrison, H.; Ablett, M.P.; Jithesh, P.V.; McKeen, H.D.; Bennett, R.; Donley, C.; Kissenpfennig, A.; McIntosh, S.; et al. Targeting treatment-resistant breast cancer stem cells with FKBPL and its peptide derivative, AD-01, via the CD44 pathway. Clin. Cancer Res. 2013, 19, 3881–3893. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, P.; Niedziółka, S.M.; Markiewicz, Ł.; Uśpieński, T.; Baran, B.; Chojnowska, K. Gli Proteins: Regulation in Development and Cancer. Cells 2019, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duan, W.; Kang, L.; Mao, J.; Yu, X.; Fan, S.; Li, L.; Tao, Y. Smoothened activates breast cancer stem-like cell and promotes tumorigenesis and metastasis of breast cancer. Biomed. Pharmacother. 2014, 68, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, S.A.; Machalek, D.A.; Shearer, R.F.; Millar, E.K.A.; Nair, R.; Schofield, P.; McLeod, D.; Cooper, C.L.; McNeil, C.M.; McFarland, A.; et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011, 71, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Ohta, Y.; Saitoh, W.; Yamashita, T.; Kanomata, N.; Moriya, T.; Kurebayashi, J. Anti-cell growth and anti-cancer stem cell activities of the non-canonical hedgehog inhibitor GANT61 in triple-negative breast cancer cells. Breast Cancer 2017, 24, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Balko, J.M.; Schwarz, L.J.; Luo, N.; Estrada, M.V.; Giltnane, J.M.; Dávila-González, D.; Wang, K.; Sánchez, V.; Dean, P.T.; Combs, S.E.; et al. Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci. Transl. Med. 2016, 8, 334ra53. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Croker, A.K.; Allan, A.L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44⁺ human breast cancer cells. Breast Cancer Res. Treat. 2012, 133, 75–87. [Google Scholar] [CrossRef]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014, 2, 78–91. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Q.; Zou, Y.; Chen, H.; Qi, L.; Chen, Y. Stem Cells and Cellular Origins of Breast Cancer: Updates in the Rationale, Controversies, and Therapeutic Implications. Front. Oncol. 2019, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T. EMT and MET in metastasis: Where are the cancer stem cells? Cancer Cell 2012, 22, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.H.; Pedersen, B.; Tønnesen, H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin. Exp. Res. 2011, 35, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kumar, I.S.; Brown, S.; Kannapan, V.; Tawari, P.E.; Tang, J.Z.; Jiang, W.; Armesilla, A.L.; Darling, J.L.; Wang, W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br. J. Cancer 2013, 109, 1876–1885. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, Y.; Oh, E.; Lee, N.; An, H.; Sung, D.; Cho, T.-M.; Seo, J.H. Disulfiram targets cancer stem-like properties and the HER2/Akt signaling pathway in HER2-positive breast cancer. Cancer Lett. 2016, 379, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, J.Y.; Lee, N.; Oh, E.; Sung, D.; Cho, T.-M.; Seo, J.H. Disulfiram suppresses cancer stem-like properties and STAT3 signaling in triple-negative breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 486, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Duarte, D.; Vale, N. Drug Repurposing in Cancer Therapy: Influence of Patient’s Genetic Background in Breast Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 4280. [Google Scholar] [CrossRef]
- Dattilo, R.; Mottini, C.; Camera, E.; Lamolinara, A.; Auslander, N.; Doglioni, G.; Muscolini, M.; Tang, W.; Planque, M.; Ercolani, C.; et al. Pyrvinium Pamoate Induces Death of Triple-Negative Breast Cancer Stem-like Cells and Reduces Metastases through Effects on Lipid Anabolism. Cancer Res. 2020, 80, 4087–4102. [Google Scholar] [CrossRef]
- Marcucci, F.; Rumio, C.; Lefoulon, F. Anti-Cancer Stem-like Cell Compounds in Clinical Development—An Overview and Critical Appraisal. Front. Oncol. 2016, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer Mechanisms of Salinomycin in Breast Cancer and Its Clinical Applications. Front. Oncol. 2021, 11, 654428. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Kanaya, N.; Wu, S.V.; Mendez, C.; Nguyen, D.; Luu, T.; Chen, S. Targeting breast cancer stem cells in triple-negative breast cancer using a combination of LBH589 and salinomycin. Breast Cancer Res. Treat. 2015, 151, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Versini, A.; Colombeau, L.; Hienzsch, A.; Gaillet, C.; Retailleau, P.; Debieu, S.; Müller, S.; Cañeque, T.; Rodriguez, R. Salinomycin Derivatives Kill Breast Cancer Stem Cells by Lysosomal Iron Targeting. Chemistry 2020, 26, 7416–7424. [Google Scholar] [CrossRef] [PubMed]
- Muntimadugu, E.; Kumar, R.; Saladi, S.; Rafeeqi, T.A.; Khan, W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf. B Biointerfaces 2016, 143, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, L.; Hu, C.; Liang, S.; Fei, X.; Yan, N.; Zhang, Y.; Zhang, F. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. Int. J. Oncol. 2016, 48, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hollmén, M.; Li, L.; Chen, Y.; Proulx, S.T.; Reker, D.; Schneider, G.; Detmar, M. New use of an old drug: Inhibition of breast cancer stem cells by benztropine mesylate. Oncotarget 2017, 8, 1007–1022. [Google Scholar] [CrossRef]
- Velázquez-Quesada, I.; Ruiz-Moreno, A.J.; Casique-Aguirre, D.; Aguirre-Alvarado, C.; Cortés-Mendoza, F.; de la Fuente-Granada, M.; García-Pérez, C.; Pérez-Tapia, S.M.; González-Arenas, A.; Segura-Cabrera, A.; et al. Pranlukast Antagonizes CD49f and Reduces Stemness in Triple-Negative Breast Cancer Cells. Drug Des. Dev. Ther. 2020, 14, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Kuo, P.-L.; Hou, M.-F.; Hung, J.-Y.; Chang, F.-R.; Hsu, Y.-C.; Huang, Y.-F.; Tsai, E.-M.; Hsu, Y.-L. Wedelolactone inhibits breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR signaling. Int. J. Oncol. 2015, 46, 555–562. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, P.; Chatterjee, R.; Jamal, Z.; Chatterji, U. Enhancing Chemosensitivity of Breast Cancer Stem Cells by Downregulating SOX2 and ABCG2 Using Wedelolactone-encapsulated Nanoparticles. Mol. Cancer Ther. 2019, 18, 680–692. [Google Scholar] [CrossRef]
- Lin, C.Y.; Barry-Holson, K.Q.; Allison, K.H. Breast cancer stem cells: Are we ready to go from bench to bedside? Histopathology 2016, 68, 119–137. [Google Scholar] [CrossRef]
- Yoshida, G.J.; Saya, H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016, 107, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise Reviews: Cancer Stem Cell Targeted Therapies: Toward Clinical Success. Stem Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, X.; Huang, J.; Chen, Y.; Zhang, J.; Zhang, B.; Shi, C.; Liu, L. Bevacizumab Addition in Neoadjuvant Treatment Increases the Pathological Complete Response Rates in Patients with HER-2 Negative Breast Cancer Especially Triple Negative Breast Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0160148. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Charafe-Jauffret, E.; Pierga, J.-Y.; Curé, H.; Lambaudie, E.; Genre, D.; Houvenaeghel, G.; Viens, P.; Ginestier, C.; Bertucci, F.; et al. Stem Cells Inhibition by Bevacizumab in Combination with Neoadjuvant Chemotherapy for Breast Cancer. J. Clin. Med. 2019, 8, 612. [Google Scholar] [CrossRef]
- Seo, A.N.; Lee, H.J.; Kim, E.J.; Jang, M.H.; Kim, Y.J.; Kim, J.H.; Kim, S.-W.; Ryu, H.S.; Park, I.A.; Im, S.-A.; et al. Expression of breast cancer stem cell markers as predictors of prognosis and response to trastuzumab in HER2-positive breast cancer. Br. J. Cancer 2016, 114, 1109–1116. [Google Scholar] [CrossRef]
- Schmidt, M.; Scheulen, M.E.; Dittrich, C.; Obrist, P.; Marschner, N.; Dirix, L.; Schmidt, M.; Rüttinger, D.; Schuler, M.; Reinhardt, C.; et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann. Oncol. 2010, 21, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Rüttinger, D.; Sebastian, M.; Hanusch, C.A.; Marschner, N.; Baeuerle, P.A.; Wolf, A.; Göppel, G.; Oruzio, D.; Schlimok, G.; et al. Phase IB study of the EpCAM antibody adecatumumab combined with docetaxel in patients with EpCAM-positive relapsed or refractory advanced-stage breast cancer. Ann. Oncol. 2012, 23, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wen, P.; Li, M.; Li, Y.; Li, X.-A. Construction of chimeric antigen receptor-modified T cells targeting EpCAM and assessment of their anti-tumor effect on cancer cells. Mol. Med. Rep. 2019, 20, 2355–2364. [Google Scholar] [CrossRef]
- Rupp, U.; Schoendorf-Holland, E.; Eichbaum, M.; Schuetz, F.; Lauschner, I.; Schmidt, P.; Sttab, A.; Hanft, G.; Huober, J.; Sinn, H.-P.; et al. Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: Final results of a phase I study. Anticancer Drugs 2007, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.; Demuth, T.; Guthrie, T.; Wen, P.Y.; Mason, W.P.; Chinnaiyan, P.; Butowski, N.; Groves, M.D.; Kesari, S.; Freedman, S.J.; et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 2012, 30, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Debeb, B.G.; Cohen, E.N.; Boley, K.; Freiter, E.M.; Li, L.; Robertson, F.M.; Reuben, J.M.; Cristofanilli, M.; Buchholz, T.A.; Woodward, W.A. Pre-clinical studies of Notch signaling inhibitor RO4929097 in inflammatory breast cancer cells. Breast Cancer Res. Treat. 2012, 134, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, S.; Badawi, M.; Mrozek, E.; Morgan, E.; Phelps, M.; Stephens, J.; Wei, L.; Kassem, M.; Ling, Y.; Lustberg, M.; et al. A phase I study of an oral selective gamma secretase (GS) inhibitor RO4929097 in combination with neoadjuvant paclitaxel and carboplatin in triple negative breast cancer. Investig. New Drugs 2020, 38, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Means-Powell, J.A.; Mayer, I.A.; Ismail-Khan, R.; Del Valle, L.; Tonetti, D.; Abramson, V.G.; Sanders, M.S.; Lush, R.M.; Sorrentino, C.; Majumder, S.; et al. A Phase Ib Dose Escalation Trial of RO4929097 (a γ-secretase inhibitor) in Combination with Exemestane in Patients with ER + Metastatic Breast Cancer (MBC). Clin. Breast Cancer 2021, 22, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.A.; Aftimos, P.; Dees, E.C.; LoRusso, P.M.; Pegram, M.D.; Awada, A.; Huang, B.; Cesari, R.; Jiang, Y.; Shaik, M.N.; et al. Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 2017, 8, 2320–2328. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yan, Z.; Zong, Q.; Fang, D.D.; Painter, C.; Zhang, Q.; Chen, E.; Lira, M.E.; John-Baptiste, A.; Christensen, J.G. Synergistic effect of the γ-secretase inhibitor PF-03084014 and docetaxel in breast cancer models. Stem Cells Transl. Med. 2013, 2, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D.; et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Perez, R.P.; Yardley, D.; Han, L.K.; Reuben, J.M.; Gao, H.; McCanna, S.; Butler, B.; Ruffini, P.A.; Liu, Y.; et al. A window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res. 2020, 22, 4. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausovà, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; de Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Schech, A.; Brodie, A.; Lewis, J.; Tkaczuk, K.; Edelman, M.J.; Tait, N.; Chumsri, S.; Bao, T.; Stearns, V. Targeting Cancer Stem Cells and Metastasis with Epigenetic Modulation and Anti-HER2 Therapy: Phase I/II Trial of Vorinostat in Combination with Lapatinib. Clin. Oncol. Res. 2020, 7, 2–12. [Google Scholar]
- Stover, D.G.; Del Alcazar, C.R.G.; Brock, J.; Guo, H.; Overmoyer, B.; Balko, J.; Xu, Q.; Bardia, A.; Tolaney, S.M.; Gelman, R.; et al. Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple-negative breast cancer. NPJ Breast Cancer 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).