Microfibre-Functionalised Silk Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. B. mori Silk Degumming and Solution Preparation
2.2. A. mylitta Silk Degumming
2.3. Microfibre Production
2.4. Self-Assembling B. mori Silk Hydrogels with and without Microfibres
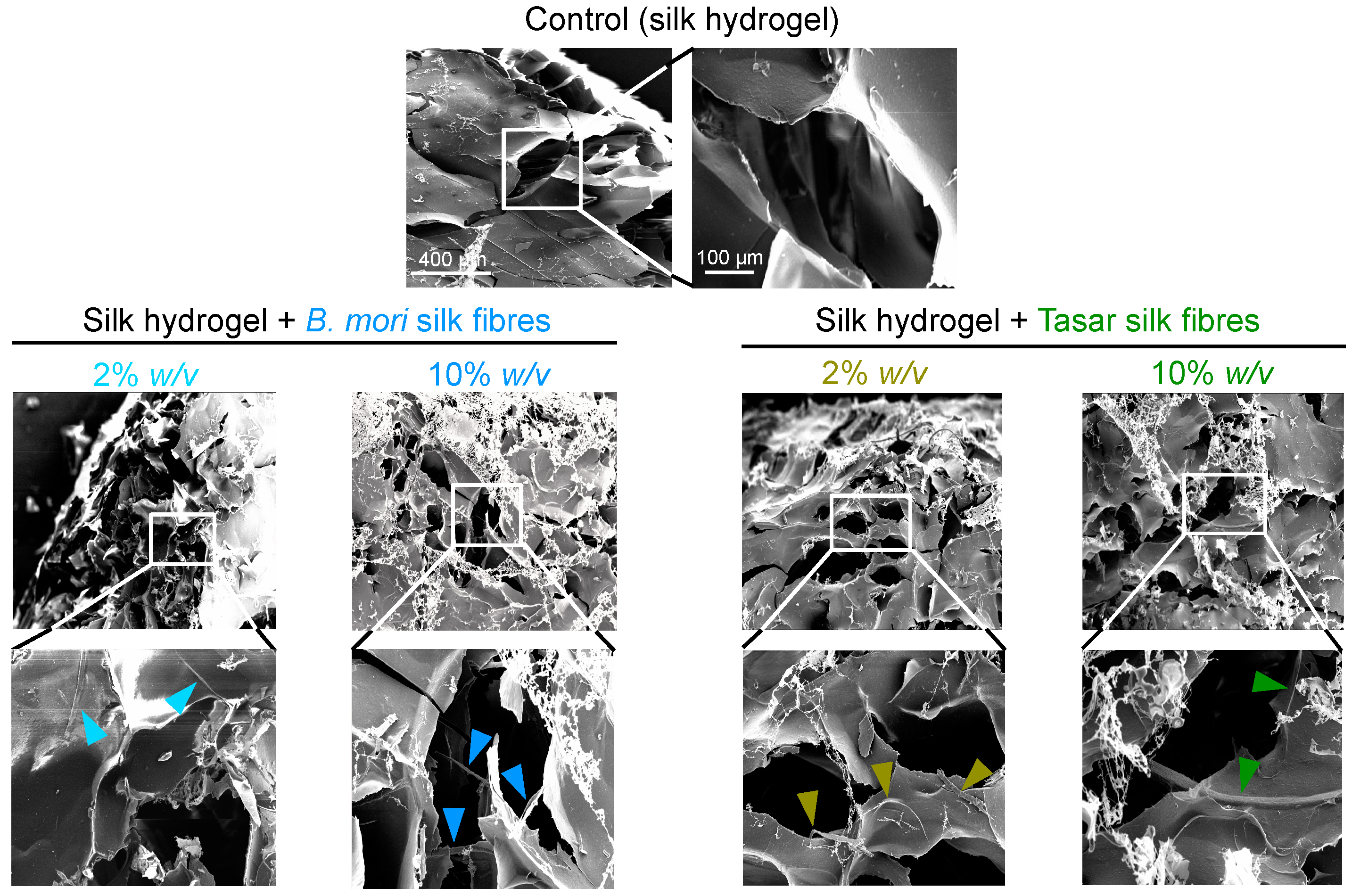
2.5. Scanning Electron Microscopy (SEM)
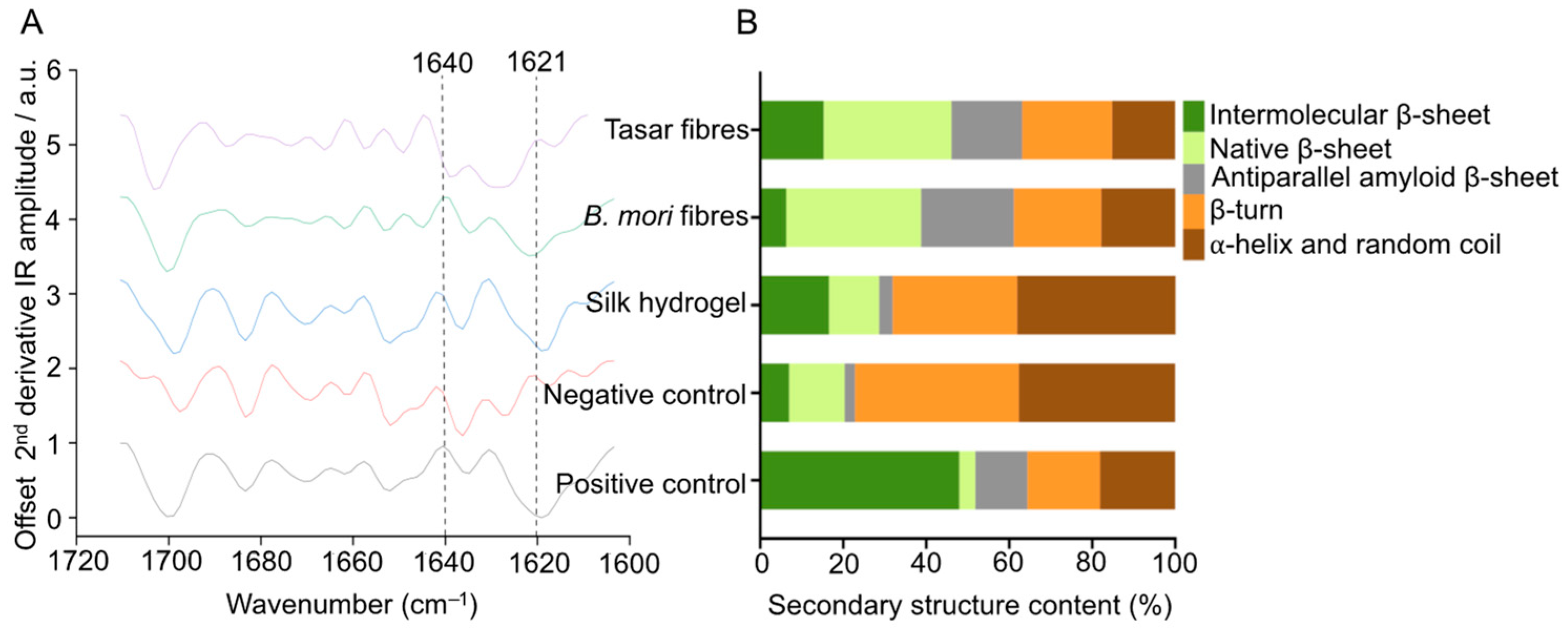
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Rheology of Silk Hydrogels
2.8. Cell Assays
2.9. Cell Staining and Image Analyses
2.10. Statistical Analyses and Presentation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2022, 9, 837464. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, H.; Sheldon, B.W.; Webster, T.J.; Yang, S.; Yang, H.; Yang, L. Surface energy-mediated fibronectin adsorption and osteoblast responses on nanostructured diamond. J. Mater. Sci. Technol. 2019, 35, 817–823. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, L.; Dou, X.; Bai, R.; Wang, H.; Deng, J.; Zhang, Y.; Sun, Q.; Li, Q.; Wang, X.; et al. Mechanically Robust Hydrogels Facilitating Bone Regeneration through Epigenetic Modulation. Adv. Sci. 2022, 9, 2203734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-directed cell behavior for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100260. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-K.; Kang, M.-L.; Park, J.H.; Lee, K.-M.; Shin, Y.M.; Lee, J.W.; Kim, H.O.; Sung, H.-J. Direct Control of Stem Cell Behavior Using Biomaterials and Genetic Factors. Stem Cells Int. 2018, 2018, 8642989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Long, L.; He, S.; Wang, Z.; Liu, Y.; Yang, L.; Chen, N.; Hu, C.; Wang, Y. Responsive multifunctional hydrogels emulating the chronic wounds healing cascade for skin repair. J. Control Release 2023, 354, 821–834. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Lyu, Y.; Liu, Y.; He, H.; Wang, H. Application of Silk-Fibroin-Based Hydrogels in Tissue Engineering. Gels 2023, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Phuagkhaopong, S.; Mendes, L.; Müller, K.; Wobus, M.; Bornhäuser, M.; Carswell, H.V.O.; Duarte, I.F.; Seib, F.P. Silk Hydrogel Substrate Stress Relaxation Primes Mesenchymal Stem Cell Behavior in 2D. ACS Appl. Mater. Interfaces 2021, 13, 30420–30433. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Grinberg, A.; Seok Gil, E.; Panilaitis, B.; Kaplan, D.L. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.-A.N.; Chao, P.-h.G.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, Z.; Lu, G.; Wang, J.; Wang, L.; Lu, Q. Amorphous Silk Fibroin Nanofiber Hydrogels with Enhanced Mechanical Properties. Macromol. Biosci. 2019, 19, 1900326. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Tan, Y.; Li, J.; Gu, C.; Li, H.; Li, B.; Liao, X. Fabrication and characterization of silk microfiber-reinforced methacrylated gelatin hydrogel with tunable properties. J. Biomater. Sci. Polym. Ed. 2018, 29, 2068–2082. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect silk: One name, many materials. Annu. Rev. Entomol. 2010, 55, 171–188. [Google Scholar] [CrossRef]
- Acharya, C.; Ghosh, S.; Kundu, S. Silk fibroin film from non-mulberry tropical tasar silkworms: A novel substrate for in vitro fibroblast culture. Acta Biomater. 2008, 5, 429–437. [Google Scholar] [CrossRef]
- Naskar, D.; Sapru, S.; Ghosh, A.K.; Reis, R.L.; Dey, T.; Kundu, S.C. Nonmulberry silk proteins: Multipurpose ingredient in bio-functional assembly. Biomed. Mater. 2021, 16, 062002. [Google Scholar] [CrossRef]
- Patra, C.; Talukdar, S.; Novoyatleva, T.; Velagala, S.R.; Muhlfeld, C.; Kundu, B.; Kundu, S.C.; Engel, F.B. Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials 2012, 33, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Perrucci, G.L.; Gowran, A.; Pompilio, G. Unchain My Heart: Integrins at the Basis of iPSC Cardiomyocyte Differentiation. Stem Cells Int. 2019, 2019, 8203950. [Google Scholar] [CrossRef] [PubMed]
- Ozay, E.I.; Vijayaraghavan, J.; Gonzalez-Perez, G.; Shanthalingam, S.; Sherman, H.L.; Garrigan, D.T., Jr.; Chandiran, K.; Torres, J.A.; Osborne, B.A.; Tew, G.N.; et al. Cymerus™ iPSC-MSCs significantly prolong survival in a pre-clinical, humanized mouse model of Graft-vs-host disease. Stem Cell Res. 2019, 35, 101401. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Wu, K.-C.; Ding, D.-C. Induced Pluripotent Stem Cell-Differentiated Chondrocytes Repair Cartilage Defect in a Rabbit Osteoarthritis Model. Stem Cells Int. 2020, 2020, 8867349. [Google Scholar] [CrossRef] [PubMed]
- Saetersmoen, M.L.; Hammer, Q.; Valamehr, B.; Kaufman, D.S.; Malmberg, K.-J. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin. Immunopathol. 2019, 41, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P. Reverse-engineered silk hydrogels for cell and drug delivery. Ther. Deliv. 2018, 9, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Manufacture and Drug Delivery Applications of Silk Nanoparticles. J. Vis. Exp. 2016, 116, e54669. [Google Scholar] [CrossRef]
- Dash, R.; Ghosh, S.K.; Kaplan, D.L.; Kundu, S.C. Purification and biochemical characterization of a 70 kDa sericin from tropical tasar silkworm, Antheraea mylitta. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 129–134. [Google Scholar] [CrossRef]
- Osama, I.; Gorenkova, N.; McKittrick, C.M.; Wongpinyochit, T.; Goudie, A.; Seib, F.P.; Carswell, H.V.O. In vitro studies on space-conforming self-assembling silk hydrogels as a mesenchymal stem cell-support matrix suitable for minimally invasive brain application. Sci. Rep. 2018, 8, 13655. [Google Scholar] [CrossRef]
- Matthew, S.; Totten, J.; Phuagkhaopong, S.; Egan, G.; Witte, K.; Perrie, Y.; Seib, F. Silk Nanoparticle Manufacture in Semi-Batch Format. ACS Biomater. Sci. Eng. 2020, 6, 6748–6759. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-p.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Choi, J.H.; Kim, P.; Youn, J.; Song, J.E.; Motta, A.; Migliaresi, C.; Khang, G. Preparation and evaluation of gellan gum hydrogel reinforced with silk fibers with enhanced mechanical and biological properties for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2021, 15, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Lee, W.; Choi, J.H.; Lee, S.; Song, J.E.; Khang, G. Fabrication and characterization of silk fibroin microfiber-incorporated bone marrow stem cell spheroids to promote cell–cell interaction and osteogenesis. ACS Omega 1802, 5. [Google Scholar] [CrossRef]
- Darshan, G.H.; Kong, D.; Gautrot, J.; Vootla, S. Physico-chemical characterization of Antheraea mylitta silk mats for wound healing applications. Sci. Rep. 2017, 7, 10344. [Google Scholar] [CrossRef]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the conformation and mechanical properties of silk fibroin hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Egan, G.; Phuagkhaopong, S.; Matthew, S.A.L.; Connolly, P.; Seib, F.P. Impact of silk hydrogel secondary structure on hydrogel formation, silk leaching and in vitro response. Sci. Rep. 2022, 12, 3729. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Folkman, J.; Moscona, A. Role of cell shape in growth control. Nature 1978, 273, 345–349. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Von Erlach, T.C.; Bertazzo, S.; Wozniak, M.A.; Horejs, C.M.; Maynard, S.A.; Attwood, S.; Robinson, B.K.; Autefage, H.; Kallepitis, C.; Del Rio Hernandez, A.; et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater. 2018, 17, 237–242. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewchuchuen, J.; Roamcharern, N.; Phuagkhaopong, S.; Bimbo, L.M.; Seib, F.P. Microfibre-Functionalised Silk Hydrogels. Cells 2024, 13, 10. https://doi.org/10.3390/cells13010010
Kaewchuchuen J, Roamcharern N, Phuagkhaopong S, Bimbo LM, Seib FP. Microfibre-Functionalised Silk Hydrogels. Cells. 2024; 13(1):10. https://doi.org/10.3390/cells13010010
Chicago/Turabian StyleKaewchuchuen, Jirada, Napaporn Roamcharern, Suttinee Phuagkhaopong, Luis M. Bimbo, and F. Philipp Seib. 2024. "Microfibre-Functionalised Silk Hydrogels" Cells 13, no. 1: 10. https://doi.org/10.3390/cells13010010
APA StyleKaewchuchuen, J., Roamcharern, N., Phuagkhaopong, S., Bimbo, L. M., & Seib, F. P. (2024). Microfibre-Functionalised Silk Hydrogels. Cells, 13(1), 10. https://doi.org/10.3390/cells13010010







