Therapeutic Approaches to Targeting Androgen Receptor Splice Variants
Abstract
:1. Introduction

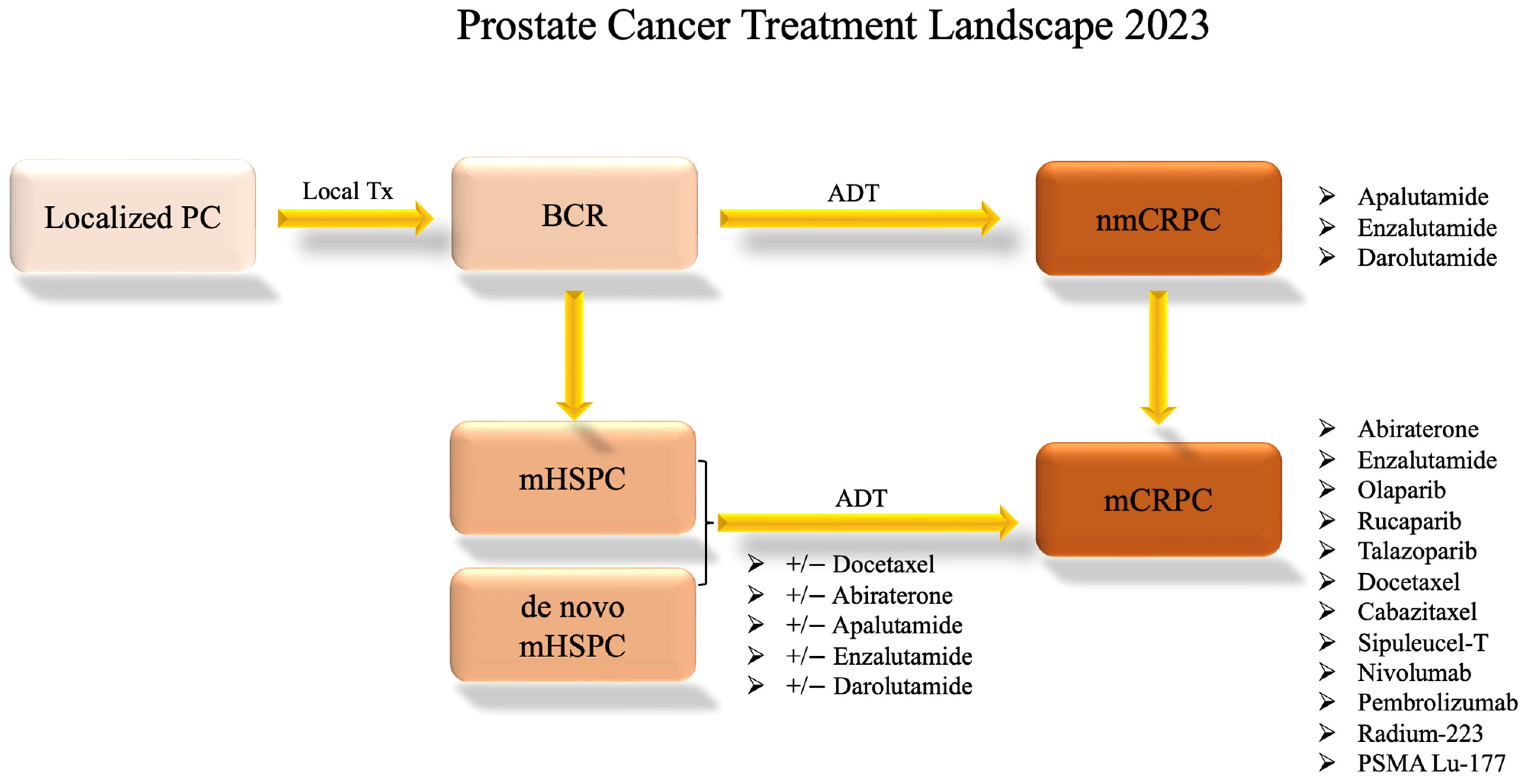
2. Prostate Cancer Treatment Landscape
3. Drugs That Target AR-Vs Directly
3.1. Niclosamide
3.2. TAS3681
3.3. EPI Compounds
3.4. PROTAC
3.5. VNPP433-3β
3.6. RIPTAC
| Therapy | Prostate Cancer Models Used in Preclinical Studies | AR-V7 Target Genes Tested | Mechanism | Compound Name, Clinical Trial Number, Reference |
|---|---|---|---|---|
| Niclosamide | LNCaP, VCaP, CWR22Rv1, PC3, C4-2, C4-2B, xenograft (CWR22Rv1) [16] | KLK3 (PSA) [16] | Inhibits AR-V activity via protein degradation. Reformulated niclosamide has higher bioavailability. | Niclosamide: NCT02532114 [17] Reformulated niclosamide: NCT02807805 [18] |
| TAS3681 | DU145, xenograft (cell line not disclosed) [21] | * N/A | Downregulates AR-V7 via an unknown mechanism (the paper yet to be published). | TAS3681: NCT02566772 [22] |
| EPI-Compounds | LNCaP, 22RV1, MDA PCa2b, PC3, Du145, VCaP, xenograft (PC3, LNCaP, VCaP) [24,25] | KLK3 (PSA), TMPRSS2 [24,25] Probasin, NKX3.1, UBE2C, AKT1, CDC20, and CYCLINA2 [25] | Inhibits protein-protein interaction of AR-FL and AR-Vs by targeting the AF-1 region of AR-NTD. EPI-7386 has improved bioavailability over EPI-506. | EPI-506: NCT02606123 EPI-7386: NCT04421222, NCT05075577 [27] |
| PROTAC | LNCaP, C4-2, CWR22Rv1, and DU145, xenograft (C4-2 and CWR22Rv1) [30] | N/A | Targets the DNA binding domain and MDM2-dependent mechanism. Clinical investigations have yet to be initiated. | Au-AR pep-PROTAC [30] |
| VNPP433-3β | LNCaP, CWR22RV1, xenograft (CWR22RV1) [32] | N/A | Promotes protein degradation of AR-FL and AR-Vs while also inhibiting MNK1/2. This is a galeterone drug derivative not yet in clinical trials. | VNPP433-3β [32] |
| RIPTAC | VCaP, xenograft (VCaP) [34] | * N/A | Selectively binds AR-FL and AR-Vs as well as an essential protein to cell viability which effectively kills cells overproducing AR. | RIPTAC [34] |
4. Drugs That May Overcome Resistance Mediated by AR-Vs through Indirect Mechanisms
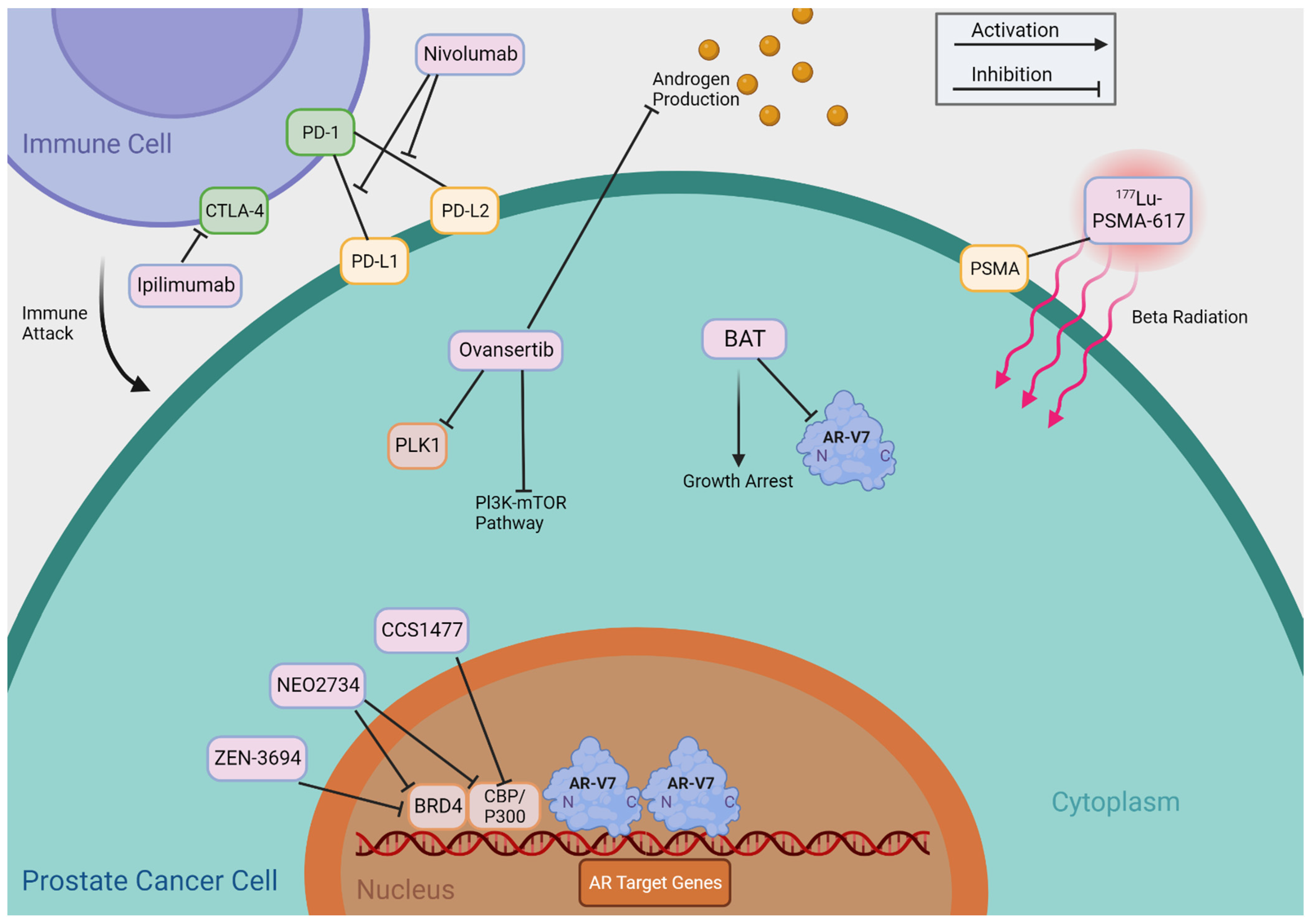
4.1. BET Inhibitors
4.2. CBP/p300 Inhibitors
4.3. PLK1 Inhibitors
4.4. Bipolar Androgen Therapy
4.5. Immune Checkpoint Inhibitors
4.6. 177Lu-PSMA-617 (Pluvicto)
| Therapy | Prostate Cancer Models Used in Preclinical Studies | Mechanism | Compound Name, Clinical Trial Number, Reference |
|---|---|---|---|
| Bromodomain and Extra Terminal (BET) Inhibitors | LNCaP, CWR22Rv1, LNCaP95 [36] | Targets the amino terminal bromodomains of BRD4, which typically interact with AR-FL and AR-Vs as a necessary step for transcription activation of AR target genes. BRD4 also elevates c-Myc, interacts with E2F, and mitigate TMPRSS2-ERG fusion. | ZEN-3694: NCT02711956 [37] NCT04986423 NCT04471974 GS-5829: NCT02607228 [44] |
| CREB-binding Protein (MCBP)/p300 Inhibitors | LNCaP, PC-3, LNCaP-abl, LAPC-4, CWR22Rv1, VCaP, LNCaP-AR, LNCaP-Bic, C4-2, LNCaP95, DU145 [45,47], xenograft (CWR22Rv1), PDX (CP-50) [47] | Targeted inhibition of CBP/p300 enzymes decreases chromatin accessibility and lowers the transcription of AR target genes. NEO2734 is a dual CBP/p300 and BET Inhibitor | CCS1477: NCT03568656 [47] NEO2734: NCT05488548 [49] |
| Polo-like Kinase 1 (PLK1) Inhibitors | RWPE-1 LNCaP, C4-2, CWR22Rv1, MR49F, LNCaP95, PC3, DU145 [51,52], PDX (LuCaP35CR) [51], PDX (LVCaP-2CR) [52] | Target inhibition of PLK1 in combination with abiraterone results in reduced AR-FL and AR-V7 protein expression potentially via cholesterol biosynthesis inhibition. Also, the drug combination induces mitotic arrest. | Ovansertib: NCT03414034 |
| Bipolar Androgen Therapy (BAT) | LNCaP, PC-3, CWR22Rv1, PrSC, LAPC-4 [53], VcaP, DU145 [56], xenograft (CWR22Rv1, LNCaP, LAPC-4) [53], LNCaP 104-S, LNCaP 104-R2, xenograft (LNCaP 104-S, 104-R1, CDXR-3) [55], Ex vivo PDX culture (LuCaP 35, 70, and 96CR) [56] | The cyclic alternation between two extremes of blood serum testosterone levels re-sensitizes CRPC to anti-androgen therapies. BAT in combination therapy may yield synergistic effects that improve outcomes for AR-V7 positive patients. | BAT: NCT02286921 [57] NCT02090114 [58] NCT04704505 NCT04558866 NCT03522064 NCT03516812 |
| Immune Checkpoint Inhibitors | Cited papers did not include preclinical studies. | Nivolumab and ipilimumab target specific molecules (PD-1 and CTLA-4 respectively) involved in regulating the immune response, allowing the immune system to better recognize and attack cancer cells. pTVG-AR (MVI-118) is a DNA vaccine encoding the ligand binding domain of AR, which induces CD8+T cell-mediated immune response against cancer cells overexpressing AR. | Nivolumab + ipilimumab: NCT02601014 [63] NCT02985957 [65] NCT05150236 NCT05655715 PTVG-AR (MVI-118): NCT02411786 [67] NCT04090528 NCT04989946 NCT03554317 |
| 177Lu-PSMA-617 (Pluvicto) | Cited papers did not include preclinical studies. | PSMA ligand linked to the radioactive isotope, 177Lu, targets PSMA-expressing cells. | NCT03511664 NCT05340374 NCT05113537 |
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cantley, R.L.; Wang, X.; Reichert, Z.R.; Chinnaiyan, A.M.; Mannan, R.; Cao, X.; Spratt, D.E.; Vaishampayan, U.N.; Alumkal, J.J.; Morgan, T.M.; et al. Metastatic prostate cancer diagnosed by fine-needle aspiration: Contemporary cytopathologic and biomarker assessment with clinical correlates. Cancer Cytopathol. 2023, 131, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Lu, C.; Luo, J.; Antonarakis, E.S. AR Splicing Variants and Resistance to AR Targeting Agents. Cancers 2021, 13, 2563. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dunn, T.A.; Wei, S.; Isharwal, S.; Veltri, R.W.; Humphreys, E.; Han, M.; Partin, A.W.; Vessella, R.L.; Isaacs, W.B.; et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009, 69, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lu, C.; Mostaghel, E.A.; Yegnasubramanian, S.; Gurel, M.; Tannahill, C.; Edwards, J.; Isaacs, W.B.; Nelson, P.S.; Bluemn, E.; et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012, 72, 3457–3462. [Google Scholar] [CrossRef]
- Watson, P.A.; Chen, Y.F.; Balbas, M.D.; Wongvipat, J.; Socci, N.D.; Viale, A.; Kim, K.; Sawyers, C.L. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 16759–16765. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Halabi, S.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Healy, P.; Anand, M.; Rothwell, C.J.; et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J. Clin. Oncol. 2019, 37, 1120–1129. [Google Scholar] [CrossRef]
- Luo, J.; Attard, G.; Balk, S.P.; Bevan, C.; Burnstein, K.; Cato, L.; Cherkasov, A.; De Bono, J.S.; Dong, Y.; Gao, A.C.; et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. Eur. Urol. 2018, 73, 715–723. [Google Scholar] [CrossRef]
- Luo, J. Development of AR-V7 as a putative treatment selection marker for metastatic castration-resistant prostate cancer. Asian J. Androl. 2016, 18, 580. [Google Scholar] [CrossRef]
- Kupelian, P.A.; Buchsbaum, J.C.; Elshaikh, M.; Reddy, C.A.; Zippe, C.; Klein, E.A. Factors affecting recurrence rates after prostatectomy or radiotherapy in localized prostate carcinoma patients with biopsy Gleason score 8 or above. Cancer 2002, 95, 2302–2307. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Guin, S.; Liaw, B.K.; Jun, T.; Ayers, K.; Patel, B.; O’Connell, T.; Deitz, M.; Klein, M.; Mullaney, T.; Prentice, T.; et al. Management of de novo metastatic hormone-sensitive prostate cancer: A comprehensive report of a single-center experience. PLoS ONE 2022, 17, e0264800. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef]
- Parikh, M.; Liu, C.; Wu, C.Y.; Evans, C.P.; Dall’Era, M.; Robles, D.; Lara, P.N.; Agarwal, N.; Gao, A.C.; Pan, C.X. Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer. Sci. Rep. 2021, 11, 6377. [Google Scholar] [CrossRef]
- Kang, B.; Mottamal, M.; Zhong, Q.; Bratton, M.; Zhang, C.; Guo, S.; Hossain, A.; Ma, P.; Zhang, Q.; Wang, G. Design, Synthesis, and Evaluation of Niclosamide Analogs as Therapeutic Agents for Enzalutamide-Resistant Prostate Cancer. Pharmaceuticals 2023, 16, 735. [Google Scholar] [CrossRef]
- Seki, M.; Minamiguchi, K.; Kajiwara, D.; Mizutani, H.; Yoshida, S.; Sasaki, E.; Utsugi, T.; Iwasawa, Y. TAS3681, a novel type of AR antagonist with AR downregulating activity, as a new targeted therapy for aberrant AR-driven prostate cancer. J. Clin. Oncol. 2018, 36, 298. [Google Scholar] [CrossRef]
- Minamiguchi, K.; Seki, M.; Aoyagi, H.; Kajiwara, D.; Mori, T.; Masuko, N.; Fujita, R.; Okajima, S.; Hayashi, Y.; Sasaki, E. TAS3681: New class of androgen receptor antagonist with androgen receptor downregulating activity. J. Clin. Oncol. 2015, 33, 266. [Google Scholar] [CrossRef]
- De Bono, J.S.; Cook, N.; Yu, E.Y.; Lara, P.L.N.; Wang, J.S.; Yamasaki, Y.; Yamamiya, I.; Gao, P.; Calleja, E.M.; Rathkopf, D.E. First-in-human study of TAS3681, an oral androgen receptor (AR) antagonist with AR and AR splice variant (AR-SV) downregulation activity, in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) refractory to abiraterone (ABI) and/or enzalutamide (ENZ) and chemotherapy (CT). J. Clin. Oncol. 2021, 39, 5031. [Google Scholar]
- Ji, Y.; Zhang, R.; Han, X.; Zhou, J. Targeting the N-terminal domain of the androgen receptor: The effective approach in therapy of CRPC. Eur. J. Med. Chem. 2023, 247, 115077. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.J.; Mawji, N.R.; Wang, J.; Wang, G.; Haile, S.; Myung, J.K.; Watt, K.; Tam, T.; Yang, Y.C.; Banuelos, C.A.; et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 2010, 17, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.K.; Banuelos, C.A.; Fernandez, J.G.; Mawji, N.R.; Wang, J.; Tien, A.H.; Yang, Y.C.; Tavakoli, I.; Haile, S.; Watt, K.; et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J. Clin. Investig. 2013, 123, 2948–2960. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Chandhasin, C.; Osbourne, E.; Luo, J.; Sadar, M.D.; Perabo, F. Targeting the N-Terminal Domain of the Androgen Receptor: A New Approach for the Treatment of Advanced Prostate Cancer. Oncologist 2016, 21, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Le Moigne, R.; Pearson, P.; Lauriault, V.; Chi, K.; Ianotti, N.; Pachynski, R.; Vogelzang, N.; Hong, N.H.; Virsik, P.; Zhou, H.-J. Preclinical and clinical pharmacology of EPI-7386, an androgen receptor N-terminal domain inhibitor for castration-resistant prostate cancer. J. Clin. Oncol. 2021, 39, 119. [Google Scholar] [CrossRef]
- Laccetti, A.L.; Chatta, G.S.; Iannotti, N.; Kyriakopoulos, C.; Villaluna, K.; Le Moigne, R.; Cesano, A. Phase 1/2 study of EPI-7386 in combination with enzalutamide (enz) compared with enz alone in subjects with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41, 179. [Google Scholar] [CrossRef]
- Gao, X.; Burris III, H.A.; Vuky, J.; Dreicer, R.; Sartor, A.O.; Sternberg, C.N.; Percent, I.J.; Hussain, M.H.; Rezazadeh Kalebasty, A.; Shen, J. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef]
- Ma, B.; Fan, Y.; Zhang, D.; Wei, Y.; Jian, Y.; Liu, D.; Wang, Z.; Gao, Y.; Ma, J.; Chen, Y.; et al. De Novo Design of an Androgen Receptor DNA Binding Domain-Targeted peptide PROTAC for Prostate Cancer Therapy. Adv. Sci. 2022, 9, e2201859. [Google Scholar] [CrossRef]
- Taplin, M.E.; Antonarakis, E.S.; Ferrante, K.J.; Horgan, K.; Blumenstein, B.; Saad, F.; Luo, J.; de Bono, J.S. Androgen Receptor Modulation Optimized for Response-Splice Variant: A Phase 3, Randomized Trial of Galeterone Versus Enzalutamide in Androgen Receptor Splice Variant-7-expressing Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 76, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Thankan, R.S.; Purushottamachar, P.; Huang, W.; Kane, M.A.; Zhang, Y.; Ambulos, N.P.; Weber, D.J.; Njar, V.C.O. Novel AR/AR-V7 and Mnk1/2 Degrader, VNPP433-3beta: Molecular Mechanisms of Action and Efficacy in AR-Overexpressing Castration Resistant Prostate Cancer In Vitro and In Vivo Models. Cells 2022, 11, 2699. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Eastman, K.J.; Yu, X.; Forbes, C.D.; Jones, K.M.; Mousseau, J.J.; Li, H.; Kayser-Bricker, K.J.; Crews, C.M. An oral androgen receptor RIPTAC for prostate cancer. J. Clin. Oncol. 2023, 41, 184. [Google Scholar] [CrossRef]
- Yu, X.; Eastman, K.J.; Raina, K.; Jones, K.M.; Forbes, C.D.; Hundt, A.; Garcia, M.; Stronk, R.; Howard, K.; McGovern, A. Prostate cancer RIPTAC™ therapeutics demonstrate activity in preclinical models of Enzalutamide-resistant prostate cancer. Cancer Res. 2023, 83, 1629. [Google Scholar] [CrossRef]
- Mandl, A.; Markowski, M.C.; Carducci, M.A.; Antonarakis, E.S. Role of bromodomain and extraterminal (BET) proteins in prostate cancer. Expert. Opin. Investig. Drugs 2023, 32, 213–228. [Google Scholar] [CrossRef]
- Li, X.; Baek, G.; Ramanand, S.G.; Sharp, A.; Gao, Y.; Yuan, W.; Welti, J.; Rodrigues, D.N.; Dolling, D.; Figueiredo, I. BRD4 promotes DNA repair and mediates the formation of TMPRSS2-ERG gene rearrangements in prostate cancer. Cell Rep. 2018, 22, 796–808. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Schweizer, M.T.; Nanus, D.M.; Pantuck, A.J.; Heath, E.I.; Campeau, E.; Attwell, S.; Norek, K.; Snyder, M.; Bauman, L.; et al. A Phase Ib/IIa Study of the Pan-BET Inhibitor ZEN-3694 in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2020, 26, 5338–5347. [Google Scholar] [CrossRef]
- Ameratunga, M.; Brana, I.; Bono, P.; Postel-Vinay, S.; Plummer, R.; Aspegren, J.; Korjamo, T.; Snapir, A.; de Bono, J.S. First-in-human Phase 1 open label study of the BET inhibitor ODM-207 in patients with selected solid tumours. Br. J. Cancer 2020, 123, 1730–1736. [Google Scholar] [CrossRef]
- Asangani, I.A.; Dommeti, V.L.; Wang, X.; Malik, R.; Cieslik, M.; Yang, R.; Escara-Wilke, J.; Wilder-Romans, K.; Dhanireddy, S.; Engelke, C.; et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014, 510, 278–282. [Google Scholar] [CrossRef]
- Faivre, E.J.; Wilcox, D.; Lin, X.; Hessler, P.; Torrent, M.; He, W.; Uziel, T.; Albert, D.H.; McDaniel, K.; Kati, W.; et al. Exploitation of Castration-Resistant Prostate Cancer Transcription Factor Dependencies by the Novel BET Inhibitor ABBV-075. Mol. Cancer Res. 2017, 15, 35–44. [Google Scholar] [CrossRef]
- Hupe, M.C.; Hoda, M.R.; Zengerling, F.; Perner, S.; Merseburger, A.S.; Cronauer, M.V. The BET-inhibitor PFI-1 diminishes AR/AR-V7 signaling in prostate cancer cells. World J. Urol. 2019, 37, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Wyce, A.; Degenhardt, Y.; Bai, Y.; Le, B.; Korenchuk, S.; Crouthamel, M.-C.; McHugh, C.F.; Vessella, R.; Creasy, C.L.; Tummino, P.J. Inhibition of BET bromodomain proteins as a therapeutic approach in prostate cancer. Oncotarget 2013, 4, 2419. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ma, J.; Wang, D.; Lin, D.; Pang, X.; Wang, S.; Zhao, Y.; Shi, L.; Xue, H.; Pan, Y. The novel BET-CBP/p300 dual inhibitor NEO2734 is active in SPOP mutant and wild-type prostate cancer. EMBO Mol. Med. 2019, 11, e10659. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Starodub, A.N.; Koh, B.D.; Xing, G.; Armstrong, A.J.; Carducci, M.A. Phase Ib study of the BET inhibitor GS-5829 as monotherapy and combined with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2022, 28, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, Z.; Kimball, H.; Qu, F.; Berlind, K.; Stopsack, K.H.; Lee, G.M.; Choueiri, T.K.; Kantoff, P.W. Abiraterone Acetate Induces CREB1 Phosphorylation and Enhances the Function of the CBP-p300 Complex, Leading to Resistance in Prostate Cancer Cells. Clin. Cancer Res. 2021, 27, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.; Williams, G.L.; Castro, J.; Battalagine, L.; Wilker, E.; Yao, L.; Schiller, S.; Toms, A.; Li, P.; Pardo, E. FT-6876, a Potent and Selective Inhibitor of CBP/p300, is Active in Preclinical Models of Androgen Receptor-Positive Breast Cancer. Target. Oncol. 2023, 18, 269–285. [Google Scholar] [CrossRef]
- Welti, J.; Sharp, A.; Brooks, N.; Yuan, W.; McNair, C.; Chand, S.N.; Pal, A.; Figueiredo, I.; Riisnaes, R.; Gurel, B.; et al. Targeting the p300/CBP Axis in Lethal Prostate Cancer. Cancer Discov. 2021, 11, 1118–1137. [Google Scholar] [CrossRef]
- He, Y.; Wei, T.; Ye, Z.; Orme, J.J.; Lin, D.; Sheng, H.; Fazli, L.; Jeffrey Karnes, R.; Jimenez, R.; Wang, L.; et al. A noncanonical AR addiction drives enzalutamide resistance in prostate cancer. Nat. Commun. 2021, 12, 1521. [Google Scholar] [CrossRef]
- Sun, R.; Yan, B.; Li, H.; Ding, D.; Wang, L.; Pang, J.; Ye, D.; Huang, H. Androgen Receptor Variants Confer Castration Resistance in Prostate Cancer by Counteracting Antiandrogen-Induced Ferroptosis. Cancer Res. 2023, 83, 3192–3204. [Google Scholar] [CrossRef]
- Strebhardt, K. Multifaceted polo-like kinases: Drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 643–660. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, X.; Shao, C.; Li, J.; Cheng, J.X.; Kuang, S.; Ahmad, N.; Ratliff, T.; Liu, X. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res. 2014, 74, 6635–6647. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.C.; Varkaris, A.; Croucher, P.J.; Ridinger, M.; Dalrymple, S.; Nouri, M.; Xie, F.; Varmeh, S.; Jonas, O.; Whitman, M.A. Plk1 Inhibitors and Abiraterone Synergistically Disrupt Mitosis and Kill Cancer Cells of Disparate Origin Independently of Androgen Receptor Signaling. Cancer Res. 2023, 83, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Vander Griend, D.J.; Antony, L.; Dalrymple, S.; De Marzo, A.M.; Drake, C.G.; Isaacs, J.T. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15085–15090. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, Y.; Yue, H.H.; Zhang, Y.T.; Shi, C.H.; Liu, F.; Bao, T.Y.; Yang, Z.Y.; Yuan, J.L.; Shao, G.X. Biphasic effect of androgens on prostate cancer cells and its correlation with androgen receptor coactivator dopa decarboxylase. J. Androl. 2007, 28, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.-P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.-P.; Lin, C.-Y.; Huo, C.; Su, L.-C. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J. Biomed. Sci. 2011, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Schweizer, M.T.; Lucas, J.M.; Coleman, I.; Nyquist, M.D.; Frank, S.B.; Tharakan, R.; Mostaghel, E.; Luo, J.; Pritchard, C.C. Supraphysiological androgens suppress prostate cancer growth through androgen receptor–mediated DNA damage. J. Clin. Investig. 2019, 129, 4245–4260. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Wang, H.; Agarwal, N.; Smith, D.C.; Schweizer, M.T.; Stein, M.N.; Assikis, V.; Twardowski, P.W.; Flaig, T.W.; Szmulewitz, R.Z.; et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J. Clin. Oncol. 2021, 39, 1371–1382. [Google Scholar] [CrossRef]
- Markowski, M.C.; Wang, H.; Sullivan, R.; Rifkind, I.; Sinibaldi, V.; Schweizer, M.T.; Teply, B.A.; Ngomba, N.; Fu, W.; Carducci, M.A.; et al. A Multicohort Open-label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur. Urol. 2021, 79, 692–699. [Google Scholar] [CrossRef]
- Markowski, M.C.; Shenderov, E.; Eisenberger, M.A.; Kachhap, S.; Pardoll, D.M.; Denmeade, S.R.; Antonarakis, E.S. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate 2020, 80, 407–411. [Google Scholar] [CrossRef]
- Markowski, M.C.; Taplin, M.-E.; Aggarwal, R.R.; Sena, L.; Wang, H.; Lalji, A.; Meyers, J.; Skaist, A.; Gupta, A.; Gomes-Alexandre, C. Overall survival (OS) and biomarker results from combat: A phase 2 study of bipolar androgen therapy (BAT) plus nivolumab for patients with metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 5064. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Pinski, J.K. Association of ARV7 expression with molecular and clinical characteristics in prostate cancer. J. Clin. Oncol. 2016, 34, 109. [Google Scholar] [CrossRef]
- Boudadi, K.; Suzman, D.L.; Anagnostou, V.; Fu, W.; Luber, B.; Wang, H.; Niknafs, N.; White, J.R.; Silberstein, J.L.; Sullivan, R.; et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018, 9, 28561–28571. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, E.; Boudadi, K.; Fu, W.; Wang, H.; Sullivan, R.; Jordan, A.; Dowling, D.; Harb, R.; Schonhoft, J.; Jendrisak, A. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: A phase-2 nonrandomized clinical trial. Prostate 2021, 81, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e483. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Krainer, M.; Saad, F.; Castellano, D.; Bedke, J.; Kwiatkowski, M.; Patnaik, A.; Procopio, G.; Wiechno, P.; Kochuparambil, S.T. Nivolumab plus ipilimumab for the treatment of post-chemotherapy metastatic castration-resistant prostate cancer (mCRPC): Additional results from the randomized phase 2 CheckMate 650 trial. J. Clin. Oncol. 2023, 41, 22. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Eickhoff, J.C.; Ferrari, A.C.; Schweizer, M.T.; Wargowski, E.; Olson, B.M.; McNeel, D.G. Multicenter Phase I Trial of a DNA Vaccine Encoding the Androgen Receptor Ligand-binding Domain (pTVG-AR, MVI-118) in Patients with Metastatic Prostate Cancer. Clin. Cancer Res. 2020, 26, 5162–5171. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Kostos, L.K.; Buteau, J.P.; Kong, G.; Yeung, T.; Di Iulio, J.; Fahey, M.T.; Fettke, H.; Furic, L.; Hofman, M.S.; Azad, A. LuCAB: A phase I/II trial evaluating cabazitaxel in combination with [177Lu] Lu-PSMA-617 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41, 278. [Google Scholar] [CrossRef]
- Paller, C.J.; Piana, D.; Eshleman, J.R.; Riel, S.; Denmeade, S.R.; Isaacsson Velho, P.; Rowe, S.P.; Pomper, M.G.; Antonarakis, E.S.; Luo, J.; et al. A pilot study of prostate-specific membrane antigen (PSMA) dynamics in men undergoing treatment for advanced prostate cancer. Prostate 2019, 79, 1597–1603. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Weckesser, M.; Roll, W.; Humberg, V.; Schlack, K.; Bögemann, M.; Bernemann, C.; Rahbar, K. Molecular analysis of circulating tumor cells of metastatic castration-resistant Prostate Cancer Patients receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7645. [Google Scholar] [CrossRef] [PubMed]
- Pathmanandavel, S.; Crumbaker, M.; Yam, A.O.; Nguyen, A.; Rofe, C.; Hovey, E.; Gedye, C.; Kwan, E.M.; Hauser, C.; Azad, A.A. 177Lu-PSMA-617 and idronoxil in men with end-stage metastatic castration-resistant prostate cancer (LuPIN): Patient outcomes and predictors of treatment response in a phase I/II trial. J. Nucl. Med. 2022, 63, 560–566. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, V.A.; Luo, J.; Paller, C.J.; Kanayama, M. Therapeutic Approaches to Targeting Androgen Receptor Splice Variants. Cells 2024, 13, 104. https://doi.org/10.3390/cells13010104
Daniels VA, Luo J, Paller CJ, Kanayama M. Therapeutic Approaches to Targeting Androgen Receptor Splice Variants. Cells. 2024; 13(1):104. https://doi.org/10.3390/cells13010104
Chicago/Turabian StyleDaniels, Violet A., Jun Luo, Channing J. Paller, and Mayuko Kanayama. 2024. "Therapeutic Approaches to Targeting Androgen Receptor Splice Variants" Cells 13, no. 1: 104. https://doi.org/10.3390/cells13010104





