Prolonged Antibiotic Use in a Preclinical Model of Gulf War Chronic Multisymptom-Illness Causes Renal Fibrosis-like Pathology via Increased micro-RNA 21-Induced PTEN Inhibition That Is Correlated with Low Host Lachnospiraceae Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
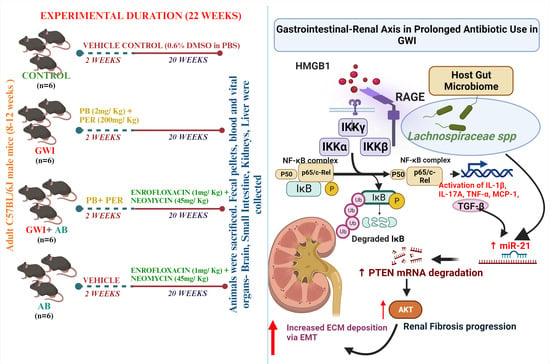
2.2. Animal Model and Care
Mouse Model of Gulf War Illness (GWI)
2.3. Microbiome Analysis
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
miR-21 Quantification and Analysis
2.5. Immunohistochemical Analysis
2.6. Immunofluorescence Staining
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Prolonged Antibiotic Administration in GWI Mice Exacerbates Renal Inflammation and Production of Pro-Inflammatory Cytokines, Namely, IL-1β and IL-17A
3.2. Prolonged Antibiotic Administration in GWI Mice Increased in the Production and mRNA Expression of Pro-Inflammatory Cytokines and Chemokines
3.3. Prolonged Antibiotic Administration Led to Augmentation of RAGE Activation Mediated by HMGB1 in GWI Mice
3.4. Prolonged Antibiotic Administration Is Associated with Worsened Renal Pathology by Increasing TGF-β Production in GWI Mice
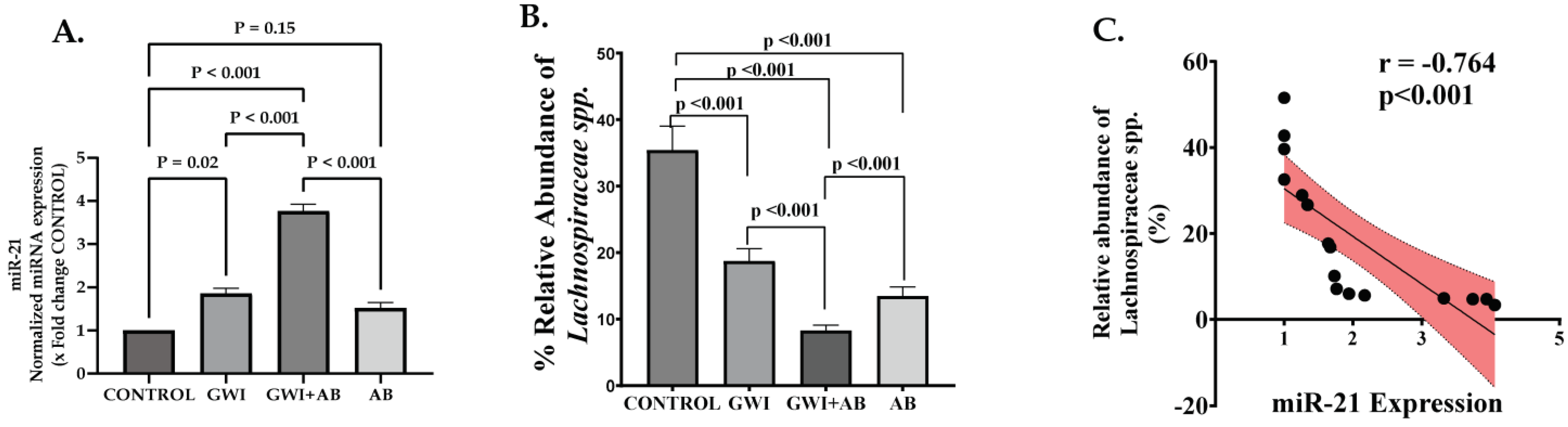
3.5. Prolonged Antibiotic Administration in GWI Mice Mediated the Upregulation of micro-RNA 21 (miR-21), a Signature miRNA for Renal Fibrosis That Targets the TGF-β/SMAD Pathway
3.6. Protein Expression of PTEN, a Target of miR-21, Is Perturbed Due to Prolonged Antibiotic Treatment in GWI Mice, Thereby Modulating AKT Signaling, as PTEN Functions as a Negative Regulator of AKT Signaling
3.7. Prolonged Antibiotic Treatment in GWI Mice Caused Increased Extracellular Matrix (ECM) Deposition and the Induction of an Epithelial–Mesenchymal Transition (EMT)-like Phenotype in the Kidney
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, F.M. Gulf war syndrome. BMJ 1999, 318, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, R.; Barrett, D.H.; Reyes, M.; Reeves, W.C. Deployment stressors and a chronic multisymptom illness among Gulf War veterans. J. Nerv. Ment. Dis. 2000, 188, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Sastre, A.; Gerkovich, M.M.; Cook, M.R. Complex factors in the etiology of Gulf War illness: Wartime exposures and risk factors in veteran subgroups. Environ. Health Perspect. 2012, 120, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Alhasson, F.; Das, S.; Seth, R.; Dattaroy, D.; Chandrashekaran, V.; Ryan, C.N.; Chan, L.S.; Testerman, T.; Burch, J.; Hofseth, L.J.; et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE 2017, 12, e0172914. [Google Scholar] [CrossRef] [PubMed]
- Janulewicz, P.A.; Seth, R.K.; Carlson, J.M.; Ajama, J.; Quinn, E.; Heeren, T.; Klimas, N.; Lasley, S.M.; Horner, R.D.; Sullivan, K.; et al. The Gut-Microbiome in Gulf War Veterans: A Preliminary Report. Int. J. Environ. Res. Public Health 2019, 16, 3751. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Saha, P.; Mondal, A.; Fanelli, B.; Seth, R.K.; Janulewicz, P.; Sullivan, K.; Lasley, S.; Horner, R.; Colwell, R.R.; et al. Obesity Worsens Gulf War Illness Symptom Persistence Pathology by Linking Altered Gut Microbiome Species to Long-Term Gastrointestinal, Hepatic, and Neuronal Inflammation in a Mouse Model. Nutrients 2020, 12, 2764. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Aftzoglou, K.; Voidarou, C.; Konstantinidis, T.; Chifiriuc, M.C.; Thodis, E.; Bezirtzoglou, E. Microbiome, Immunosenescence, and Chronic Kidney Disease. Front. Med. 2021, 8, 661203. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic. Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Lan, H.Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr. Opin. Nephrol. Hypertens 2003, 12, 25–29. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Z.; Zeng, R.; Yao, Y. HMGB1 in kidney diseases. Life Sci. 2020, 259, 118203. [Google Scholar] [CrossRef]
- Lynch, J.; Nolan, S.; Slattery, C.; Feighery, R.; Ryan, M.P.; McMorrow, T. High-mobility group box protein 1: A novel mediator of inflammatory-induced renal epithelial-mesenchymal transition. Am. J. Nephrol. 2010, 32, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- He, X.; Cheng, R.; Huang, C.; Takahashi, Y.; Yang, Y.; Benyajati, S.; Chen, Y.; Zhang, X.A.; Ma, J.X. A novel role of LRP5 in tubulointerstitial fibrosis through activating TGF-beta/Smad signaling. Signal Transduct. Target. Ther. 2020, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, F.; Liu, Y.; Zheng, L.; Jiang, X.; Tian, H.; Wang, X.; Song, X.; Yu, Y.; Wang, D. Nur77 ameliorates age-related renal tubulointerstitial fibrosis by suppressing the TGF-beta/Smads signaling pathway. FASEB J. 2022, 36, e22124. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Matoba, K.; Kawanami, D.; Takeda, Y.; Akamine, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. ROCK2 regulates TGF-beta-induced expression of CTGF and profibrotic genes via NF-kappaB and cytoskeleton dynamics in mesangial cells. Am. J. Physiol. Renal Physiol. 2019, 317, F839–F851. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Zhang, J.; Wang, M.; Lanting, L.; Yuan, H.; Rossi, J.J.; Natarajan, R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. USA 2007, 104, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chung, A.C.; Dong, Y.; Yang, W.; Zhong, X.; Lan, H.Y. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-beta/Smad3-Azin1 pathway. Kidney Int. 2013, 84, 1129–1144. [Google Scholar] [CrossRef]
- Chung, Y.H.; Huang, G.K.; Kang, C.H.; Cheng, Y.T.; Kao, Y.H.; Chien, Y.S. MicroRNA-26a-5p Restoration Ameliorates Unilateral Ureteral Obstruction-Induced Renal Fibrosis in Mice Through Modulating TGF-beta Signaling. Lab. Investig. 2023, 103, 100131. [Google Scholar] [CrossRef]
- Lyu, H.; Li, X.; Wu, Q.; Hao, L. Overexpression of microRNA-21 mediates Ang II-induced renal fibrosis by activating the TGF-beta1/Smad3 pathway via suppressing PPARalpha. J. Pharmacol. Sci. 2019, 141, 70–78. [Google Scholar] [CrossRef]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef]
- Liao, W.; Liang, P.; Liu, B.; Xu, Z.; Zhang, L.; Feng, M.; Tang, Y.; Xu, A. MicroRNA-140-5p Mediates Renal Fibrosis Through TGF-beta1/Smad Signaling Pathway by Directly Targeting TGFBR1. Front. Physiol. 2020, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Yang, S.; Abraham, E.; Agarwal, A.; Liu, G. Identification of a microRNA signature in renal fibrosis: Role of miR-21. Am. J. Physiol. Renal Physiol. 2011, 301, F793–F801. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.R.; Luo, S.G.; Lin, X.; Wang, J.; Liu, Y. Silenced miR-21 inhibits renal interstitial fibrosis via targeting ERK1/2 signaling pathway in mice. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Artelt, N.; Kindt, F.; Stracke, S.; Rettig, R.; Lendeckel, U.; Chadjichristos, C.E.; Kavvadas, P.; Chatziantoniou, C.; Endlich, K.; et al. MiR-21 is up-regulated in urinary exosomes of chronic kidney disease patients and after glomerular injury. J. Cell. Mol. Med. 2019, 23, 4839–4843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiong, M.; Fang, L.; Jiang, L.; Wen, P.; Dai, C.; Zhang, C.Y.; Yang, J. miR-21-containing microvesicles from injured tubular epithelial cells promote tubular phenotype transition by targeting PTEN protein. Am. J. Pathol. 2013, 183, 1183–1196. [Google Scholar] [CrossRef]
- Guo, J. Effect of miR-21 on Renal Fibrosis Induced by Nano-SiO(2) in Diabetic Nephropathy Rats via PTEN/AKT Pathway. J. Nanosci. Nanotechnol. 2021, 21, 1079–1084. [Google Scholar] [CrossRef]
- Zhao, S.; Li, W.; Yu, W.; Rao, T.; Li, H.; Ruan, Y.; Yuan, R.; Li, C.; Ning, J.; Li, S.; et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 2021, 11, 8660–8673. [Google Scholar] [CrossRef]
- Ernandez, T.; Mayadas, T.N. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009, 76, 262–276. [Google Scholar] [CrossRef]
- Ramseyer, V.D.; Garvin, J.L. Tumor necrosis factor-alpha: Regulation of renal function and blood pressure. Am. J. Physiol. Renal Physiol. 2013, 304, F1231–F1242. [Google Scholar] [CrossRef]
- Asavarut, P.; Zhao, H.; Gu, J.; Ma, D. The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol. Taiwan 2013, 51, 28–33. [Google Scholar] [CrossRef]
- Kimono, D.; Bose, D.; Seth, R.K.; Mondal, A.; Saha, P.; Janulewicz, P.; Sullivan, K.; Lasley, S.; Horner, R.; Klimas, N.; et al. Host Akkermansia muciniphila Abundance Correlates with Gulf War Illness Symptom Persistence via NLRP3-Mediated Neuroinflammation and Decreased Brain-Derived Neurotrophic Factor. Neurosci. Insights 2020, 15, 2633105520942480. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Mondal, A.; Saha, P.; Kimono, D.; Sarkar, S.; Seth, R.K.; Janulewicz, P.; Sullivan, K.; Horner, R.; Klimas, N.; et al. TLR Antagonism by Sparstolonin B Alters Microbial Signature and Modulates Gastrointestinal and Neuronal Inflammation in Gulf War Illness Preclinical Model. Brain Sci. 2020, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Q.; Jin, Q.; Yang, L.; Mao, H.; Qu, P.; Guo, J.; Zhang, B.; Ma, F.; Wang, Y.; et al. Targeting HMGB1: A Potential Therapeutic Strategy for Chronic Kidney Disease. Int. J. Biol. Sci. 2023, 19, 5020–5035. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Bottinger, E.P. TGF-beta in renal injury and disease. Semin. Nephrol. 2007, 27, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liu, H.; Zhang, D.; Liu, Y.; Wang, C.; Liu, F.; Chen, J. HMGB1 Enhances the AGE-Induced Expression of CTGF and TGF-beta via RAGE-Dependent Signaling in Renal Tubular Epithelial Cells. Am. J. Nephrol. 2015, 41, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kang, H.Y.; Kim, G.; Park, J.; Nam, B.Y.; Park, J.T.; Han, S.H.; Kang, S.W.; Yoo, T.H. Soluble receptors for advanced glycation end-products prevent unilateral ureteral obstruction-induced renal fibrosis. Front. Pharmacol. 2023, 14, 1172269. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-beta1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediat. Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.; Chen, H.Y.; Meng, X.M.; Lan, H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1668–1681. [Google Scholar] [CrossRef]
- Dey, N.; Ghosh-Choudhury, N.; Kasinath, B.S.; Choudhury, G.G. TGFbeta-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS ONE 2012, 7, e42316. [Google Scholar] [CrossRef]
- Higgins, D.F.; Ewart, L.M.; Masterson, E.; Tennant, S.; Grebnev, G.; Prunotto, M.; Pomposiello, S.; Conde-Knape, K.; Martin, F.M.; Godson, C. BMP7-induced-Pten inhibits Akt and prevents renal fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chen, F.; Chen, D.; Liang, H. DNMT3a negatively regulates PTEN to activate the PI3K/AKT pathway to aggravate renal fibrosis. Cell Signal. 2022, 96, 110352. [Google Scholar] [CrossRef] [PubMed]
- Loh, A.H.; Cohen, A.H. Drug-induced kidney disease--pathology and current concepts. Ann. Acad. Med. Singap. 2009, 38, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Ghane Shahrbaf, F.; Assadi, F. Drug-induced renal disorders. J. Renal Inj. Prev. 2015, 4, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Jones, M.M.; Huttner, B.; Stoddard, G.; Brown, K.A.; Stevens, V.W.; Greene, T.; Sauer, B.; Madaras-Kelly, K.; Rubin, M.; et al. Trends in Antibiotic Use and Nosocomial Pathogens in Hospitalized Veterans with Pneumonia at 128 Medical Centers, 2006–2010. Clin. Infect. Dis. 2015, 61, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.R.; Koyama, A.K.; Choudhury, D.; Yu, W.; Pavkov, M.E.; Nee, R.; Cheung, A.K.; Norris, K.C.; Yan, G. Age-Related Association between Multimorbidity and Mortality in US Veterans with Incident Chronic Kidney Disease. Am. J. Nephrol. 2022, 53, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M. Inflammatory Mediators and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 381–406. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Bustos, C.; Hernandez-Presa, M.A.; Lorenzo, O.; Plaza, J.J.; Egido, J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J. Immunol. 1998, 161, 430–439. [Google Scholar] [CrossRef]
- Lopez-Franco, O.; Suzuki, Y.; Sanjuan, G.; Blanco, J.; Hernandez-Vargas, P.; Yo, Y.; Kopp, J.; Egido, J.; Gomez-Guerrero, C. Nuclear factor-kappa B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am. J. Pathol. 2002, 161, 1497–1505. [Google Scholar] [CrossRef]
- Sanz, A.B.; Justo, P.; Sanchez-Nino, M.D.; Blanco-Colio, L.M.; Winkles, J.A.; Kreztler, M.; Jakubowski, A.; Blanco, J.; Egido, J.; Ruiz-Ortega, M.; et al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J. Am. Soc. Nephrol. 2008, 19, 695–703. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Lin, L.; Hu, K. NF-kappaB and tPA Signaling in Kidney and Other Diseases. Cells 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yao, L.; Li, J. Role of MCP-1/CCR2 axis in renal fibrosis: Mechanisms and therapeutic targeting. Medicine 2023, 102, e35613. [Google Scholar] [CrossRef]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Viedt, C.; Orth, S.R. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: Does it more than simply attract monocytes? Nephrol. Dial. Transplant. 2002, 17, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.C. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-beta in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Sarkar, S.; Alhasson, F.; Kimono, D.; Albadrani, M.; Seth, R.K.; Xiao, S.; Porter, D.E.; Scott, G.I.; Brooks, B.; Nagarkatti, M.; et al. Microcystin exposure worsens nonalcoholic fatty liver disease associated ectopic glomerular toxicity via NOX-2-MIR21 axis. Environ. Toxicol. Pharmacol. 2020, 73, 103281. [Google Scholar] [CrossRef]
- Hennino, M.F.; Buob, D.; Van der Hauwaert, C.; Gnemmi, V.; Jomaa, Z.; Pottier, N.; Savary, G.; Drumez, E.; Noel, C.; Cauffiez, C.; et al. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci. Rep. 2016, 6, 27209. [Google Scholar] [CrossRef]
- Chen, C.; Lu, C.; Qian, Y.; Li, H.; Tan, Y.; Cai, L.; Weng, H. Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis. Sci. Rep. 2017, 7, 17737. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Gao, Y.B.; Zhang, N.; Zou, D.W.; Wang, P.; Zhu, Z.Y.; Li, J.Y.; Zhou, S.N.; Wang, S.C.; Wang, Y.Y.; et al. miR-21 overexpression enhances TGF-beta1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol. Cell. Endocrinol. 2014, 392, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Bu, F.T.; Li, J.J.; Zhang, Y.F.; Jia, P.C.; You, H.M.; Wu, S.; Wu, Y.Y.; Zhu, S.; Huang, C.; et al. MicroRNA-195-3p promotes hepatic stellate cell activation and liver fibrosis by suppressing PTEN expression. Toxicol. Lett. 2022, 355, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, H.; Qiu, T.; Dai, J.; Zhang, Y.; Chen, J.; Cai, H. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-kappaB activation. Aging Cell 2019, 18, e12858. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Jiao, B.; Du, H.; Tran, M.; Zhou, D.; Wang, Y. Myeloid PTEN deficiency aggravates renal inflammation and fibrosis in angiotensin II-induced hypertension. J. Cell. Physiol. 2022, 237, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 2004, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bulow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Xue, J.; Xiao, T.; Wei, S.; Sun, J.; Zou, Z.; Shi, M.; Sun, Q.; Dai, X.; Wu, L.; Li, J.; et al. miR-21-regulated M2 polarization of macrophage is involved in arsenicosis-induced hepatic fibrosis through the activation of hepatic stellate cells. J. Cell. Physiol. 2021, 236, 6025–6041. [Google Scholar] [CrossRef]
- Jiao, B.; An, C.; Tran, M.; Du, H.; Wang, P.; Zhou, D.; Wang, Y. Pharmacological Inhibition of STAT6 Ameliorates Myeloid Fibroblast Activation and Alternative Macrophage Polarization in Renal Fibrosis. Front. Immunol. 2021, 12, 735014. [Google Scholar] [CrossRef]
- Jiao, B.; An, C.; Du, H.; Tran, M.; Wang, P.; Zhou, D.; Wang, Y. STAT6 Deficiency Attenuates Myeloid Fibroblast Activation and Macrophage Polarization in Experimental Folic Acid Nephropathy. Cells 2021, 10, 3057. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Jiao, B.; Du, H.; Tran, M.; Song, B.; Wang, P.; Zhou, D.; Wang, Y. Jumonji domain-containing protein-3 (JMJD3) promotes myeloid fibroblast activation and macrophage polarization in kidney fibrosis. Br. J. Pharmacol. 2023, 180, 2250–2265. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Du, J. Potential role of Akt signaling in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xie, Y.; Xiao, Y.; Zeng, W.; Gong, Z.; Du, J. Longitudinal analysis of fecal microbiome and metabolome during renal fibrotic progression in a unilateral ureteral obstruction animal model. Eur. J. Pharmacol. 2020, 886, 173555. [Google Scholar] [CrossRef]
- Luo, M.; Cai, J.; Luo, S.; Hong, X.; Xu, L.; Lin, H.; Chen, X.; Fu, W. Causal effects of gut microbiota on the risk of chronic kidney disease: A Mendelian randomization study. Front. Cell. Infect. Microbiol. 2023, 13, 1142140. [Google Scholar] [CrossRef]
- Cabana-Puig, X.; Bond, J.M.; Wang, Z.; Dai, R.; Lu, R.; Lin, A.; Oakes, V.; Rizzo, A.; Swartwout, B.; Abdelhamid, L.; et al. Phenotypic Drift in Lupus-Prone MRL/lpr Mice: Potential Roles of MicroRNAs and Gut Microbiota. Immunohorizons 2022, 6, 36–46. [Google Scholar] [CrossRef]






| Gene | Primer Sequence (5′–3′) | Melting Temperature (Tm) | |
|---|---|---|---|
| Forward Sequence | Reverse Sequence | ||
| GAPDH | CGACTTCAACAGCAACTCCCACTCTTCC | TGGGTGGTCCAGGGTTTCTTACTCCTT | 62 °C |
| IL-1β | CCTCGGCCAAGACAGGTCGC | TGCCCATCAGAGGCAAGGAGGA | 59.2 °C |
| IL-17A | TGAGCTTCCCAGATCACAGA | TCCAGAAGGCCCTCAGACTA | 52.8 °C |
| TNF-α | CGTCAGCCCGATTTGCTATCT | CGGACTCCGCAAAGTCAAG | 52.8 °C |
| MCP | CACAGTTGCCGGCTGGAGCAT | GTAGCAGCAGGTGAGTGGGGC | 59.2 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, A.; Bose, D.; Saha, P.; Roy, S.; More, M.; Skupsky, J.; Klimas, N.G.; Chatterjee, S. Prolonged Antibiotic Use in a Preclinical Model of Gulf War Chronic Multisymptom-Illness Causes Renal Fibrosis-like Pathology via Increased micro-RNA 21-Induced PTEN Inhibition That Is Correlated with Low Host Lachnospiraceae Abundance. Cells 2024, 13, 56. https://doi.org/10.3390/cells13010056
Trivedi A, Bose D, Saha P, Roy S, More M, Skupsky J, Klimas NG, Chatterjee S. Prolonged Antibiotic Use in a Preclinical Model of Gulf War Chronic Multisymptom-Illness Causes Renal Fibrosis-like Pathology via Increased micro-RNA 21-Induced PTEN Inhibition That Is Correlated with Low Host Lachnospiraceae Abundance. Cells. 2024; 13(1):56. https://doi.org/10.3390/cells13010056
Chicago/Turabian StyleTrivedi, Ayushi, Dipro Bose, Punnag Saha, Subhajit Roy, Madhura More, Jonathan Skupsky, Nancy G. Klimas, and Saurabh Chatterjee. 2024. "Prolonged Antibiotic Use in a Preclinical Model of Gulf War Chronic Multisymptom-Illness Causes Renal Fibrosis-like Pathology via Increased micro-RNA 21-Induced PTEN Inhibition That Is Correlated with Low Host Lachnospiraceae Abundance" Cells 13, no. 1: 56. https://doi.org/10.3390/cells13010056