Therapeutic Effects of a Novel Aptamer on Coronaviral Infection-Induced Lung Injury and Systemic Inflammatory Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. LPS-Induced Acute Lung Injury
2.3. Viral Challenge and Apta-1 Treatment
2.4. Viral Titers
2.5. Assessment of Lung Injury
2.6. Immunohistochemistry Staining
2.7. Quantitative Reverse Transcription PCR (RT-qPCR)
2.8. Western Blotting
2.9. Serum and Plasma Collection and Tests
2.10. Statistical Analysis
3. Results
3.1. Apta-1 Reduces Airway LPS-Induced Acute Lung Injury
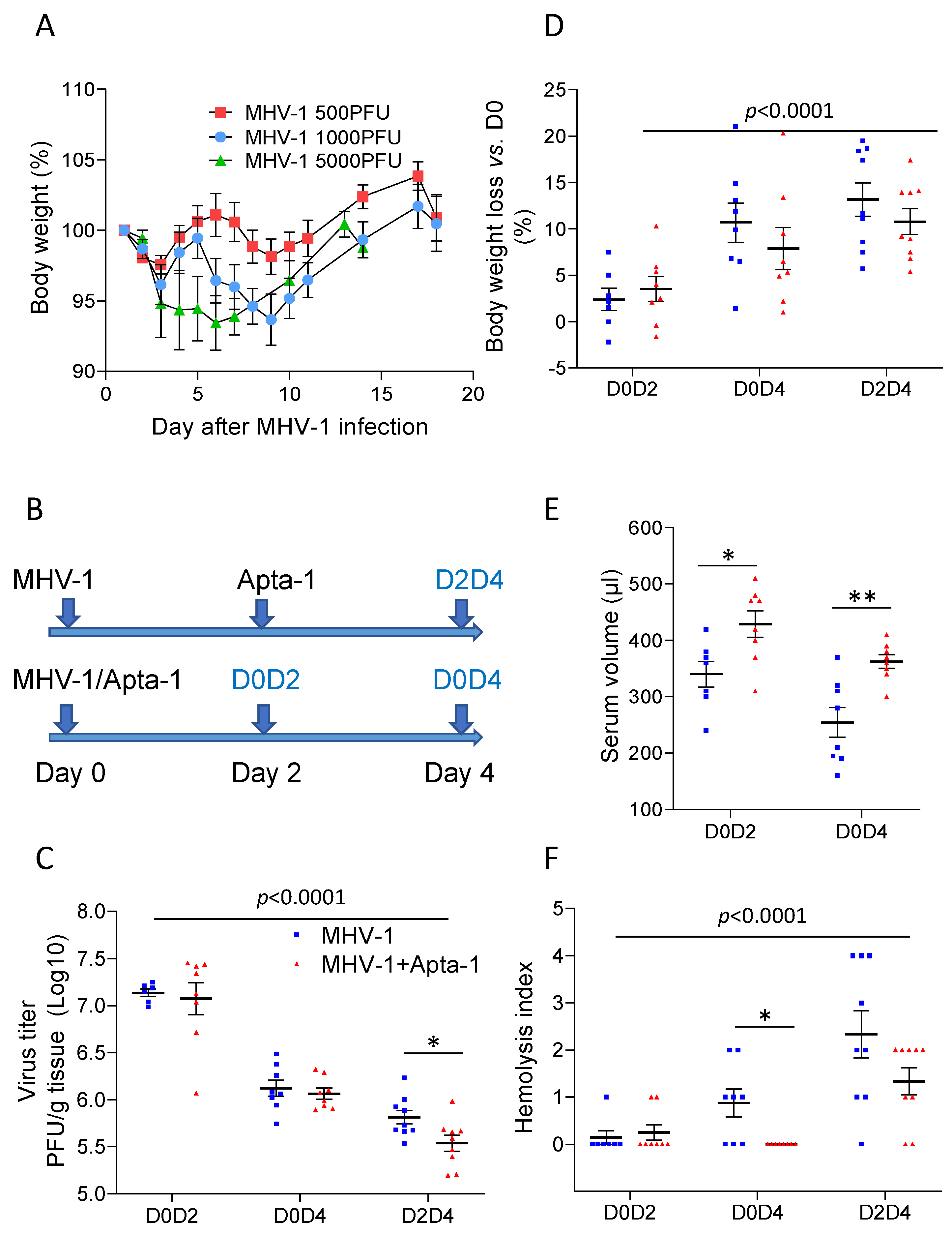
3.2. Apta-1 Treatment Reduces MHV-1 Coronaviral Titer, Loss of Blood Volume, and Hemolysis
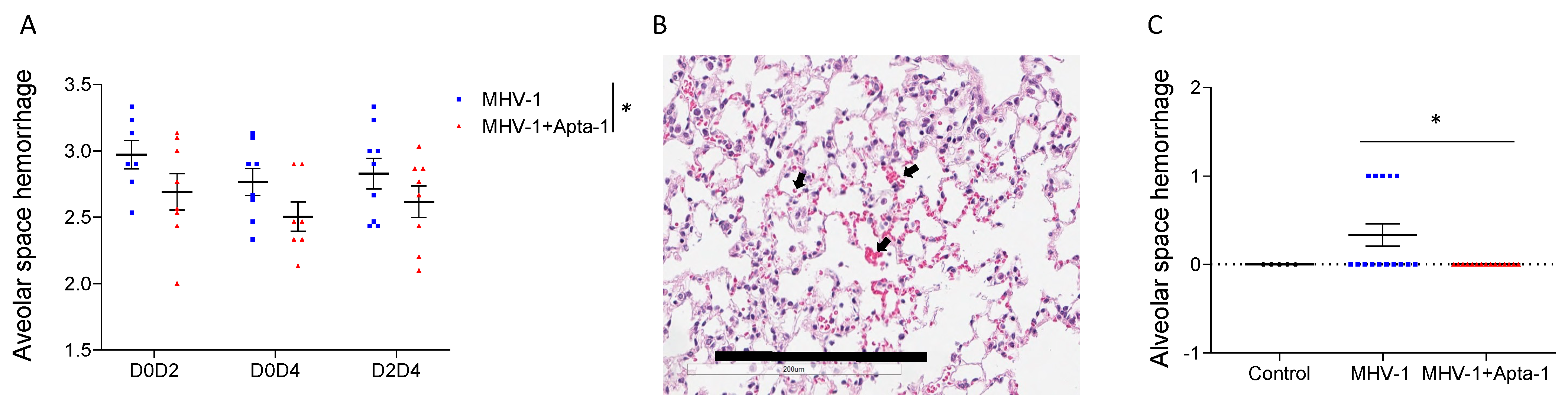
3.3. Apta-1 Treatment Reduced Alveolar Hemorrhage and Cleavage of PAR-1
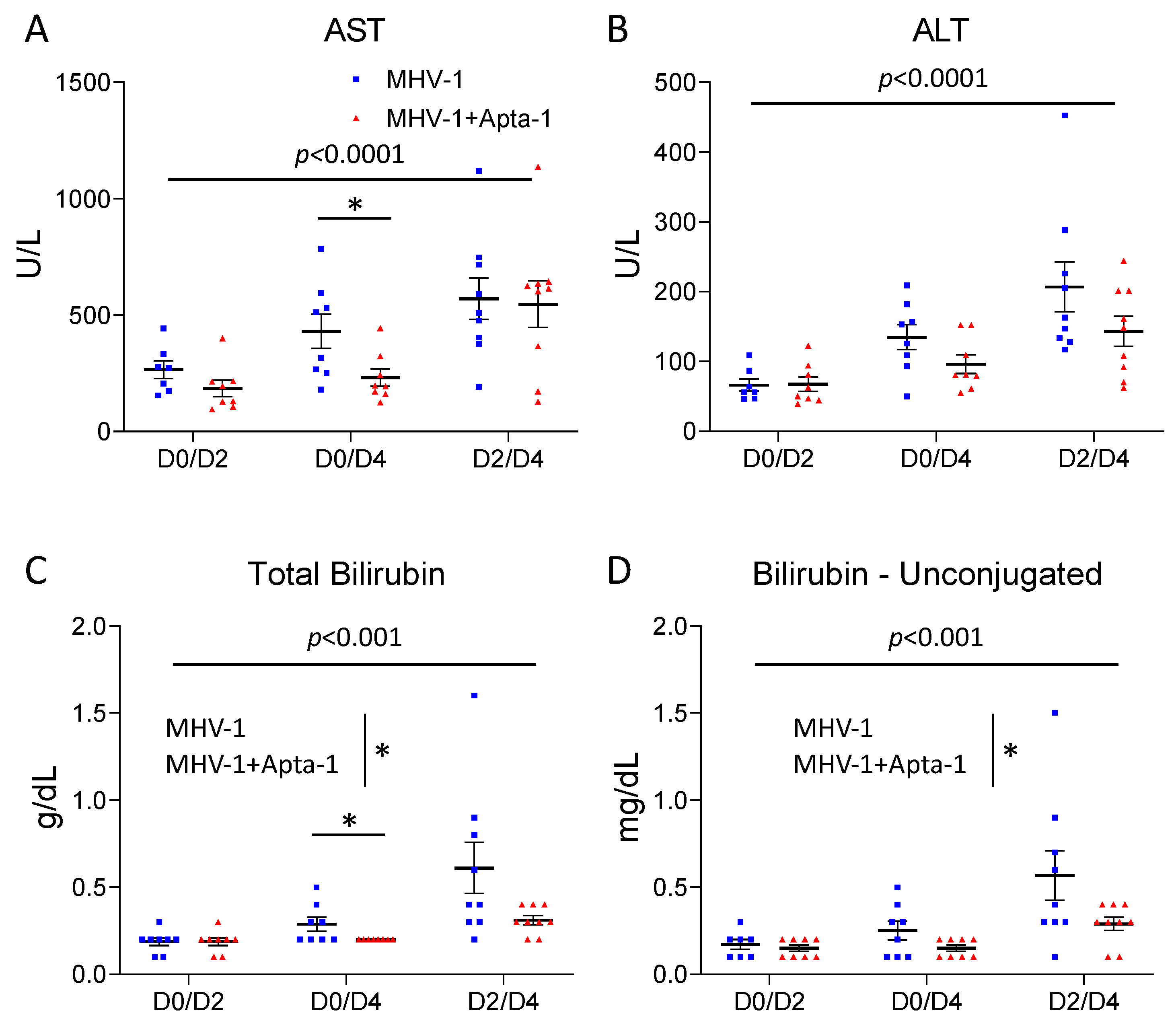
3.4. Apta-1 Treatment Reduced MHV-1 Viral Infection-Induced Systemic Inflammatory Responses
4. Discussion
4.1. Animal Models for Corona Viral Infection
4.2. Apta-1, a Novel Anti-Inflammatory Aptamer
4.3. Apta-1 Reduces Coronaviral Infection-Induced Pulmonary Hemorrhage and PAR-1 Cleavage
4.4. Apta-1 Reduces Coronaviral Infection-Induced Systemic Inflammatory Responses
4.5. Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.; Hui, D.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, N.; Baig, E.; Ma, X.; Zhang, J.; He, W.; Rowe, A.; Habal, M.; Liu, M.; Shalev, I.; Downey, G.P.L.; et al. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 2006, 80, 10382–10394. [Google Scholar] [CrossRef]
- Leibowitz, J.L.; Srinivasa, R.; Williamson, S.T.; Chua, M.M.; Liu, M.; Wu, S.; Kang, H.; Ma, X.; Zhang, J.; Shalev, I.; et al. Genetic determinants of mouse hepatitis virus strain 1 pneumovirulence. J. Virol. 2010, 84, 9278–9291. [Google Scholar] [CrossRef]
- Han, B.; Ma, X.; Zhang, J.; Zhang, Y.; Bai, X.; Hwang, D.M.; Liu, M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab. Investig. 2012, 92, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Z.; Bartczak, A.; Zhang, J.; Khattar, R.; Chen, L.; Liu, M.F.; Edwards, A.; Levy, G.; McGilvray, I.D. Proteasome inhibition in vivo promotes survival in a lethal murine model of severe acute respiratory syndrome. J. Virol. 2010, 84, 12419–12428. [Google Scholar] [CrossRef]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef]
- Ng, E.W.; Shima, D.T.; Calias, P.; Cunningham, E.T., Jr.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Pourhajibagher, M.; Raoofian, R.; Tabarzad, M.; Bahador, A. Therapeutic applications of nucleic acid aptamers in microbial infections. J. Biomed. Sci. 2020, 27, 6. [Google Scholar] [CrossRef]
- Zou, X.; Wu, J.; Gu, J.; Shen, L.; Mao, L. Application of Aptamers in virus detection and antiviral therapy. Front. Microbiol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Jedlina, L.; Chutna Olin, O.; Fransen, K.; Lindstam, M.; Grenegard, M. The RNA Aptamer, APTA-1 Targeting Evolutionarily Conserved Motif on Thrombin Cures Endotoxemia and Reduces Thrombosis in Animal Models by Inhibiting Aggregation and Secretion of Platelets. Res. Pract. Thromb. Haemost. 2023, 7, 101366. [Google Scholar] [CrossRef]
- Jedlina, L.; Olin, O.; Bylock, A.; Casslén, V.; Eriksson, M.; Brewinska-Olchowik, M.; Piwocka, K.; Lindstam, M. A novel protein-binding RNA aptamer, Apta-1—A new therapeutic tool to combat sepsis. Crit. Care 2020, 24, 506. [Google Scholar]
- Jedlina, L.C.V.; Chutná Olin, O.; Bylock, A.; Lindstam, M. Promising Therapeutic Effects of a Novel RNA Aptamer, Apta-1, in a Severe Systemic Inflammation Model on Non-human Primates. Res. Pract. Thromb. Haemost. 2020, 4. [Google Scholar]
- Toba, H.; Tomankova, T.; Wang, Y.; Bai, X.; Cho, H.R.; Guan, Z.; Adeyi, O.A.; Tian, F.; Keshavjee, S.; Liu, M. XB130 deficiency enhances lipopolysaccharide-induced septic response and acute lung injury. Oncotarget 2016, 7, 25420–25431. [Google Scholar] [CrossRef]
- Brake, M.A.; Ivanciu, L.; Maroney, S.A.; Martinez, N.D.; Mast, A.E.; Westrick, R.J. Assessing Blood Clotting and Coagulation Factors in Mice. Curr. Protoc. Mouse Biol. 2019, 9, e61. [Google Scholar] [CrossRef]
- He, X.; Han, B.; Bai, X.; Zhang, Y.; Cypel, M.; Mura, M.; Keshavjee, S.; Liu, M. PTX3 as a potential biomarker of acute lung injury: Supporting evidence from animal experimentation. Intensive Care Med. 2010, 36, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, F.; Zhong, S.; Ding, R.; Su, J.; Li, X. Astaxanthin attenuates ferroptosis via Keap1-Nrf2/HO-1 signaling pathways in LPS-induced acute lung injury. Life Sci. 2022, 311 Pt A, 121091. [Google Scholar] [CrossRef]
- Fukatsu, M.; Ohkawara, H.; Wang, X.; Alkebsi, L.; Furukawa, M.; Mori, H.; Fukami, M.; Fukami, S.I.; Sano, T.; Takahashi, H.; et al. The suppressive effects of Mer inhibition on inflammatory responses in the pathogenesis of LPS-induced ALI/ARDS. Sci. Signal. 2022, 15, eabd2533. [Google Scholar] [CrossRef]
- Lang, S.; Li, L.; Wang, X.; Sun, J.; Xue, X.; Xiao, Y.; Zhang, M.; Ao, T.; Wang, J. CXCL10/IP-10 Neutralization Can Ameliorate Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome in Rats. PLoS ONE 2017, 12, e0169100. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Park, M.; Turrini, C.; Tunstall, T.; Thwaites, R.; Mauri, T.; Ragazzi, R.; Ruggeri, P.; Hansel, T.T.; Caramori, G.; et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Mizgerd, J.P.; Spieker, M.R.; Doerschuk, C.M. Early response cytokines and innate immunity: Essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J. Immunol. 2001, 166, 4042–4048. [Google Scholar] [CrossRef]
- Frantzeskaki, F.; Armaganidis, A.; Orfanos, S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration 2017, 93, 212–225. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Ward, N.S.; Waxman, A.B.; Homer, R.J.; Mantell, L.L.; Einarsson, O.; Du, Y.; Elias, J.A. Interleukin-6-induced protection in hyperoxic acute lung injury. Am. J. Respir. Cell Mol. Biol. 2000, 22, 535–542. [Google Scholar] [CrossRef]
- Voiriot, G.; Razazi, K.; Amsellem, V.; Tran Van Nhieu, J.; Abid, S.; Adnot, S.; Dessap, A.M.; Maitre, B. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir. Res. 2017, 18, 64. [Google Scholar] [CrossRef]
- Bhatia, M.; Zemans, R.L.; Jeyaseelan, S. Role of chemokines in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 46, 566–572. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.S.; Sarma, P.; Batra, G.; Joshi, R.; Kaur, H.; Sharma, A.R.; Prakash, A.; Medhi, B. A Comprehensive Review of Animal Models for Coronaviruses: SARS-CoV-2, SARS-CoV, and MERS-CoV. Virol. Sin. 2020, 35, 290–304. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Sharma, A.R.; Prakash, S.; Medhi, B. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, F.; Qi, F.; et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef]
- Hassan, A.O.; Case, J.B.; Winkler, E.S.; Thackray, L.B.; Kafai, N.M.; Bailey, A.L.; McCune, B.T.; Fox, J.M.; Chen, R.E.; Alsoussi, W.B.; et al. A SARS-CoV-2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell 2020, 182, 744–753.e4. [Google Scholar] [CrossRef]
- Israelow, B.; Song, E.; Mao, T.; Lu, P.; Meir, A.; Liu, F.; Alfajaro, M.M.; Wei, J.; Dong, H.; Homer, R.J.; et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J. Exp. Med. 2020, 217, e20201241. [Google Scholar] [CrossRef]
- Barfod, A.; Persson, T.; Lindh, J. In vitro selection of RNA aptamers against a conserved region of the Plasmodium falciparum erythrocyte membrane protein 1. Parasitol. Res. 2009, 105, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.C.; Johns, R.H.; Lasky, J.A.; Shan, B.; Scotton, C.J.; Laurent, G.J.; Chambers, R.C. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am. J. Pathol. 2005, 166, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.J.; Tang, S.E.; Pao, H.P.; Wu, S.Y.; Liao, W.I. Protease-Activated Receptor-1 Antagonist Protects Against Lung Ischemia/Reperfusion Injury. Front. Pharmacol. 2021, 12, 752507. [Google Scholar] [CrossRef] [PubMed]
- Rovai, E.S.; Alves, T.; Holzhausen, M. Protease-activated receptor 1 as a potential therapeutic target for COVID-19. Exp. Biol. Med. 2021, 246, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.-K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fett, C.; Mack, M.; Ten Eyck, P.P.; Meyerholz, D.K.; Perlman, S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J. Immunol. 2017, 198, 4046–4053. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lindstam, M.; Hwang, D.; Jedlina, L.; Liu, M. Therapeutic Effects of a Novel Aptamer on Coronaviral Infection-Induced Lung Injury and Systemic Inflammatory Responses. Cells 2024, 13, 422. https://doi.org/10.3390/cells13050422
Wang Y, Lindstam M, Hwang D, Jedlina L, Liu M. Therapeutic Effects of a Novel Aptamer on Coronaviral Infection-Induced Lung Injury and Systemic Inflammatory Responses. Cells. 2024; 13(5):422. https://doi.org/10.3390/cells13050422
Chicago/Turabian StyleWang, Yingchun, Mikael Lindstam, David Hwang, Luiza Jedlina, and Mingyao Liu. 2024. "Therapeutic Effects of a Novel Aptamer on Coronaviral Infection-Induced Lung Injury and Systemic Inflammatory Responses" Cells 13, no. 5: 422. https://doi.org/10.3390/cells13050422






