Abstract
Juvenile Idiopathic Arthritis (JIA), the leading childhood rheumatic condition, has a chronic course in which persistent disease activity leads to long-term consequences. In the era of biologic therapy and tailored treatment, precise disease activity assessment and aggressive intervention for high disease activity are crucial for improved outcomes. As inflammation is a fundamental aspect of JIA, evaluating it reflects disease severity. Recently, there has been growing interest in investigating cellular immune inflammation indices such as the neutrophil-to-lymphocyte ratio (NLR) and systemic immune inflammation index (SII) as measures of disease severity. The aim of this retrospective study was to explore the potential of the SII in reflecting both inflammation and disease severity in children with JIA. The study comprised 74 JIA patients and 50 healthy controls. The results reveal a notable increase in median SII values corresponding to disease severity, exhibiting strong correlations with traditional inflammatory markers, including CRP and ESR (ρ = 0.714, ρ = 0.661), as well as the JADAS10 score (ρ = 0.690). Multiple regression analysis revealed the SII to be independently associated with JADAS10. Furthermore, the SII accurately distinguished patients with high disease activity from other severity groups (AUC = 0.827, sensitivity 81.5%, specificity 66%). These findings suggest that integrating the SII as an additional measure holds potential for assessing disease activity in JIA.
1. Introduction
Affecting up to 4 per 1000 children, JIA constitutes the most common rheumatic disease in children [1,2], being characterized by disordered immunity and chronic inflammation [3,4]. Generally acknowledged as a clinical syndrome comprising various disease subsets, it involves multiple inflammatory processes that converge into a common pathway [5,6]. Cases characterized by persistent active disease may lead to damage to articular cartilage and underlying bone [5,7,8]. Over the last decade, several advancements pertaining to the pathogenesis of JIA have led to the emergence of biological disease-modifying antirheumatic drugs, significantly improving disease outcomes [9,10,11]. Prompt diagnosis and therapeutic interventions are considered overarching principles in disease management [12,13]. Treatment decisions are guided by the ongoing and systematic assessment of disease activity [14]. In this regard, research has focused on identifying accurate disease activity measurements, with various clinical (various joint count types, pain measures, and global assessment scales) and laboratory (acute phase reactants, several cytokines) measures being proposed [15,16]. Aside from individual measures, composite disease activity measures, such as the Juvenile Arthritis Disease Activity Score (JADAS) and its variants, developed by Consolaro et al., have gained increased popularity [17,18,19].
Regarding laboratory measures, most of them have focused on quantifying protein responses to inflammation, such as C-reactive protein (CRP), the erythrocyte sedimentation ratio (ESR), and several antibodies [20,21,22,23]. However, there is a need for more nuanced assessments that go beyond traditional markers. In light of the advances in understanding the pathogenesis of JIA, which currently place more emphasis on innate immunity than in the past, it seems reasonable to consider evaluating indices that quantify the cellular response to inflammation as measures of disease activity [24,25]. In adult inflammatory arthritis, multiple studies have described the role of several cellular inflammation markers, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, and systemic immune–inflammation index (SII), in reflecting both systemic inflammation and disease severity [25,26,27,28,29,30,31]. In children, there is a dearth of studies with a primary focus on the NLR [32]. However, research focusing on JIA’s pathogenesis has brought attention to the pivotal role of platelets in developing this disease [33,34]. The SII, a composite index that integrates the information of both NLR components and platelets, could, therefore, bring relevant information regarding the cellular response in JIA. Against this background, we sought to investigate the role of the SII as a potential inflammatory marker and its association with disease activity in children with JIA.
2. Materials and Methods
2.1. Study Design and Patient Selection
In this retrospective cross-sectional study, we enrolled children diagnosed with Juvenile Idiopathic Arthritis in the Rheumatology Department of the tertiary care Pediatric Emergency Hospital “Louis Turcanu” in Timisoara, Romania. We analyzed the medical charts of 93 consecutive patients who were admitted here during the period from January 2014 to October 2023. The inclusion criteria were (1) diagnosis of Juvenile Idiopathic Arthritis and (2) age under 18 years. Exclusion criteria were (1) systemic JIA, (2) arthritis due to any other illnesses, such as reactive arthritis, septic arthritis, vasculitis, acute rheumatic fever, malignancy, inflammatory bowel disease, and trauma; (3) active infections at the moment of admission or during the last two weeks prior to admission; and (4) patients with incomplete data. Upon review of the medical charts, we identified 74 patients who fulfilled the study criteria. All patients were diagnosed with Juvenile Idiopathic Arthritis according to the International League Associations for Rheumatology (ILAR) classification criteria [6]. Oligoarticular involvement was considered if fewer than five joints were affected, while polyarticular involvement was considered otherwise, based on the number of affected joints. In addition, 50 control subjects were included for comparison after reviewing their medical records. The exclusion criteria for control subjects comprised a diagnosis of inflammatory or autoimmune disease, acute or chronic infection, malignancy, and the use of medications known to affect complete blood count (CBC) parameters, such as corticosteroids.
This study received approval from the hospital’s Institutional Review Board and complied with the Declaration of Helsinki and its later amendments. Informed consent was waived due to the retrospective nature of the study.
2.2. Collection of Clinical Data and Assessment of Disease Activity
The following patient data were retrieved: age, gender, discharge diagnosis, ILAR category of JIA, disease duration, type of articular involvement, extra-articular disease manifestations, and current medication. Additionally, several disease activity parameters were recorded: active joint count, physician global rating of disease activity (measured on a 10 cm visual analog scale, where 0 means no activity and 10 signifies maximum activity), parent/patient global rating of wellbeing (measured on a 10 cm VAS, where 0 means very well and 10 very poor). Patients were categorized into three study groups according to their JADAS10 score [17]. The JADAS10 score was calculated as the arithmetic sum of the physician global rating of disease activity, parent/patient global rating of wellbeing, active joint count (with 10 as the maximum score in patients with 10 or more active joints), and the ESR, normalized to a 0–10 scale, using the formula (ESR-20)/10. The low-disease-activity (LDA) group comprised children with JADAS10 scores ranging from 1.1 to 2 for oligoarticular involvement and 1.1 to 3.8 for polyarticular involvement. The moderate-disease-activity (MDA) group consisted of children with JADAS10 scores ranging from 2.1 to 4.2 for oligoarticular involvement and 3.9 to 10.5 for polyarticular involvement. The high-disease-activity (HDA) group encompassed children with JADAS10 scores exceeding 4.2 for oligoarticular involvement and 10.5 for polyarticular involvement [35].
The blood samples collected upon hospital admission and analyzed for this study comprised a complete blood count (CBC) conducted using an automated hematology analyzer (Sysmex XN-550, Sysmex Corporation, Kobe, Japan) and CRP and ferritin performed using an automatic analyzer (Hitachi 747, Hitachi, Tokyo, Japan). Fibrinogen levels were determined using the Clauss method on an ACL Top Analyzer and ESR with the Westergren method. In addition, the following two hematological indices were computed based on the available CBC taken upon admission: the NLR (absolute neutrophil count/absolute lymphocyte count) and SII (absolute platelet count × NLR) [36,37].
2.3. Statistical Analysis
Statistical analyses were performed using the IBM Statistical Package for Social Sciences software (version 28, Armonk, NY, USA). The three study groups were characterized using descriptive statistics (percentage, median, range of quarters (IQR)). Visual (histograms, probability plots) and analytical methods (Shapiro–Wilk test) were employed to assess the normality of data distribution. Due to their abnormal distribution, numerical variables were expressed as medians (25th and 75th interquartile ranges (IQRs)) and compared using the Kruskal–Wallis test. Dunn’s test was conducted as a post hoc test to evaluate the statistical significance of distinctions among pairs of patient groups regarding SII values. Categorical variables were presented as numbers (percentages), and a Chi-squared test or Fisher’s exact test was performed, as appropriate, to compare these variables among research groups. The correlation between the two hematological indices, the NLR and SII, and several disease activity measures was evaluated using Spearman’s rank correlation coefficient (ρ). Linear regression was applied to identify associations between inflammation markers and JADAS10. For the univariate regression analysis, the concurrent medication variable was categorized into three groups: (1) no medication, (2) NSAIDs, and (3) immunosuppressants. While exploring predictor variables, we identified instances where certain combinations resulted in sparse data. Therefore, bootstrapping was performed to evaluate the robustness of the estimates and enhance the stability of our results. ROC curves were used to characterize the performance of several inflammatory markers in discriminating high disease activity. Youden’s index, calculated as sensitivity + specificity − 1, was used to estimate cutoff values for different biomarkers, while the area under the curve (AUC) in the ROC analysis was determined to compare the results. A p-value (two-tailed) <0.05 was deemed statistically significant.
3. Results
3.1. General Characteristics of the Study Population
Data from 74 children aged 1 to 18 years diagnosed with JIA were included in the present study, alongside 50 healthy controls matched for age and gender. The JIA patients were divided into three study groups based on disease activity status (35.1% with low disease activity, 28.4% with moderate disease activity, and 36.5% with high disease activity). Demographic data and disease characteristics are illustrated in Table 1. The median age of the entire study population was 13 [interquartile range (IQR): 9, 15.6] years, with a median disease duration of 1.2 (IQR: 0.6, 2.7) years. Gender distribution did not reveal significant variations across study groups (p = 0.136). Across the entire study population, the most common ILAR subtypes were enthesitis-related arthritis (ERA) (32.4.%) and oligoarticular JIA (29.7%); the majority of patients had oligoarticular involvement (60.8%), with no significant variations between disease activity groups. As expected, groups with more pronounced disease severity displayed a significant increase in all assessed disease activity parameters and biochemical inflammatory markers (Table 1).

Table 1.
Demographic and clinical characteristics by disease activity status.
3.2. Comparison of Hematological Parameters and Indices across Groups of Disease Activity
There was a significant gradual increase in the absolute count of white blood cells, neutrophils, and platelets with increased disease severity, as seen in Table 2. Conversely, hemoglobin levels were lower in the more severe study groups. The same trend of a gradual increase in disease severity was observed for both hematological indices, the NLR, and the SII (p < 0.001) across JIA groups. Compared to the control group, these differences were significant only regarding HDA and MDA patients. However, in comparing LDA and the control group, the only significant difference observed was a lower hemoglobin level among LDA patients. Nevertheless, there was a tendency towards a higher white WBC count, platelet count, and SII among LDA patients, although these differences did not reach statistical significance.

Table 2.
Hematological comparison by disease activity groups.
As illustrated in Figure 1, there were significant differences in median SII values among all three study groups.
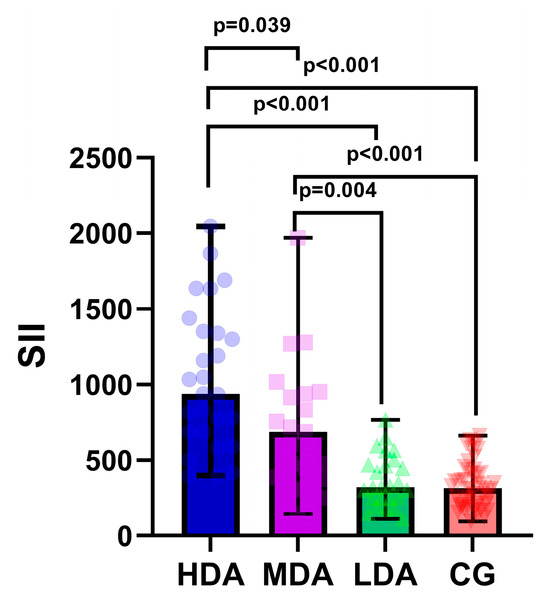
Figure 1.
Boxplot diagram of systemic immune–inflammation index (SII) across study groups. LDA, low disease activity; MDA, moderate disease activity; HDA, high disease activity. The individual values are represented as semi-transparent circles, triangles, and rhombi.
3.3. Correlation Analysis of Hematological Indices with Disease Activity Parameters
Spearman correlation analysis was performed to characterize the relationship between hematological indices, disease core set variables, and the JADAS10 score (Table 3). Significant positive correlations were observed between the NLR, SII, and all disease activity parameters, with a stronger correlation noted for the SII. The strongest correlation for both hematological indices was observed with CRP, while the weakest was observed with the active joint count. Regarding the median JADAS10 score, a strong correlation was observed exclusively with the SII (ρ = 0.697).

Table 3.
Correlation between hematological indices and disease activity indices in JIA.
3.4. Relationship between SII and JADAS10
Furthermore, we employed linear regression to evaluate the association between the SII and JADAS10, as shown in Table 4. In the univariate analysis, we found associations between JADAS10 and the SII, CRP, and fibrinogen. However, following multiple linear regression, only CRP and the SII maintained significance as independent factors associated with JADAS10. Bootstrapping was employed to increase the stability of parameter estimates in the presence of a relatively limited number of observations.

Table 4.
Regression analysis of factors related to JADAS10.
In addition, we assessed the diagnostic performance of the SII for identifying high disease activity by comparing it with various hematological parameters and the NLR. The AUC was calculated, and optimal cutoff values were determined using the Youden Index derived from the receiver operating characteristic (ROC) curve (Table 5). As illustrated in Table 5 and Figure 2, the most significant accuracy for high disease activity was displayed by CRP (AUC = 0.841, 81% sensitivity, and 79% specificity), followed closely by the SII (AUC = 0.827, 82% sensitivity, and 66% specificity). Platelet count also displayed borderline excellent discrimination ability (AUC = 0.809, 77% sensitivity, and 62% specificity), while neutrophils and the NLR presented acceptable discrimination ability (AUC = 0.729 and AUC = 0.761, respectively).

Table 5.
Comparison of hematological parameters and indices in discriminating high disease activity.
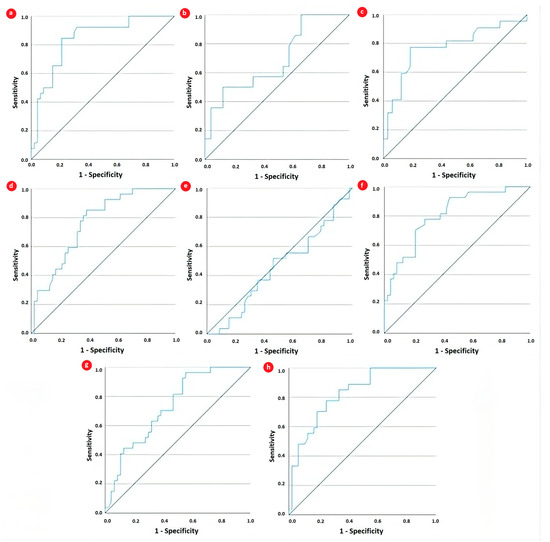
Figure 2.
Receiver operating characteristic curve analysis for evaluating the performance of (a) CRP, (b) fibrinogen, (c) ferritin, (d) neutrophils, (e) lymphocytes, (f) platelets, (g) NLR, and (h) SII in discriminating high disease activity in JIA patients.
4. Discussion
In this retrospective study, we explored, for the first time, the clinical applicability of the SII in reflecting inflammatory burden and disease activity in real-world JIA patients. Our results show a significant positive correlation between the SII, a cellular inflammation marker, and disease activity markers, encompassing both clinical (JADAS10) and laboratory (CRP and ESR) measures. Furthermore, the SII exhibited excellent accuracy in distinguishing patients with high disease activity from other severity groups and demonstrated an independent, albeit modest, association with high disease activity in our study group.
Similar to other chronic diseases, JIA can manifest periods of disease activity and remission [38]. Ongoing advancements in JIA treatment have significantly improved the prognosis of this chronic condition, emphasizing the importance of early diagnosis and intervention as overarching principles in disease management [12,39]. There is a growing demand for more personalized treatment approaches, ensuring that children with unfavorable prognostic factors and those experiencing high disease activity receive early and aggressive interventions [40]. Consequently, the measurement of disease activity becomes a fundamental component in managing this condition [17]. Research efforts are focused on identifying the most effective measures of disease activity, a challenging task given the heterogeneity of JIA [41]. A proper evaluation of disease activity includes quantifying inflammatory responses [42]. Juvenile Idiopathic Arthritis, like most autoimmune rheumatic diseases, is accompanied by chronic inflammation [43]. This non-specific, multidimensional process is initiated, among other factors, by excessive production of inflammatory cytokines [44,45]. Most studies have focused on protein responses to pro-inflammatory cytokines, such as the conventional acute phase reactants CRP and ESR [20,21,22,23]. However, recent studies have raised concerns that ESR and CRP levels may not accurately reflect clinical disease activity in Juvenile Idiopathic Arthritis compared to their performance in adult inflammatory arthritis [46,47]. However, in addition to eliciting protein responses, inflammation also triggers cellular responses, causing changes in one or more cellular lineages within the hematopoietic system [48]. In contrast to earlier literature that emphasized dysregulated adaptive immunity, specifically the involvement of autoreactive Th1 and Th17 subsets, more recent studies highlight the significance of innate immunity in the immunopathology of JIA [24]. In this context, cellular responses to inflammation imply the release and migration of neutrophils and large platelets from the bone marrow to both circulating pools and sites of inflammation [49,50]. Due to limitations in accessing synovial inflammatory cells, particularly in children, some studies have shifted their focus to peripheral blood cells and observed changes in the biology of neutrophils and platelets in JIA patients [24,51,52,53]. In light of the role of blood cell interactions in inflammation and immune responses, several cellular immune inflammation markers have been shown to reflect a systemic inflammatory response [29,54]. These markers were initially studied in oncology and later extended to chronic inflammatory diseases, including rheumatic conditions [55,56,57,58]. Beyond the established role of the neutrophil-to-lymphocyte ratio, recent studies have investigated the applicability of the systemic immune–inflammation index in the context of inflammatory arthritis [26,31,59]. They found the SII was able to strongly predict disease activity, joint damage, and radiographic progression in rheumatoid arthritis [59]. Nevertheless, studies exploring its value in JIA are currently lacking.
To investigate the potential value of using the SII in children with JIA, we analyzed a study population stratified into low, moderate, and high disease activity according to the JADAS10 score. No statistically significant differences were found among the study groups concerning demographic characteristics (p = 0.569 for age, p = 0.230 for gender). In characterizing our patients, the most frequently observed ILAR subtypes were ERA (32.4%) and oligoarticular (29.7%), representing a notably higher rate of ERA compared to that found in most European epidemiological studies [60]. This discrepancy may stem from the tendency to refer more severe cases to our tertiary care center, while some of the milder oligoarticular cases are often managed on an outpatient basis in local healthcare centers.
As expected, cellular modifications reflecting the degree of systemic inflammation became progressively more evident with increasing disease severity. Peripheral leukocyte, neutrophil, and platelet counts gradually increased while hemoglobin levels decreased. This is in keeping with previous studies that noted neutrophilia and thrombocytosis as signs of active disease [61,62]. In our examination of the two hematological indices, both the NLR and SII showed a notable, gradual increase with the severity of the disease. The median NLR value of the entire study population was 1.63 (IQR: 1.19, 2.31), which is slightly smaller than the previous values reported in active JIA of 2.50 ± 1.89 [61] and 2.11 ± 1.19 [32]. The observed difference in NLR values may be influenced by the differing proportions of the JIA subtype and disease severity in the two studies and the difference in descriptive units. To the best of our knowledge, there are no reports of SII values in children with JIA. However, the SII value for our study population (592, IQR: 343, 942) was comparable to that reported by Satis et al. in adults with rheumatoid arthritis (667 ± 33) [54]. When comparing the CBC across study groups, statistically significant differences were observed between HAD, MDA, and controls. Additionally, a tendency towards a lower WBC count, platelet count, and SII was noted in the LDA group compared to the controls, although this difference did not reach statistical significance.
In order to determine whether the two hematological indices reflected the degree of systemic inflammation in our study population, we applied correlation analysis. We found both indices positively correlated with conventional inflammation markers, CRP, and ESR. However, the correlation was notably robust only for the SII, suggesting its potential as a reliable marker of inflammation. To further investigate the role of the SII in relation to disease activity, we performed a logistic regression analysis to ascertain its capacity to identify children with high disease activity. Timely identification of such patients allows for tailoring medications based on disease activity, leading to more optimal disease control. Multiple regression analysis revealed the SII to be independently associated with JADAS10. Furthermore, the SII demonstrated excellent predictive performance in identifying high disease activity status in ROC analysis. At a cutoff value of 586, it yielded a sensitivity of 81.5% and a specificity of 766%, with an AUC of 0.827, similar to CRP. As ESR was already incorporated into the JADAS10, which served as the classification criterion for disease activity status, we omitted it from regression and ROC analyses. In distinguishing children with high disease activity status, we also observed that the diagnostic AUC of the SII outperformed that of its individual component cell lineages. These findings suggest that the combined information provided by the SII may offer a more comprehensive assessment of inflammatory state and disease activity than its components in children with JIA. However, given the relatively small number of patients, the cutoff values cannot be generalized and should be regarded only as exploratory results.
It is important to consider potential limitations when interpreting the findings of the present study. First, due to the study’s retrospective nature, selection bias may be inherent. Second, the relatively small sample size of patients can be attributed to both the single-center design of the study and the lower frequency of the disease compared to adult arthritis. This may result in the limited extrapolation and robustness of the study results.
Furthermore, it restricted our ability to draw firm conclusions regarding the specific characteristics and outcomes associated with each subtype of JIA. Third, most patients were undergoing treatment, potentially influencing certain laboratory results; nonetheless, it is noteworthy that only 2 out of the 81 patients received oral corticosteroids in low doses at the time of the study. Therefore, prospective, multicenter studies would facilitate a more robust statistical assessment.
Our study also has several strengths. Firstly, as far as we know, this study is the first to assess the relationship between the SII and disease activity in patients with JIA. Secondly, patients reflect real-life settings, and the investigated hematologic index incurs no additional costs, as it is derived from the universally conducted complete blood count.
In conclusion, this study unveiled a gradual increase in the SII corresponding to disease severity in children with JIA. Moreover, the SII demonstrated an independent association with high disease activity status. In an era of ongoing efforts to explore chemokines and other biological markers, there is a tendency to somewhat downplay the significance of routine blood analyses, which are widely accessible. Given that not every healthcare center is equipped with advanced technology, we aimed to highlight the complementary value of routine blood work, such as the complete blood count. Considering that the SII reflects alterations in various inflammatory cell lineages implicated in the pathogenesis of JIA, our findings encourage us to consider the SII as an additional instrument in evaluating disease activity in children with JIA. Nevertheless, due to the relatively small sample size, our results should be regarded as exploratory rather than definitive, and additional multicentric studies are required to validate and reinforce these findings.
Author Contributions
Conceptualization, D.-M.N. and A.-I.M.; methodology, G.-F.B.; software, R.A. and D.-M.N.; validation I.J., M.-A.B., and O.M.; formal analysis, D.-M.N. and A.-I.M.; investigation, A.-I.M. and D.-M.N.; resources, A.-I.M.; data curation, I.J. and M.-A.B.; writing—original draft preparation, D.-M.N. and A.-C.S.; writing—review and editing, G.-F.B., A.-C.S., and O.M.; visualization, R.A.; supervision, O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Pediatric Emergency Hospital “Louis Turcanu” in Timisoara, Romania (Protocol No. 142328/19 October 2023).
Informed Consent Statement
Patient consent was waived because of the retrospective nature of the study.
Data Availability Statement
Data can be made available upon reasonable request due to ethical restrictions.
Acknowledgments
We acknowledge the use of BioRender.com for creating the graphical abstract included in this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeller, L.; Tyrrell, P.N.; Wang, S.; Fischer, N.; Haas, J.P.; Hügle, B. α2-fraction and haptoglobin as biomarkers for disease activity in oligo- and polyarticular juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2022, 20, 66. [Google Scholar] [CrossRef]
- Manners, P.J.; Bower, C. Worldwide prevalence of juvenile arthritis why does it vary so much? J. Rheumatol. 2002, 29, 1520–1530. [Google Scholar] [PubMed]
- El-Gazzar, I.; Hanan, M.; Gheita, T.; Abeer, N.; Enas, A.; Rash, B.; Sanaa, K. Tumor necrosis factor-α -308 A/G gene polymorphism in children with juvenile idiopathic arthritis: Relation to disease activity, damage, and functional status. Clin. Rheumatol. 2017, 36, 1757–1763. [Google Scholar] [CrossRef]
- Moncrieffe, H.; Prahalad, S.; Thompson, S.D. Genetics of juvenile idiopathic arthritis: New tools bring new approaches. Curr. Opin. Rheumatol. 2014, 26, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Abu Shady, M.M.; Fathy, H.A.; Ali, A.; Youness, E.R.; Fathy, G.A. Association of neopterin as a marker of immune system activation and juvenile rheumatoid arthritis activity. J. de Pediatr. 2015, 91, 352–357. [Google Scholar] [CrossRef]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Phelan, J.D.; Thompson, S.D. Genomic progress in pediatric arthritis: Recent work and future goals. Curr. Opin. Rheumatol. 2006, 18, 482–489. [Google Scholar] [CrossRef]
- Marzan, K.A.B.; Shaham, B. Early juvenile idiopathic arthritis. Rheum. Dis. Clin. N. Am. 2012, 38, 355–372. [Google Scholar] [CrossRef]
- Ringold, S.; Angeles-Han, S.T.; Beukelman, T.; Lovell, D.; Cuello, C.A.; Becker, M.L.; Colbert, R.A.; Feldman, B.M.; Ferguson, P.J.; Gewanter, H.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res. 2019, 71, 717–734. [Google Scholar] [CrossRef]
- Ruperto, N.; Martini, A. Current medical treatments for juvenile idiopathic arthritis. Front. Pharmacol. 2011, 2, 60. [Google Scholar] [CrossRef][Green Version]
- Bouayed, K.; Hamraoui, D.; Mikou, N.; Sakhi, A.; Hilmi, W. Biotherapy in juvenile idiopathic arthritis Moroccan patients: A single-center experience. Pan Afr. Med. J. 2022, 41, 135. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.A.; Giannini, E.H.; Spalding, S.J.; Hashkes, P.J.; O'Neil, K.M.; Zeft, A.S.; Szer, I.S.; Ringold, S.; Brunner, H.I.; Schanberg, L.E.; et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012, 64, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.; Wessels, J.A.M.; van der Straaten, R.J.H.M.; Brinkman, D.M.C.; Suijlekom-Smit, L.W.A.; Kamphuis, S.S.M.; Girschick, H.J.; Wouters, C.; Schilham, M.W.; le Cessie, S.; et al. Time to treatment is an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Care Res. 2009, 61, 46–51. [Google Scholar] [CrossRef]
- Backström, M.; Tynjälä, P.; Aalto, K.; Ylijoki, H.; Putto-Laurila, A.; Grönlund, M.-M.; Kärki, J.; Keskitalo, P.; Sard, S.; Pohjankoski, H.; et al. Defining new clinically derived criteria for high disease activity in non-systemic juvenile idiopathic arthritis: A Finnish multicentre study. Rheumatol. Adv. Pract. 2018, 2, rky044. [Google Scholar] [CrossRef] [PubMed]
- Nordal, E.B.; Zak, M.; Aalto, K.; Berntson, L.; Fasth, A.; Herlin, T.; Lahdenne, P.; Nielsen, S.; Peltoniemi, S.; Straume, B.; et al. Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population-based setting. Ann. Rheum. Dis. 2012, 71, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Luca, N.J.; Feldman, B.M. Disease activity measures in paediatric rheumatic diseases. Int. J. Rheumatol. 2013, 2013, 715352. [Google Scholar] [CrossRef] [PubMed]
- Consolaro, A.; Ruperto, N.; Bazso, A.; Pistorio, A.; Magni-Manzoni, S.; Filocamo, G.; Malattia, C.; Viola, S.; Martini, A.; Ravelli, A. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009, 61, 658–666. [Google Scholar] [CrossRef]
- Consolaro, A.; Bracciolini, G.; Ruperto, N.; Pistorio, A.; Magni-Manzoni, S.; Malattia, C.; Pederzoli, S.; Davì, S.; Martini, A.; Ravelli, A.; et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: Defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum. 2012, 64, 2366–2374. [Google Scholar] [CrossRef]
- Consolaro, A.; Ruperto, N.; Bracciolini, G.; Frisina, A.; Gallo, M.C.; Pistorio, A.; Verazza, S.; Negro, G.; Gerloni, V.; Goldenstein-Schainberg, C.; et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the Juvenile Arthritis Disease Activity Score. Ann. Rheum. Dis. 2014, 73, 1380–1383. [Google Scholar] [CrossRef]
- Ahn, J.G. Role of Biomarkers in Juvenile Idiopathic Arthritis. J. Rheum. Dis. 2020, 27, 233–240. [Google Scholar] [CrossRef]
- Sarkar, S.; Alam, M.; Das, G.; Datta, S. Inflammatory Markers and Disease Activity in Juvenile Idiopathic Arthritis. Indian J. Pediatr. 2017, 84, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Yang, Y.H.; Wang, L.C.; Lee, J.H.; Shen, E.Y.; Chiang, B.L. Comparative usefulness of C-reactive protein and erythrocyte sedimentation rate in juvenile rheumatoid arthritis. Clin. Exp. Rheumatol. 2007, 25, 782–785. [Google Scholar]
- Gilliam, B.E.; Chauhan, A.K.; Low, J.M.; Moore, T.L. Measurement of biomarkers in juvenile idiopathic arthritis patients and their significant association with disease severity: A comparative study. Clin. Exp. Rheumatol. 2008, 26, 492–497. [Google Scholar] [PubMed]
- Parackova, Z.; Zentsova, I.; Horvath, R.; Malcova, H.; Cebecauerova, D.; Sediva, A.; Klocperk, A. Immunomodulation of neutrophils and platelets by TNF blockage in patients with juvenile idiopathic arthritis. Clin. Immunol. 2022, 245, 109170. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, S.; Ahmad, M.M.; Renuka, P.; Anupama, K.R.; Renuka, K. Characterization of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 1457–1467. [Google Scholar] [CrossRef]
- Choe, J.-Y.; Lee, C.U.; Kim, S.-K. Association between Novel Hematological Indices and Measures of Disease Activity in Patients with Rheumatoid Arthritis. Medicina 2023, 59, 117. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13877. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Suh, C.-H.; Jung, J.-Y.; Kim, A.-R.; Yang, J.W.; Kim, H.-A. The neutrophil-to-lymphocyte ratio could be a good diagnostic marker and predictor of relapse in patients with adult-onset Still’s disease: A STROBE-compliant retrospective observational analysis. Medicine 2017, 96, e7546. [Google Scholar] [CrossRef]
- Targońska-Stępniak, B.; Grzechnik, K. The Usefulness of Cellular Immune Inflammation Markers and Ultrasound Evaluation in the Assessment of Disease Activity in Patients with Spondyloarthritis. J. Clin. Med. 2023, 12, 5463. [Google Scholar] [CrossRef]
- Yorulmaz, A.; Hayran, Y.; Akpinar, U.; Yalcin, B. Systemic Immune-Inflammation Index (SII) Predicts Increased Severity in Psoriasis and Psoriatic Arthritis. Curr. Health Sci. J. 2020, 46, 352–357. [Google Scholar]
- Wu, J.; Yan, L.; Chai, K. Systemic immune-inflammation index is associated with disease activity in patients with ankylosing spondylitis. J. Clin. Lab. Anal. 2021, 35, e23964. [Google Scholar] [CrossRef]
- Güneş, A.; Ece, A.; Şen, V.; Uluca, Ü.; Aktar, F.; Tan, İ.; Yel, S.; Yolbaş, İ. Correlation of mean platelet volume, neutrophil-to-lymphocyte ratio, and disease activity in children with juvenile ıdiopathic arthritis. Int. J. Clin. Exp. Med. 2015, 8, 11337–11341. [Google Scholar]
- Luo, S.; Clarke, S.L.N.; Ramanan, A.V.; Thompson, S.D.; Langefeld, C.D.; Marion, M.C.; Grom, A.A.; Schooling, C.M.; Gaunt, T.R.; Yeung, S.L.A.; et al. Platelet Glycoprotein Ib α-Chain as a Putative Therapeutic Target for Juvenile Idiopathic Arthritis: A Mendelian Randomization Study. Arthritis Rheumatol. 2021, 73, 693–701. [Google Scholar] [CrossRef]
- Punnen, K.A.; Kumar, N.; Nair, S.C.; Jayaseelan, V.; Kumar, T.S. Platelet Microparticles Level in Juvenile Idiopathic Arthritis: A Pediatric Population-Based Cross-Sectional Study in a Tertiary Care Center. Indian J. Rheumatol. 2019, 14, 182–186. [Google Scholar] [CrossRef]
- Consolaro, A.; Giancane, G.; Ravelli, A. Clinical Outcome Measures in Pediatric Rheumatic Diseases. In Textbook of Pediatric rheumatology, 8th ed.; Petty, R.E., Laxer, R.M., Lindsley, C.B., Wedderburn, L.R., Mellins, E.D., Fuhlbrigge, R.C., Eds.; Elsevier Saunders Company: Philadelphia, PA, USA, 2022; p. 84. [Google Scholar]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Laxer, R.M.; Wedderburn, L.R. Juvenile Idiopathic Arthritis. In Textbook of Pediatric Rheumatology, 7th ed.; Petty, R.E., Laxer, R.M., Lindsley, C.B., et al., Eds.; Elsevier Saunders Company: Philadelphia, PA, USA, 2016; pp. 188–203. [Google Scholar]
- Bartoli, M.; Tarò, M.; Magni-Manzoni, S.; Pistorio, A.; Traverso, F.; Viola, S.; Magnani, A.; Gasparini, C.; Martini, A.; Ravelli, A. The magnitude of early response to methotrexate therapy predicts long-term outcome of patients with juvenile idiopathic arthritis. Ann. Rheum. Dis. 2008, 67, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.A.; Ruperto, N.; Giannini, E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J. Rheumatol. 2004, 31, 2290–2294. [Google Scholar]
- McErlane, F.; Beresford, M.W.; Baildam, E.M.; Thomson, W.; Hyrich, K.L. Recent developments in disease activity indices and outcome measures for juvenile idiopathic arthritis. Rheumatology 2013, 52, 1941–1951. [Google Scholar] [CrossRef]
- Taha, S.I.; Samaan, S.F.; Ibrahim, R.A.; Moustafa, N.M.; El-Sehsah, E.M.; Youssef, M.K. Can Complete Blood Count Picture Tell Us More About the Activity of Rheumatological Diseases? Clin. Med. Insights: Arthritis Musculoskelet. Disord. 2022, 15, 11795441221089182. [Google Scholar] [CrossRef]
- Gruszewska, E.; Sienkiewicz, M.; Abramowicz, P.; Konstantynowicz, J.; Gudowska-Sawczuk, M.; Chrostek, L.; Cylwik, B. Serum profile of transferrin isoforms in juvenile idiopathic arthritis: A preliminary study. Rheumatol. Int. 2018, 38, 1235–1240. [Google Scholar] [CrossRef]
- Hahn, Y.-S.; Kim, J.-G. Pathogenesis and clinical manifestations of juvenile rheumatoid arthritis. Korean J. Pediatr. 2010, 53, 921–930. [Google Scholar] [CrossRef]
- Gheita, T.; Kamel, S.; Helmy, N.; El-Laithy, N.; Monir, A. Omega-3 fatty acids in juvenile idiopathic arthritis: Effect on cytokines (IL-1 and TNF-a), disease activity and response criteria. Clin. Rheumatol. 2012, 31, 363–366. [Google Scholar] [CrossRef]
- Aletaha, D.; Nell, V.P.; Stamm, T.; Uffmann, M.; Pflugbeil, S.; Machold, K.; Smolen, J.S. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: Validation of a clinical activity score. Arthritis Res. Ther. 2005, 7, R796–R806. [Google Scholar] [CrossRef]
- Wolfe, F. Comparative usefulness of C-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. J. Rheumatol. 1997, 24, 1477–1485. [Google Scholar]
- López-Verdugo, F.; Furuzawa-Carballeda, J.; Romero-Hernández, F.; Coss-Adame, E.; Valdovinos, M.A.; Priego-Ranero, A.; Olvera-Prado, H.; Narváez-Chavez, S.; Peralta-Figueroa, J.; Torres-Villalobos, G. Hematological indices as indicators of silent inflammation in achalasia patients: A cross-sectional study. Medicine 2020, 99, e19326. [Google Scholar] [CrossRef]
- Malengier-Devlies, B.; Metzemaekers, M.; Wouters, C.; Proost, P.; Matthys, P. Neutrophil Homeostasis and Emergency Granulopoiesis: The Example of Systemic Juvenile Idiopathic Arthritis. Front. Immunol. 2021, 12, 766620. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Sandoo, A.; Stavropoulos-Kalinoglou, A.; Kitas, G.D. Mean platelet volume in patients with rheumatoid arthritis: The effect of antiTNF-α therapy. Rheumatol. Int. 2010, 30, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Glaser, A.; Lythgoe, H.; Ong, J.; Beresford, M.W.; Midgley, A.; Wright, H.L. Neutrophil activation signature in juvenile idiopathic arthritis indicates the presence of low-density granulocytes. Rheumatology 2018, 57, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Henderlight, M.; Do, T.; Yasin, S.; Grom, A.A.; DeLay, M.; Thornton, S.; Schulert, G.S. Neutrophils from Children With Systemic Juvenile Idiopathic Arthritis Exhibit Persistent Proinflammatory Activation Despite Long-Standing Clinically Inactive Disease. Front. Immunol. 2018, 9, 2995. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Petty, H.R.; Tang, Y.; Frank, M.B.; Tessier, P.A.; Dozmorov, I.; Jiang, K.; Kindzelski, A.; Chen, Y.; Cadwell, C.; et al. Evidence for chronic, peripheral activation of neutrophils in polyarticular juvenile rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R154. [Google Scholar] [CrossRef]
- Satis, S. New Inflammatory Marker Associated with Disease Activity in Rheumatoid Arthritis: The Systemic ImmuneInflammation Index. Curr. Health Sci. J. 2021, 47, 553–557. [Google Scholar] [PubMed]
- Targońska-Stępniak, B.; Grzechnik, K.; Kolarz, K.; Gągoł, D.; Majdan, M. Systemic Inflammatory Parameters in Patients with Elderly-Onset Rheumatoid Arthritis (EORA) and Young-Onset Rheumatoid Arthritis (YORA)—An Observational Study. J. Clin. Med. 2021, 10, 1204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, J.; Lu, Z.; Chen, M.; Fang, X.; Wang, G. High neutrophil-to-lymphocyte ratio is associated with increased carotid artery intima-media thickness in type 2 diabetes. J. Diabetes Investig. 2017, 8, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Cho, H.J.; Lee, H.Y.; Ki, Y.J.; Jeon, E.S.; Hwang, K.K.; Chae, S.C.; Baek, S.H.; Kang, S.M.; Choi, D.J.; et al. NeutrophilLymphocyte Ratio in Patients with Acute Heart Failure Predicts In-Hospital and Long-Term Mortality. J. Clin. Med. 2020, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Lin, F.; Ren, Y.; Liu, D.; Zhong, R.; Liang, Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS 2017, 125, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Jung, J.-Y.; Suh, C.-H.; Kim, H.-A. Systemic immune-inflammation index combined with ferritin can serve as a reliable assessment score for adult-onset Still’s disease. Clin. Rheumatol. 2021, 40, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Colbert, R.A. Classification of juvenile spondyloarthritis: Enthesitis-related arthritis and beyond. Nat. Rev. Rheumatol. 2010, 6, 477–485. [Google Scholar] [CrossRef]
- Çalışkan, O.; Güngör, Z.B.; Ekmekçi, Ö.B.; Ekmekçi, H.; Barut, K.; Şahin, S.; Yıldız, A.A.; Dümür, Ş.; Kasapçopur, Ö.; Kucur, M. Evaluation of the Serum Visfatin and Adiponectin Levels Related with the Activity of Juvenile Idiopathic Arthritis. J. Acad. Res. Med. 2021, 11, 120–125. [Google Scholar] [CrossRef]
- Vakili, M.; Ziaee, V.; Moradinejad, M.H.; Raeeskarami, S.R.; Kompani, F.; Rahamooz, T. Changes of Platelet Indices in Juvenile Idiopathic Arthritis in Acute Phase and After Two Months Treatment. Iran. J. Pediatr. 2016, 26, e5006. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).