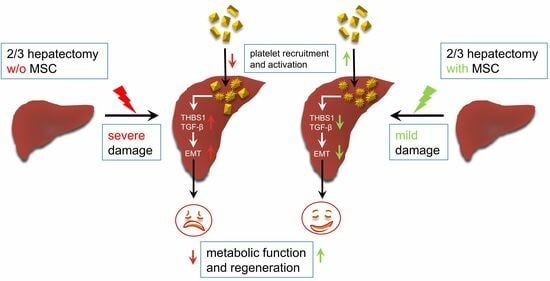
Approaching Thrombospondin-1 as a Potential Target for Mesenchymal Stromal Cells to Support Liver Regeneration after Partial Hepatectomy in Mouse and Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Trials
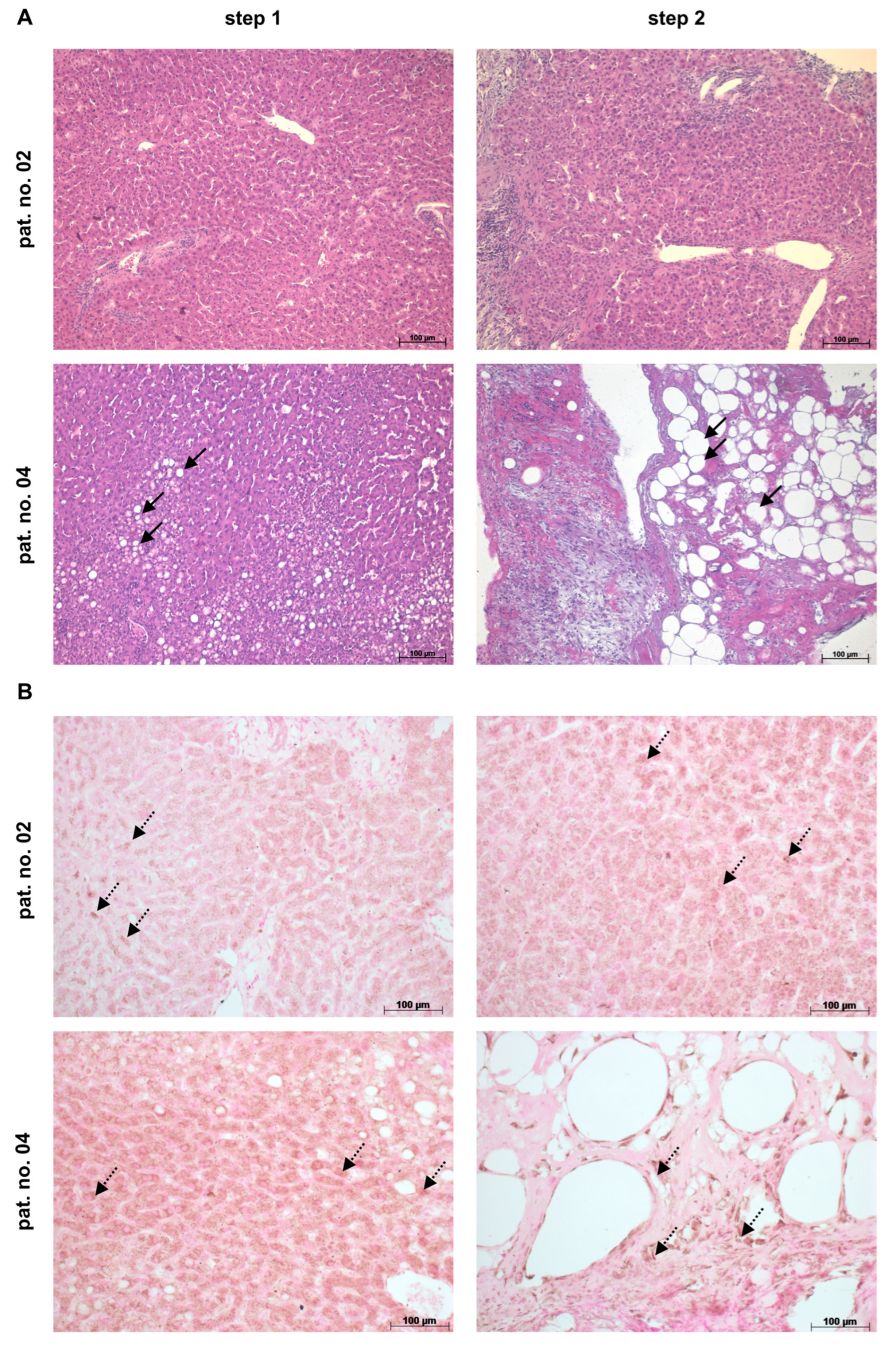
2.2. Human Liver and Blood Samples from Patients after ALPPS
2.3. Analyses of Mouse Plasma and Liver Tissue Samples
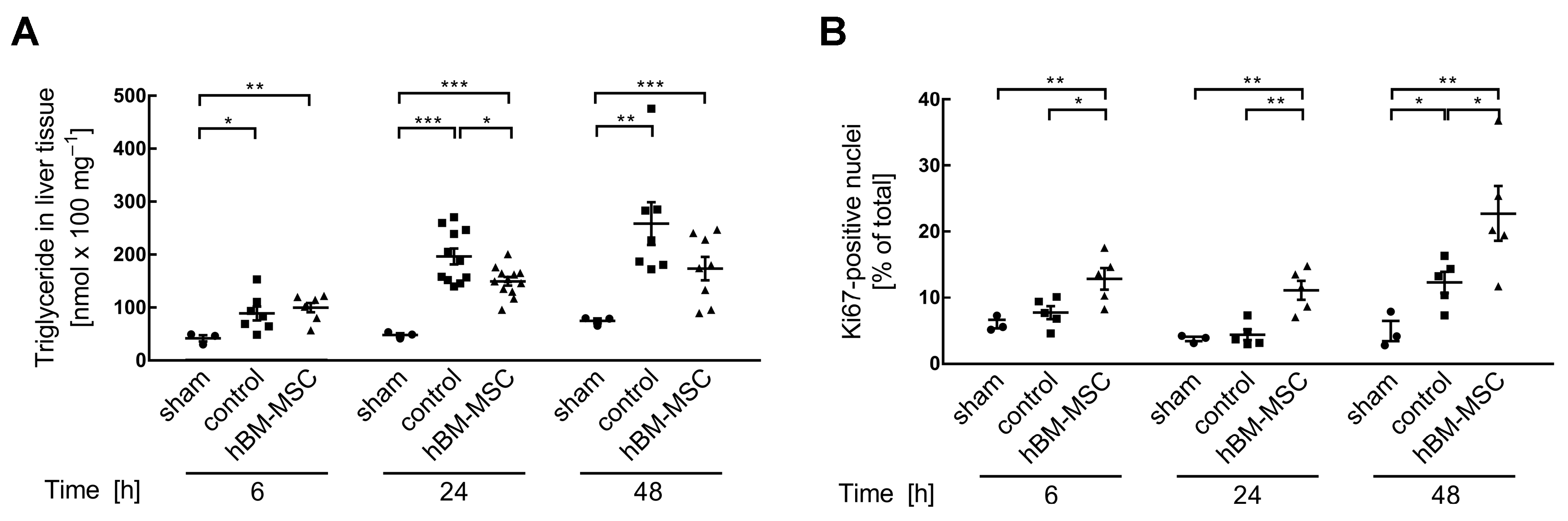
2.4. Quantification of Ki67-Positive Nuclei
2.5. sqRT-PCR Analyses
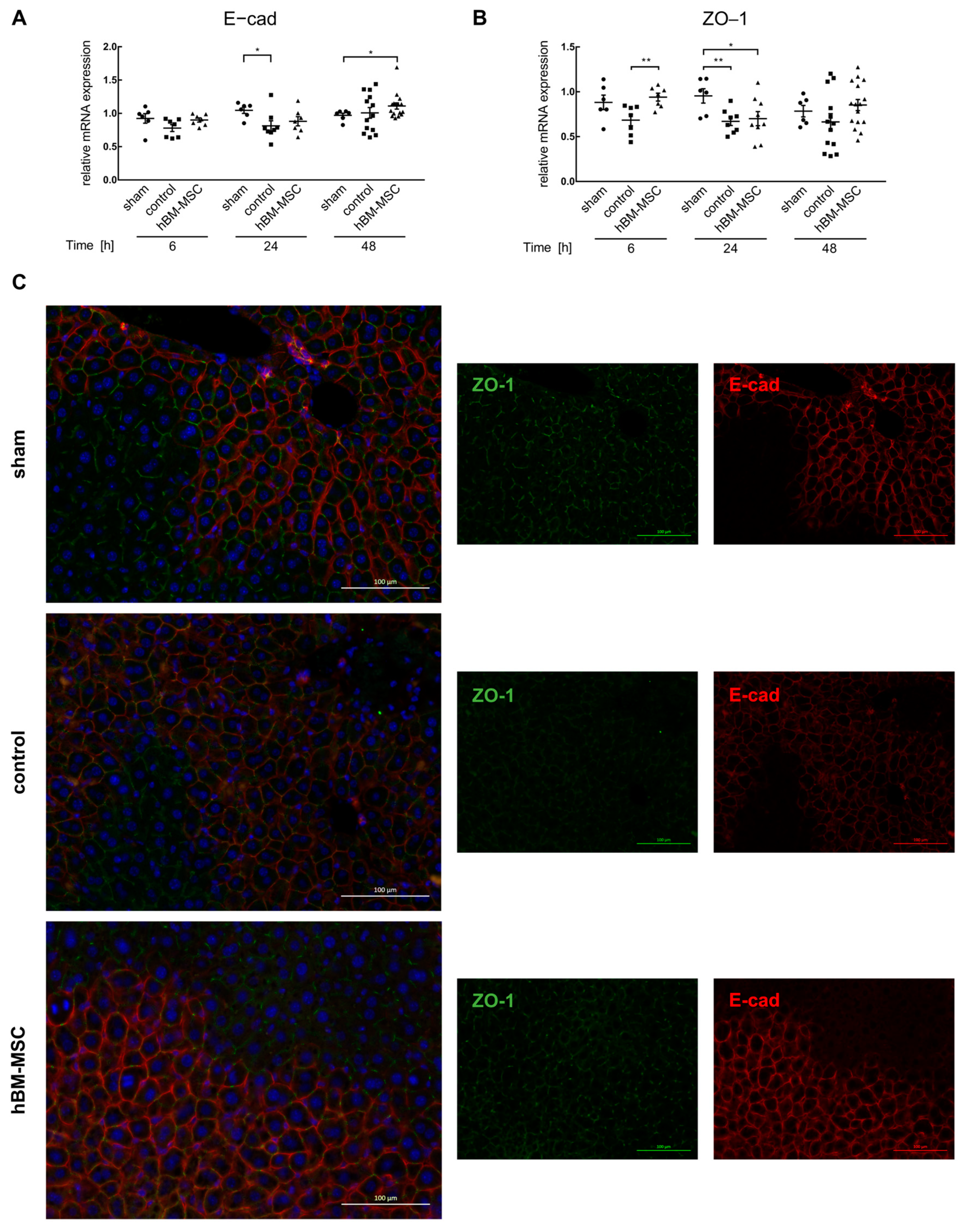
2.6. Immunofluorescent Co-Detection of THBS1 with CD42b, of THBS1 with CD11b, of E-cadherin (E-cad) and Zonula Occludens-1 (ZO-1), and of N-cadherin and Glutamine Synthetase (GS) in Mouse Liver
2.7. Immunofluorescent Co-Detection of Cytochrome P450 Subtype 2E1 (CYP2E1) and Glutamine Synthetase (GS) in Human Liver
2.8. Immunohistochemical Detection of THBS1 in Human Liver
2.9. Flow Cytometry of Platelets in Mouse Liver
2.10. Statistics
3. Results
3.1. MSCs Support Post-Surgery Restoration of Tissue Homeostasis in the Mouse Liver
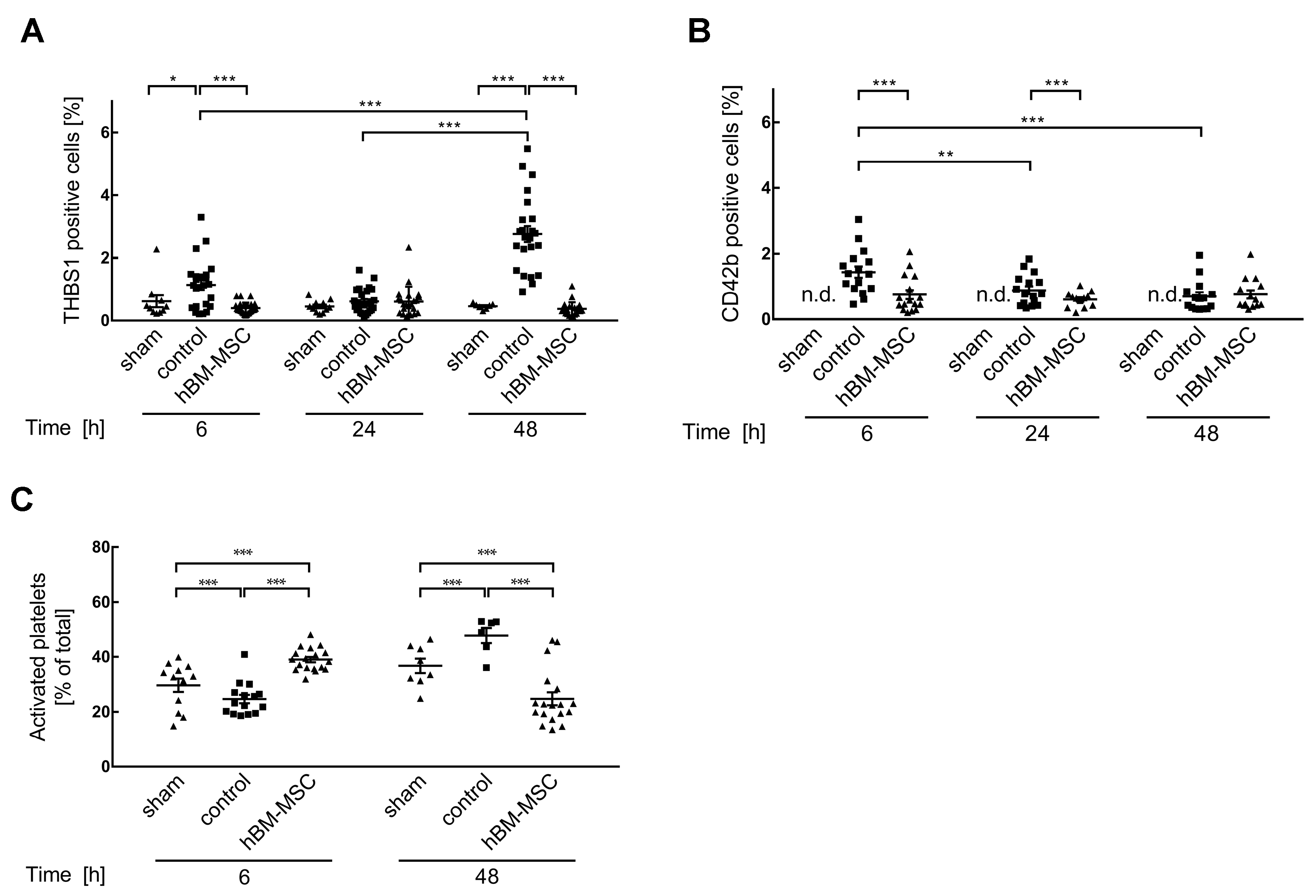
3.2. MSCs Decrease Liver Tissue TGF-β3 and THBS1
3.3. MSCs Promote Activation of Platelets in the Liver
3.4. THBS1 Might Mediate Hepatic Complications after Liver Surgery in Humans
3.5. High THBS1 Coincides with Functional Impairment of the Liver
4. Discussion
4.1. MSCs Support Mouse Liver Regeneration after Partial Hepatectomy by Mitigating THBS1
4.2. THBS1 Might Be a Therapeutic Target of MSCs in Humans after Complex Liver Resections
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagener, G. Assessment of hepatic function, operative candidacy, and medical management after liver resection in the patient with underlying liver disease. Semin. Liver Dis. 2013, 33, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Ruzzenente, A.; Conci, S.; Valdegamberi, A.; Iacono, C. How much remnant is enough in liver resection? Dig. Surg. 2012, 29, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Balaphas, A.; Combescure, C.; Morel, P.; Gonelle-Gispert, C.; Buhler, L. Systematic review and meta-analysis of thrombocytopenia as a predictor of post-hepatectomy liver failure. HPB 2019, 21, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, D.; Pereyra, D.; Veldhuis, Z.; Adelmeijer, J.; Ottens, P.; Kopec, A.K.; Starlinger, P.; Lisman, T.; Luyendyk, J.P. Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans. Blood 2019, 133, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Alkozai, E.M.; Nijsten, M.W.; de Jong, K.P.; de Boer, M.T.; Peeters, P.M.; Slooff, M.J.; Porte, R.J.; Lisman, T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann. Surg. 2010, 251, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Yang, J.; Yang, J.Y.; Wang, W.T.; Yan, L.N. Low immediate postoperative platelet count is associated with hepatic insufficiency after hepatectomy. World J. Gastroenterol. 2014, 20, 11871–11877. [Google Scholar] [CrossRef]
- Riediger, C.; Bachmann, J.; Hapfelmeier, A.; Friess, H.; Kleeff, J.; Mueller, M.W. Low postoperative platelet count is associated with negative outcome after liver resection for hepatocellular carcinoma. Hepato-Gastroenterol. 2014, 61, 1313–1320. [Google Scholar]
- Kawasaki, T.; Murata, S.; Takahashi, K.; Nozaki, R.; Ohshiro, Y.; Ikeda, N.; Pak, S.; Myronovych, A.; Hisakura, K.; Fukunaga, K.; et al. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J. Hepatol. 2010, 53, 648–654. [Google Scholar] [CrossRef]
- Meyer, J.; Balaphas, A.; Fontana, P.; Morel, P.; Robson, S.C.; Sadoul, K.; Gonelle-Gispert, C.; Buhler, L. Platelet Interactions with Liver Sinusoidal Endothelial Cells and Hepatic Stellate Cells Lead to Hepatocyte Proliferation. Cells 2020, 9, 1243. [Google Scholar] [CrossRef]
- Starlinger, P.; Haegele, S.; Offensperger, F.; Oehlberger, L.; Pereyra, D.; Kral, J.B.; Schrottmaier, W.C.; Badrnya, S.; Reiberger, T.; Ferlitsch, A.; et al. The profile of platelet alpha-granule released molecules affects postoperative liver regeneration. Hepatology 2016, 63, 1675–1688. [Google Scholar] [CrossRef]
- Starlinger, P.; Schauer, D.; Alidzanovic, L.; Zikeli, S.; Gebhardt, K.; Luf, F.; Fleischmann, E.; Perisanidis, B.; Gruenberger, B.; Gruenberger, T.; et al. Clinical evidence for thrombospondin-1 as a relevant suppressor of liver regeneration. J. Hepatol. 2013, 58, 1053–1054. [Google Scholar] [CrossRef]
- Hayashi, H.; Sakai, K.; Baba, H.; Sakai, T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology 2012, 55, 1562–1573. [Google Scholar] [CrossRef]
- Choi, S.S.; Diehl, A.M. Epithelial-to-mesenchymal transitions in the liver. Hepatology 2009, 50, 2007–2013. [Google Scholar] [CrossRef]
- Oh, S.H.; Swiderska-Syn, M.; Jewell, M.L.; Premont, R.T.; Diehl, A.M. Liver regeneration requires Yap1-TGFbeta-dependent epithelial-mesenchymal transition in hepatocytes. J. Hepatol. 2018, 69, 359–367. [Google Scholar] [CrossRef]
- Adas, G.; Koc, B.; Adas, M.; Duruksu, G.; Subasi, C.; Kemik, O.; Kemik, A.; Sakiz, D.; Kalayci, M.; Purisa, S.; et al. Effects of mesenchymal stem cells and VEGF on liver regeneration following major resection. Langenbecks Arch. Surg. 2016, 401, 725–740. [Google Scholar] [CrossRef]
- Papanikolaou, I.G.; Katselis, C.; Apostolou, K.; Feretis, T.; Lymperi, M.; Konstadoulakis, M.M.; Papalois, A.E.; Zografos, G.C. Mesenchymal Stem Cells Transplantation following Partial Hepatectomy: A New Concept to Promote Liver Regeneration-Systematic Review of the Literature Focused on Experimental Studies in Rodent Models. Stem Cells Int. 2017, 2017, 7567958. [Google Scholar] [CrossRef]
- Tautenhahn, H.M.; Bruckner, S.; Baumann, S.; Winkler, S.; Otto, W.; von Bergen, M.; Bartels, M.; Christ, B. Attenuation of Postoperative Acute Liver Failure by Mesenchymal Stem Cell Treatment Due to Metabolic Implications. Ann. Surg. 2016, 263, 546–556. [Google Scholar] [CrossRef]
- Wabitsch, S.; Benzing, C.; Krenzien, F.; Splith, K.; Haber, P.K.; Arnold, A.; Nosser, M.; Kamali, C.; Hermann, F.; Gunther, C.; et al. Human Stem Cells Promote Liver Regeneration After Partial Hepatectomy in BALB/C Nude Mice. J. Surg. Res. 2019, 239, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Liu, X.; Ma, Y.; Ge, Y.; Zhang, Q.; Liu, B.; Wang, H. Adipose-Derived Stem Cells Protect Ischemia-Reperfusion and Partial Hepatectomy by Attenuating Endoplasmic Reticulum Stress. Front. Cell Dev. Biol. 2020, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, H.M.; Bruckner, S.; Uder, C.; Erler, S.; Hempel, M.; von Bergen, M.; Brach, J.; Winkler, S.; Pankow, F.; Gittel, C.; et al. Mesenchymal stem cells correct haemodynamic dysfunction associated with liver injury after extended resection in a pig model. Sci. Rep. 2017, 7, 2617. [Google Scholar] [CrossRef] [PubMed]
- Nickel, S.; Vlaic, S.; Christ, M.; Schubert, K.; Henschler, R.; Tautenhahn, F.; Burger, C.; Kuhne, H.; Erler, S.; Roth, A.; et al. Mesenchymal stromal cells mitigate liver damage after extended resection in the pig by modulating thrombospondin-1/TGF-beta. NPJ Regen. Med. 2021, 6, 84. [Google Scholar] [CrossRef]
- Stock, P.; Bruckner, S.; Ebensing, S.; Hempel, M.; Dollinger, M.M.; Christ, B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat. Protoc. 2010, 5, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.; Borkham-Kamphorst, E.; Stock, P.; Bruckner, S.; Dollinger, M.; Weiskirchen, R.; Christ, B. Human mesenchymal stem cells towards non-alcoholic steatohepatitis in an immunodeficient mouse model. Exp. Cell Res. 2014, 326, 230–239. [Google Scholar] [CrossRef]
- Christ, B.; Dollinger, M.M. The generation of hepatocytes from mesenchymal stem cells and engraftment into the liver. Curr. Opin. Organ. Transplant. 2011, 16, 69–75. [Google Scholar] [CrossRef]
- Nickel, S.; Christ, M.; Schmidt, S.; Kosacka, J.; Kuhne, H.; Roderfeld, M.; Longerich, T.; Tietze, L.; Bosse, I.; Hsu, M.J.; et al. Human Mesenchymal Stromal Cells Resolve Lipid Load in High Fat Diet-Induced Non-Alcoholic Steatohepatitis in Mice by Mitochondria Donation. Cells 2022, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Schoffski, P.; Trautwein, C.; Luedde, T.; Ganser, A.; Manns, M.P.; von Depka, M. Plasma P-selectin levels are elevated in patients with chronic liver disease. Blood Coagul. Fibrinolysis 2003, 14, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Jeong, D.; Kim, G.H.; Go, S.; Song, J.; Moon, E.; Huh, Y.H.; Kim, D. Super-resolution imaging of platelet-activation process and its quantitative analysis. Sci. Rep. 2021, 11, 10511. [Google Scholar] [CrossRef]
- Shteyer, E.; Liao, Y.; Muglia, L.J.; Hruz, P.W.; Rudnick, D.A. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 2004, 40, 1322–1332. [Google Scholar] [CrossRef]
- Walldorf, J.; Hillebrand, C.; Aurich, H.; Stock, P.; Hempel, M.; Ebensing, S.; Fleig, W.E.; Seufferlein, T.; Dollinger, M.M.; Christ, B. Propranolol impairs liver regeneration after partial hepatectomy in C57Bl/6-mice by transient attenuation of hepatic lipid accumulation and increased apoptosis. Scand. J. Gastroenterol. 2010, 45, 468–476. [Google Scholar] [CrossRef]
- Jiao, Z.; Ma, Y.; Zhang, Q.; Wang, Y.; Liu, T.; Liu, X.; Piao, C.; Liu, B.; Wang, H. The adipose-derived mesenchymal stem cell secretome promotes hepatic regeneration in miniature pigs after liver ischaemia-reperfusion combined with partial resection. Stem Cell Res. Ther. 2021, 12, 218. [Google Scholar] [CrossRef]
- Almalé, L.; García-Álvaro, M.; Martínez-Palacián, A.; García-Bravo, M.; Lazcanoiturburu, N.; Addante, A.; Roncero, C.; Sanz, J.; López, M.; Bragado, P.; et al. c-Met Signaling Is Essential for Mouse Adult Liver Progenitor Cells Expansion After Transforming Growth Factor-beta-Induced Epithelial-Mesenchymal Transition and Regulates Cell Phenotypic Switch. Stem Cells 2019, 37, 1108–1118. [Google Scholar] [CrossRef]
- Adams, J.C. Thrombospondin-1. Int. J. Biochem. Cell Biol. 1997, 29, 861–865. [Google Scholar] [CrossRef]
- Shiravand, Y.; Walter, U.; Jurk, K. Fine-Tuning of Platelet Responses by Serine/Threonine Protein Kinases and Phosphatases-Just the Beginning. Hamostaseologie 2021, 41, 206–216. [Google Scholar] [CrossRef]
- Yadav, S.; Storrie, B. The cellular basis of platelet secretion: Emerging structure/function relationships. Platelets 2017, 28, 108–118. [Google Scholar] [CrossRef]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef]
- Gebhardt, R.; Matz-Soja, M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J. Gastroenterol. 2014, 20, 8491–8504. [Google Scholar] [CrossRef]
- Jungermann, K. Zonation of metabolism and gene expression in liver. Histochem. Cell Biol. 1995, 103, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Zhang, L.; Li, Q.; Li, Y.; Ding, F.H.; Li, X. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci. 2021, 265, 118821. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.R.; Wang, J.L.; Tang, Z.T.; Wang, Y.; Zhou, G.; Liu, Y.; Ren, H.Z.; Shi, X.L. Mesenchymal Stem Cells Improve Glycometabolism and Liver Regeneration in the Treatment of Post-hepatectomy Liver Failure. Front. Physiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Apostolou, K.G.; Papanikolaou, I.G.; Katselis, C.; Feretis, T.; Kletsas, D.; Konstadoulakis, M.M.; Lymperi, M.; Saetta, A.A.; Tsikalakis, S.; Agrogiannis, G.; et al. Undifferentiated Adipose Tissue Stem Cell Transplantation Promotes Hepatic Regeneration, Ameliorates Histopathologic Damage of the Liver, and Upregulates the Expression of Liver Regeneration- and Liver-Specific Genes in a Rat Model of Partial Hepatectomy. Stem Cells Int. 2018, 2018, 1393607. [Google Scholar] [CrossRef] [PubMed]
- Liska, V.; Slowik, P.; Eggenhofer, E.; Treska, V.; Renner, P.; Popp, F.C.; Mirka, H.; Kobr, J.; Sykora, R.; Schlitt, H.J.; et al. Intraportal injection of porcine multipotent mesenchymal stromal cells augments liver regeneration after portal vein embolization. In Vivo 2009, 23, 229–235. [Google Scholar]
- Ezquer, F.; Bahamonde, J.; Huang, Y.L.; Ezquer, M. Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy. Stem Cell Res. Ther. 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, P.; Yu, C.; Shi, Q.; Wei, S.; Li, Y.; Qi, H.; Cao, Q.; Guo, C.; Wu, X.; et al. Hypoxic bone marrow mesenchymal stromal cells-derived exosomal miR-182-5p promotes liver regeneration via FOXO1-mediated macrophage polarization. FASEB J. 2022, 36, e22553. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Liu, T.; Zhao, Q.; Ji, Y.; Bai, J.; Wang, H.; Yao, R.; Zhou, X.; Chen, Y.; Xu, J. Genetic landscape and immune mechanism of monocytes associated with the progression of acute-on-chronic liver failure. Hepatol. Int. 2023, 17, 676–688. [Google Scholar] [CrossRef]
- Bronson, S.M.; Westwood, B.; Cook, K.L.; Emenaker, N.J.; Chappell, M.C.; Roberts, D.D.; Soto-Pantoja, D.R. Discrete Correlation Summation Clustering Reveals Differential Regulation of Liver Metabolism by Thrombospondin-1 in Low-Fat and High-Fat Diet-Fed Mice. Metabolites 2022, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Min-DeBartolo, J.; Schlerman, F.; Akare, S.; Wang, J.; McMahon, J.; Zhan, Y.; Syed, J.; He, W.; Zhang, B.; Martinez, R.V. Thrombospondin-I is a critical modulator in non-alcoholic steatohepatitis (NASH). PLoS ONE 2019, 14, e0226854. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Kang, Y.; Huang, S.; Xiao, Y. Circulating THBS1: A Risk Factor for Nonalcoholic Fatty Liver Disease in Obese Children. Ann. Nutr. Metab. 2023, 79, 16–28. [Google Scholar] [CrossRef]
- Gwag, T.; Reddy Mooli, R.G.; Li, D.; Lee, S.; Lee, E.Y.; Wang, S. Macrophage-derived thrombospondin 1 promotes obesity-associated non-alcoholic fatty liver disease. JHEP Rep. 2021, 3, 100193. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Yao-Borengasser, A.; Bodles, A.M.; Rasouli, N.; Phanavanh, B.; Nolen, G.T.; Kern, E.M.; Nagarajan, R.; Spencer, H.J., 3rd; Lee, M.J.; et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 2008, 57, 432–439. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, T.; Sorenson, C.M.; Sheibani, N.; Liu, B. Myeloid-Derived TSP1 (Thrombospondin-1) Contributes to Abdominal Aortic Aneurysm Through Suppressing Tissue Inhibitor of Metalloproteinases-1. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e350–e366. [Google Scholar] [CrossRef]
- Kumar, R.; Mickael, C.; Kassa, B.; Sanders, L.; Hernandez-Saavedra, D.; Koyanagi, D.E.; Kumar, S.; Pugliese, S.C.; Thomas, S.; McClendon, J.; et al. Interstitial macrophage-derived thrombospondin-1 contributes to hypoxia-induced pulmonary hypertension. Cardiovasc. Res. 2020, 116, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Mahon, B.P. Allogeneic mesenchymal stem cells: Agents of immune modulation. J. Cell. Biochem. 2011, 112, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Pavlovic, D.; Djonov, V.; Volarevic, V. Therapeutic potential of mesenchymal stem cells in the treatment of acute liver failure. World J. Gastroenterol. 2022, 28, 3627–3636. [Google Scholar] [CrossRef]
- Starlinger, P.; Haegele, S.; Wanek, D.; Zikeli, S.; Schauer, D.; Alidzanovic, L.; Fleischmann, E.; Gruenberger, B.; Gruenberger, T.; Brostjan, C. Plasma thrombospondin 1 as a predictor of postoperative liver dysfunction. Br. J. Surg. 2015, 102, 826–836. [Google Scholar] [CrossRef]
- Matsuo, R.; Nakano, Y.; Ohkohchi, N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann. Surg. 2011, 253, 759–763. [Google Scholar] [CrossRef]
- Han, S. Possible roles of platelets in liver transplantation: Regeneration and cancer recurrence. Anesth. Pain. Med. 2021, 16, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Lejmi, E.; Fontana, P.; Morel, P.; Gonelle-Gispert, C.; Buhler, L. A focus on the role of platelets in liver regeneration: Do platelet-endothelial cell interactions initiate the regenerative process? J. Hepatol. 2015, 63, 1263–1271. [Google Scholar] [CrossRef]
- Takahashi, K.; Liang, C.; Oda, T.; Ohkohchi, N. Platelet and liver regeneration after liver surgery. Surg. Today 2020, 50, 974–983. [Google Scholar] [CrossRef]
- Starlinger, P.; Luyendyk, J.P.; Groeneveld, D.J. Hemostasis and Liver Regeneration. Semin. Thromb. Hemost. 2020, 46, 735–742. [Google Scholar] [CrossRef]
- Kuroda, S.; Tashiro, H.; Kobayashi, T.; Hashimoto, M.; Mikuriya, Y.; Ohdan, H. Administration of antithrombin III attenuates posthepatectomy liver failure in hepatocellular carcinoma. Dig. Surg. 2015, 32, 173–180. [Google Scholar] [CrossRef]
- Pereyra, D.; Offensperger, F.; Klinglmueller, F.; Haegele, S.; Oehlberger, L.; Gruenberger, T.; Brostjan, C.; Starlinger, P. Early prediction of postoperative liver dysfunction and clinical outcome using antithrombin III-activity. PLoS ONE 2017, 12, e0175359. [Google Scholar] [CrossRef]
- Jesudas, R.; Takemoto, C.M. Where have all the platelets gone? HIT, DIC, or something else? Hematol. Am. Soc. Hematol. Educ. Program 2023, 2023, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Maiwall, R. Platelets in acute liver failure: An innocent bystander or instigator? Hepatol. Int. 2022, 16, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M.; Chauhan, A. The role of platelet mediated thromboinflammation in acute liver injury. Front. Immunol. 2022, 13, 1037645. [Google Scholar] [CrossRef]
- Lisman, T.; Luyendyk, J.P. Platelets as Modulators of Liver Diseases. Semin. Thromb. Hemost. 2018, 44, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Kamali, C.; Kamali, K.; Brunnbauer, P.; Splith, K.; Pratschke, J.; Schmelzle, M.; Krenzien, F. Extended liver resection in mice: State of the art and pitfalls-a systematic review. Eur. J. Med. Res. 2021, 26, 6. [Google Scholar] [CrossRef]
- Kaur, S.; Roberts, D.D. Emerging functions of thrombospondin-1 in immunity. Semin. Cell Dev. Biol. 2024, 155, 22–31. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tietze, L.; Christ, M.; Yu, J.; Stock, P.; Nickel, S.; Schulze, A.; Bartels, M.; Tautenhahn, H.-M.; Christ, B. Approaching Thrombospondin-1 as a Potential Target for Mesenchymal Stromal Cells to Support Liver Regeneration after Partial Hepatectomy in Mouse and Humans. Cells 2024, 13, 529. https://doi.org/10.3390/cells13060529
Tietze L, Christ M, Yu J, Stock P, Nickel S, Schulze A, Bartels M, Tautenhahn H-M, Christ B. Approaching Thrombospondin-1 as a Potential Target for Mesenchymal Stromal Cells to Support Liver Regeneration after Partial Hepatectomy in Mouse and Humans. Cells. 2024; 13(6):529. https://doi.org/10.3390/cells13060529
Chicago/Turabian StyleTietze, Lysann, Madlen Christ, Jiyeon Yu, Peggy Stock, Sandra Nickel, Annelie Schulze, Michael Bartels, Hans-Michael Tautenhahn, and Bruno Christ. 2024. "Approaching Thrombospondin-1 as a Potential Target for Mesenchymal Stromal Cells to Support Liver Regeneration after Partial Hepatectomy in Mouse and Humans" Cells 13, no. 6: 529. https://doi.org/10.3390/cells13060529