Further Characterization of the Antiviral Transmembrane Protein MARCH8
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. DNA Constructs
2.3. Phylogenetic Analysis of the MARCH Proteins
2.4. Immunoblot Assays
2.5. Production of Pseudotyped Virus and Infectivity Assays
2.6. Immunofluorescence Microscopy
2.7. Primary Cell Culture
2.8. CRISPR-Cas9 Lentiviral Transduction of MDMs
2.9. Cell-Free and Cell-to-Cell Infectivity Assays
2.10. Statistical Analysis
3. Results
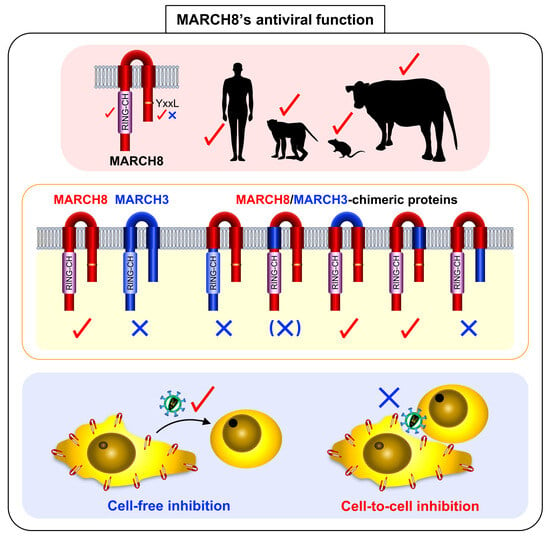
3.1. Antiviral Function of MARCH8 Is Conserved among Mammals
3.2. The RING-CH Domain and Tyrosine Motif in Mammalian MARCH8 Are Required for Antiviral Function
3.3. The N-Terminal and C-Terminal CTs of the Human MARCH8 Protein Are Responsible for the Antiviral Activity
3.4. MARCH8 Is Unable to Block Cell-to-Cell HIV-1 Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohmura-Hoshino, M.; Matsuki, Y.; Aoki, M.; Goto, E.; Mito, M.; Uematsu, M.; Kakiuchi, T.; Hotta, H.; Ishido, S. Inhibition of MHC class II expression and immune responses by c-MIR. J. Immunol. 2006, 177, 341–354. [Google Scholar] [CrossRef]
- Lapaque, N.; Jahnke, M.; Trowsdale, J.; Kelly, A.P. The HLA-DRα Chain Is Modified by Polyubiquitination. J. Biol. Chem. 2009, 284, 7007–7016. [Google Scholar] [CrossRef] [PubMed]
- Bartee, E.; Eyster, C.A.; Viswanathan, K.; Mansouri, M.; Donaldson, J.G.; Fruh, K. Membrane-Associated RING-CH proteins associate with Bap31 and target CD81 and CD44 to lysosomes. PLoS ONE 2010, 5, e15132. [Google Scholar] [CrossRef]
- Tze, L.E.; Horikawa, K.; Domaschenz, H.; Howard, D.R.; Roots, C.M.; Rigby, R.J.; Way, D.A.; Ohmura-Hoshino, M.; Ishido, S.; Andoniou, C.E.; et al. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med. 2011, 208, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Eyster, C.A.; Cole, N.B.; Petersen, S.; Viswanathan, K.; Fruh, K.; Donaldson, J.G. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol. Biol. Cell 2011, 22, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Van de Kooij, B.; Verbrugge, I.; de Vries, E.; Gijsen, M.; Montserrat, V.; Maas, C.; Neefjes, J.; Borst, J. Ubiquitination by the membrane-associated RING-CH-8 (MARCH-8) ligase controls steady-state cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptor 1. J. Biol. Chem. 2013, 288, 6617–6628. [Google Scholar] [CrossRef]
- Chen, R.; Li, M.; Zhang, Y.; Zhou, Q.; Shu, H.B. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1beta-induced NF-kappaB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2012, 109, 14128–14133. [Google Scholar] [CrossRef]
- Fujita, H.; Iwabu, Y.; Tokunaga, K.; Tanaka, Y. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J. Cell Sci. 2013, 126, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Zhang, Y.; Koyama, T.; Tobiume, M.; Tsunetsugu-Yokota, Y.; Yamaoka, S.; Fujita, H.; Tokunaga, K. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins. Nat. Med. 2015, 21, 1502–1507. [Google Scholar] [CrossRef]
- Zhang, Y.; Tada, T.; Ozono, S.; Kishigami, S.; Fujita, H.; Tokunaga, K. MARCH8 inhibits viral infection by two different mechanisms. eLife 2020, 9, e57763. [Google Scholar] [CrossRef]
- Tada, T.; Zhang, Y.; Fujita, H.; Tokunaga, K. MARCH8: The tie that binds to viruses. FEBS J. 2022, 289, 3642–3654. [Google Scholar] [CrossRef] [PubMed]
- Lun, C.M.; Waheed, A.A.; Majadly, A.; Powell, N.; Freed, E.O. Mechanism of Viral Glycoprotein Targeting by Membrane-Associated RING-CH Proteins. mBio 2021, 12, e00219-21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ozono, S.; Tada, T.; Tobiume, M.; Kameoka, M.; Kishigami, S.; Fujita, H.; Tokunaga, K. MARCH8 Targets Cytoplasmic Lysine Residues of Various Viral Envelope Glycoproteins. Microbiol. Spectr. 2022, 10, e0061821. [Google Scholar] [CrossRef]
- Villalón-Letelier, F.; Brooks, A.G.; Londrigan, S.L.; Reading, P.C. MARCH8 Restricts Influenza A Virus Infectivity but Does Not Downregulate Viral Glycoprotein Expression at the Surface of Infected Cells. mBio 2021, 12, e0148421. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, F.; Ren, L.; Zhao, F.; Huang, Y.; Wei, L.; Wang, Y.; Wang, C.; Fan, Z.; Mei, S.; et al. MARCH8 inhibits influenza A virus infection by targeting viral M2 protein for ubiquitination-dependent degradation in lysosomes. Nat. Commun. 2021, 12, 4427. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, S.; Zhang, X.; Khan, I.; Ahmad, I.; Zhou, Y.; Li, S.; Shi, J.; Wang, Y.; Zheng, Y.H. MARCH8 Inhibits Ebola Virus Glycoprotein, Human Immunodeficiency Virus Type 1 Envelope Glycoprotein, and Avian Influenza Virus H5N1 Hemagglutinin Maturation. mBio 2020, 11, e01882-20. [Google Scholar] [CrossRef] [PubMed]
- Umthong, S.; Lynch, B.; Timilsina, U.; Waxman, B.; Ivey, E.B.; Stavrou, S. Elucidating the Antiviral Mechanism of Different MARCH Factors. mBio 2021, 12, e03264-20. [Google Scholar] [CrossRef]
- Yu, C.; Bai, Y.; Tan, W.; Bai, Y.; Li, X.; Zhou, Y.; Zhai, J.; Xue, M.; Tang, Y.-D.; Zheng, C.; et al. Human MARCH1, 2, and 8 block Ebola virus envelope glycoprotein cleavage via targeting furin P domain. J. Med. Virol. 2024, 96, e29445. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, N.; Otsuka, N.; Matsuo, K.; Shiino, T.; Kojima, A.; Kurata, T.; Sakai, K.; Yamamoto, N.; Isomura, S.; Dhole, T.N.; et al. An infectious DNA clone of HIV type 1 subtype C. AIDS Res. Hum. Retroviruses 1999, 15, 1321–1324. [Google Scholar] [CrossRef]
- Ozono, S.; Zhang, Y.; Tobiume, M.; Kishigami, S.; Tokunaga, K. Super-rapid quantitation of the production of HIV-1 harboring a luminescent peptide tag. J. Biol. Chem. 2020, 295, 13023–13030. [Google Scholar] [CrossRef]
- Zhang, Y.; Tada, T.; Ozono, S.; Yao, W.; Tanaka, M.; Yamaoka, S.; Kishigami, S.; Fujita, H.; Tokunaga, K. Membrane-associated RING-CH (MARCH) 1 and 2 are MARCH family members that inhibit HIV-1 infection. J. Biol. Chem. 2019, 294, 3397–3405. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, X.; Zhang, D.; Zhao, F.; Fan, Z.; Hu, S.; Mei, S.; Huang, Y.; Sun, H.; Wei, L.; et al. The Engineered MARCH8-Resistant Vesicular Stomatitis Virus Glycoprotein Enhances Lentiviral Vector Transduction. Hum. Gene Ther. 2021, 32, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.; Liu, X. MARCH2 is upregulated in HIV-1 infection and inhibits HIV-1 production through envelope protein translocation or degradation. Virology 2018, 518, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Dufloo, J.; Bruel, T.; Schwartz, O. HIV-1 cell-to-cell transmission and broadly neutralizing antibodies. Retrovirology 2018, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Bracq, L.; Xie, M.; Benichou, S.; Bouchet, J. Mechanisms for Cell-to-Cell Transmission of HIV-1. Front. Immunol. 2018, 9, 260. [Google Scholar] [CrossRef]
- Phillips, D.M. The role of cell-to-cell transmission in HIV infection. AIDS 1994, 8, 719–731. [Google Scholar] [CrossRef]
- Carr, J.M.; Hocking, H.; Li, P.; Burrell, C.J. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 1999, 265, 319–329. [Google Scholar] [CrossRef]
- Chen, P.; Hubner, W.; Spinelli, M.A.; Chen, B.K. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 2007, 81, 12582–12595. [Google Scholar] [CrossRef]
- Martin, N.; Sattentau, Q. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr. Opin. HIV AIDS 2009, 4, 143–149. [Google Scholar] [CrossRef]
- Zhong, P.; Agosto, L.M.; Munro, J.B.; Mothes, W. Cell-to-cell transmission of viruses. Curr. Opin. Virol. 2013, 3, 44–50. [Google Scholar] [CrossRef]
- Sourisseau, M.; Sol-Foulon, N.; Porrot, F.; Blanchet, F.; Schwartz, O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 2007, 81, 1000–1012. [Google Scholar] [CrossRef]
- Jolly, C.; Sattentau, Q.J. Human immunodeficiency virus type 1 virological synapse formation in T cells requires lipid raft integrity. J. Virol. 2005, 79, 12088–12094. [Google Scholar] [CrossRef] [PubMed]
- Monel, B.; Beaumont, E.; Vendrame, D.; Schwartz, O.; Brand, D.; Mammano, F. HIV Cell-to-Cell Transmission Requires the Production of Infectious Virus Particles and Does Not Proceed through Env-Mediated Fusion Pores. J. Virol. 2012, 86, 3924–3933. [Google Scholar] [CrossRef]
- Dale, B.M.; McNerney, G.P.; Thompson, D.L.; Hubner, W.; de Los Reyes, K.; Chuang, F.Y.; Huser, T.; Chen, B.K. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe 2011, 10, 551–562. [Google Scholar] [CrossRef]
- Kumar, S.; Barouch-Bentov, R.; Xiao, F.; Schor, S.; Pu, S.; Biquand, E.; Lu, A.; Lindenbach, B.D.; Jacob, Y.; Demeret, C.; et al. MARCH8 Ubiquitinates the Hepatitis C Virus Nonstructural 2 Protein and Mediates Viral Envelopment. Cell Rep. 2019, 26, 1800–1814.e5. [Google Scholar] [CrossRef] [PubMed]
- Barouch-Bentov, R.; Neveu, G.; Xiao, F.; Beer, M.; Bekerman, E.; Schor, S.; Campbell, J.; Boonyaratanakornkit, J.; Lindenbach, B.; Lu, A.; et al. Hepatitis C Virus Proteins Interact with the Endosomal Sorting Complex Required for Transport (ESCRT) Machinery via Ubiquitination To Facilitate Viral Envelopment. mBio 2016, 7, e01456-16. [Google Scholar] [CrossRef]
- Khalil, M.I.; Yang, C.; Vu, L.; Chadha, S.; Nabors, H.; James, C.D.; Morgan, I.M.; Pyeon, D. The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer. J. Virol. 2024, 98, e01726-23. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Yang, C.; Vu, L.; Chadha, S.; Nabors, H.; Welbon, C.; James, C.D.; Morgan, I.M.; Spanos, W.C.; Pyeon, D. HPV upregulates MARCHF8 ubiquitin ligase and inhibits apoptosis by degrading the death receptors in head and neck cancer. PLOS Pathog. 2023, 19, e1011171. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Q.; Zhao, Z.; Zhai, J.; Xue, M.; Tang, Y.-D.; Wang, C.; Zheng, C. The emerging roles of MARCH8 in viral infections: A double-edged Sword. PLoS Pathog. 2023, 19, e1011619. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, T.; Zhang, Y.; Kong, D.; Tanaka, M.; Yao, W.; Kameoka, M.; Ueno, T.; Fujita, H.; Tokunaga, K. Further Characterization of the Antiviral Transmembrane Protein MARCH8. Cells 2024, 13, 698. https://doi.org/10.3390/cells13080698
Tada T, Zhang Y, Kong D, Tanaka M, Yao W, Kameoka M, Ueno T, Fujita H, Tokunaga K. Further Characterization of the Antiviral Transmembrane Protein MARCH8. Cells. 2024; 13(8):698. https://doi.org/10.3390/cells13080698
Chicago/Turabian StyleTada, Takuya, Yanzhao Zhang, Dechuan Kong, Michiko Tanaka, Weitong Yao, Masanori Kameoka, Takamasa Ueno, Hideaki Fujita, and Kenzo Tokunaga. 2024. "Further Characterization of the Antiviral Transmembrane Protein MARCH8" Cells 13, no. 8: 698. https://doi.org/10.3390/cells13080698