State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future?
Abstract
:1. Introduction
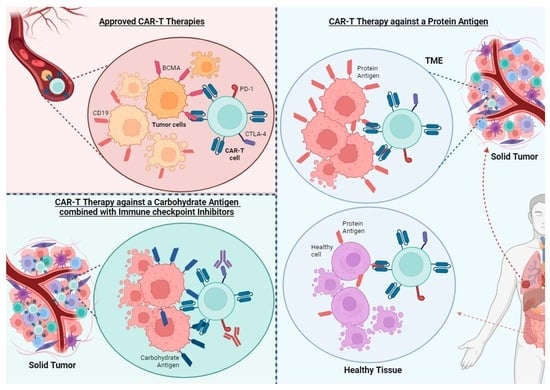
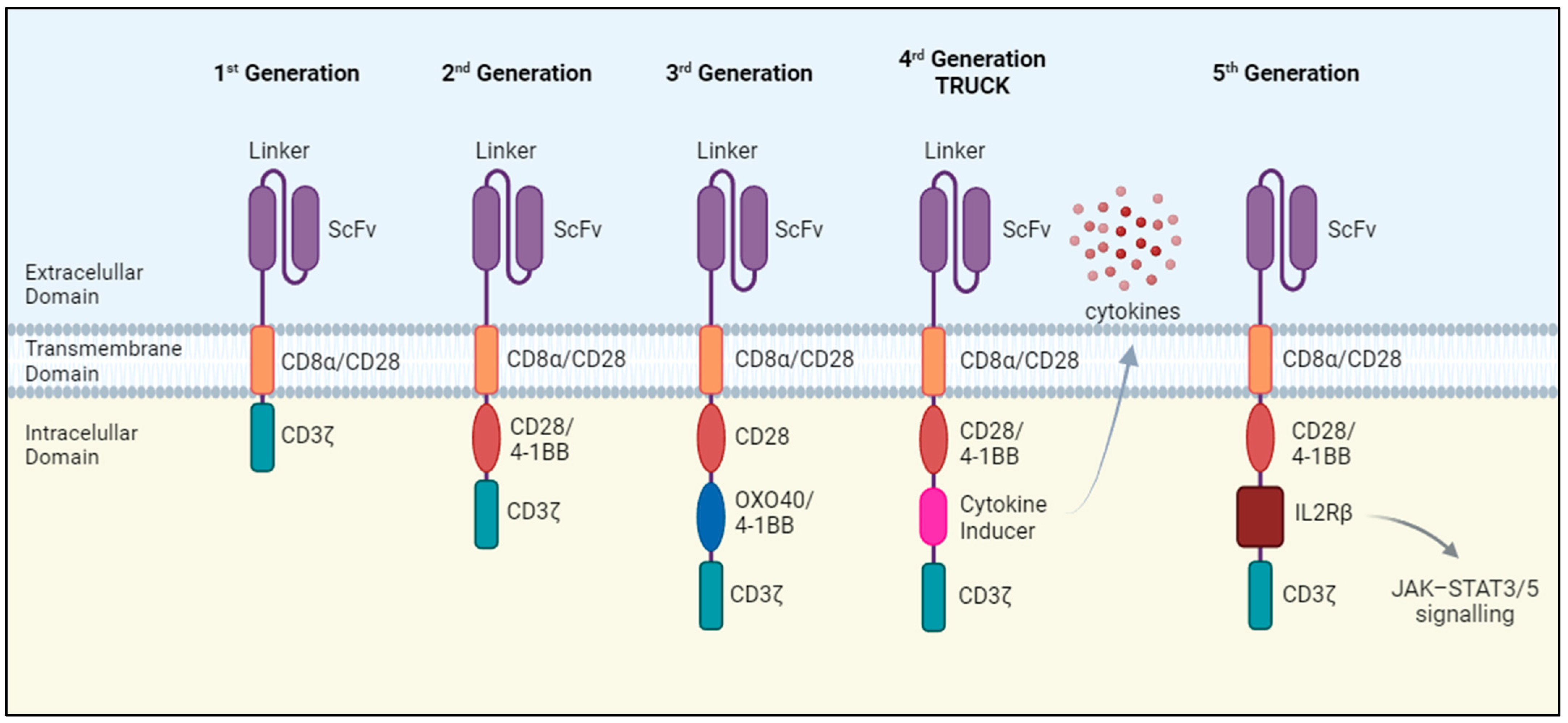
2. Approved CAR-T Cell Therapies
3. Challenges and Limitations of CAR-T Cell Therapy in Solid Tumors
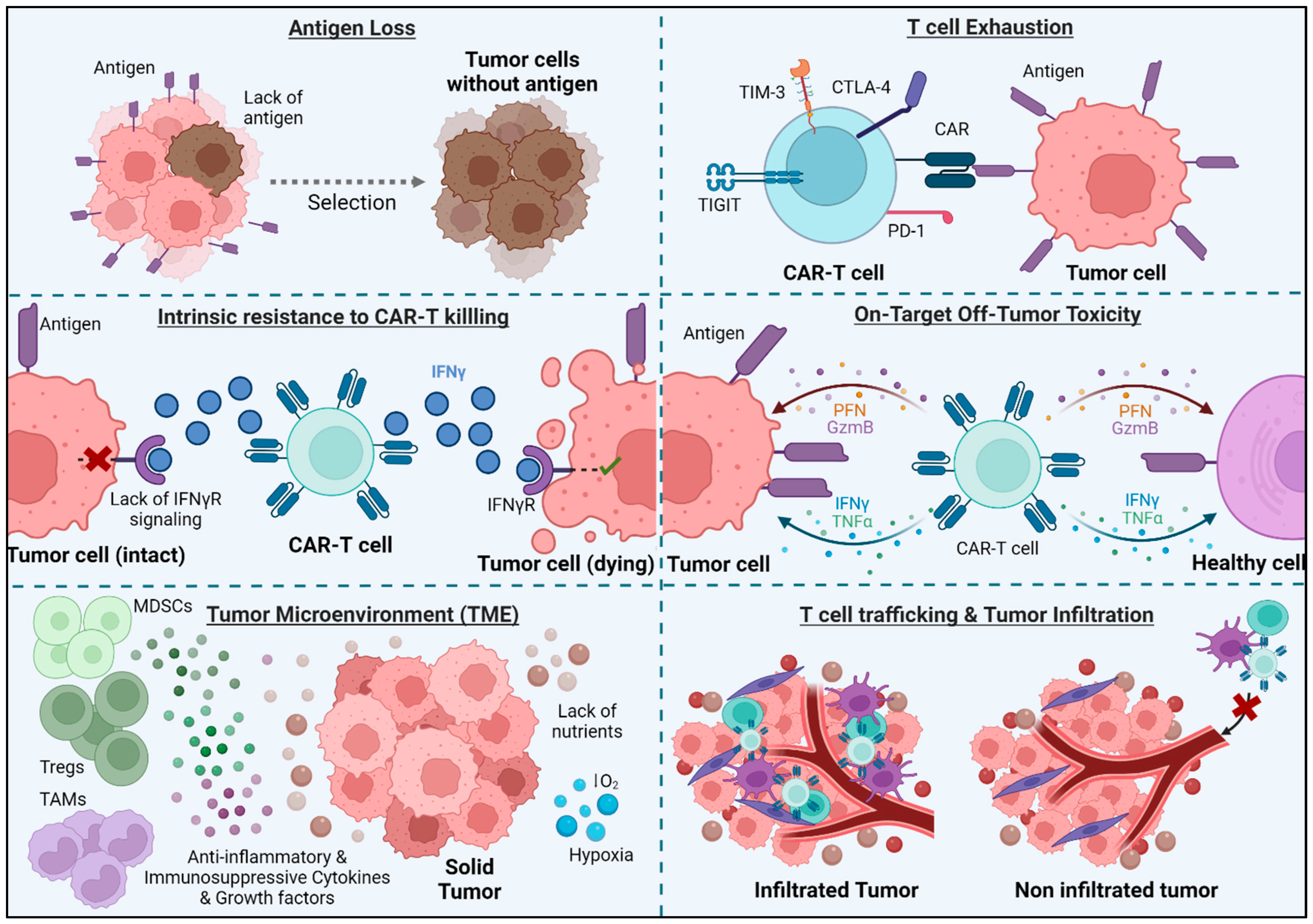
3.1. Antigen Loss
3.2. T Cell Exhaustion
3.3. T Cell Trafficking and Tumor Infiltration
3.3.1. Local Administration
3.3.2. Expression of Chemokine Receptors
3.3.3. CAR-T Cells Engineered to Better Penetrate the Tumor Stroma
3.4. Immunosuppressive Tumor Microenvironment (TME)
3.5. Intrinsic Resistance to CAR-T Killing
3.6. Combination Strategies to Improve CAR-T Therapies
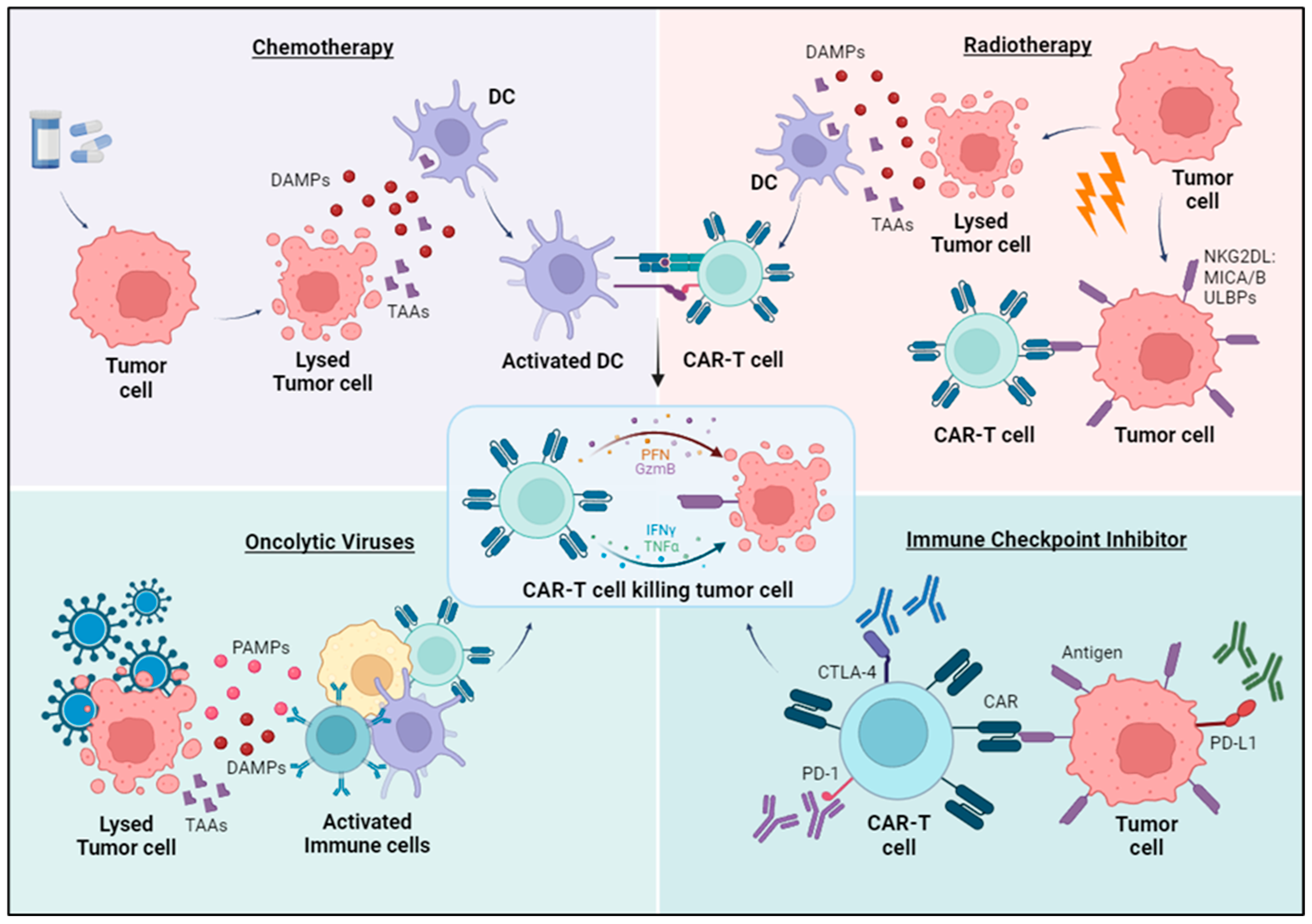
3.6.1. Chemotherapy
3.6.2. Radiotherapy
3.6.3. Oncolytic Viruses
3.6.4. Immune Checkpoints Inhibitors
3.7. On-Target Off-Tumor Toxicity
4. CAR-T Cell Targets in Solid Tumors
4.1. Protein Antigens
4.1.1. HER2
4.1.2. EGFR
4.1.3. CEA
4.1.4. ROR1
4.1.5. NKG2D Ligands
4.1.6. B7-H3
4.1.7. CD70
4.1.8. CLDN18.2
4.1.9. Mesothelin
4.2. Carbohydrate Antigens
4.2.1. Heparan Sulfate Proteoglycans (HSPGs)
4.2.2. Mucin Glycans
4.2.3. Gangliosides
4.2.4. Blood-Group Related Lewis Antigens
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A Phase I Study on Adoptive Immunotherapy Using Gene-Modified T Cells for Ovarian Cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef] [PubMed]
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. CD28 Costimulation Improves Expansion and Persistence of Chimeric Antigen Receptor-Modified T Cells in Lymphoma Patients. J. Clin. Investig. 2011, 121, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, L.; Zhang, Y.; Qin, Y.; Guan, Y.; Zhang, T.; Liu, C.; Zhou, J. Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies. Front. Oncol. 2019, 9, 1237. [Google Scholar] [CrossRef]
- Zhong, X.S.; Matsushita, M.; Plotkin, J.; Riviere, I.; Sadelain, M. Chimeric Antigen Receptors Combining 4-1BB and CD28 Signaling Domains Augment PI 3 Kinase/AKT/Bcl-X L Activation and CD8 T Cell-Mediated Tumor Eradication. Mol. Ther. 2010, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Koneru, M.; Purdon, T.J.; Spriggs, D.; Koneru, S.; Brentjens, R.J. IL-12 Secreting Tumor-Targeted Chimeric Antigen Receptor T Cells Eradicate Ovarian Tumors in Vivo. Oncoimmunology 2015, 4, e994446. [Google Scholar] [CrossRef] [PubMed]
- Zannikou, M.; Duffy, J.T.; Levine, R.N.; Seblani, M.; Liu, Q.; Presser, A.; Arrieta, V.A.; Chen, C.J.; Sonabend, A.M.; Horbinski, C.M.; et al. IL15 Modification Enables CAR T Cells to Act as a Dual Targeting Agent against Tumor Cells and Myeloid-Derived Suppressor Cells in GBM. J. Immunother. Cancer 2023, 11, e006239. [Google Scholar] [CrossRef]
- Glienke, W.; Dragon, A.C.; Zimmermann, K.; Martyniszyn-Eiben, A.; Mertens, M.; Abken, H.; Rossig, C.; Altvater, B.; Aleksandrova, K.; Arseniev, L.; et al. GMP-Compliant Manufacturing of TRUCKs: CAR T Cells Targeting GD2 and Releasing Inducible IL-18. Front. Immunol. 2022, 13, 839783. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The Fourth Generation of CARs. Expert. Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.H.; Saso, K.; Butler, M.O.; Minden, M.D.; Hirano, N. A Novel Chimeric Antigen Receptor Containing a JAK-STAT Signaling Domain Mediates Superior Antitumor Effects. Nat. Med. 2018, 24, 352–359. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Eyre, T.A.; Cheah, C.Y.; Wang, M.L. Therapeutic Options for Relapsed/Refractory Mantle Cell Lymphoma. Blood 2022, 139, 666–677. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from Car T-Cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Rafiq, S.; Brentjens, R.J. Tumors Evading CARs—The Chase Is On. Nat. Med. 2018, 24, 1492–1493. [Google Scholar] [CrossRef]
- Larson, S.M.; Walthers, C.M.; Ji, B.; Ghafouri, S.N.; Naparstek, J.; Trent, J.; Chen, J.M.; Roshandell, M.; Harris, C.; Khericha, M.; et al. CD19/CD20 Bispecific Chimeric Antigen Receptor (CAR) in Naive/Memory T Cells for the Treatment of Relapsed or Refractory Non-Hodgkin Lymphoma. Cancer Discov. 2023, 13, 580–597. [Google Scholar] [CrossRef]
- Weber, E.W.; Parker, K.R.; Sotillo, E.; Lynn, R.C.; Anbunathan, H.; Lattin, J.; Good, Z.; Belk, J.A.; Daniel, B.; Klysz, D.; et al. Transient Rest Restores Functionality in Exhausted CAR-T Cells through Epigenetic Remodeling. Science 2021, 372, eaba1786. [Google Scholar] [CrossRef]
- Gumber, D.; Wang, L.D. Improving CAR-T Immunotherapy: Overcoming the Challenges of T Cell Exhaustion. EBioMedicine 2022, 77, 103941. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A Phase i Trial of Regional Mesothelin-Targeted Car t-Cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti–Pd-1 Agent Pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef]
- Tracy, S.I.; Venkatesh, H.; Hekim, C.; Heltemes-Harris, L.M.; Knutson, T.P.; Bachanova, V.; Farrar, M.A. Combining Nilotinib and PD-L1 Blockade Reverses CD4+ T-Cell Dysfunction and Prevents Relapse in Acute B-Cell Leukemia. Blood 2022, 140, 335–348. [Google Scholar] [CrossRef]
- Liu, X.; Wen, J.; Yi, H.; Hou, X.; Yin, Y.; Ye, G.; Wu, X.; Jiang, X. Split Chimeric Antigen Receptor-Modified T Cells Targeting Glypican-3 Suppress Hepatocellular Carcinoma Growth with Reduced Cytokine Release. Ther. Adv. Med. Oncol. 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Cha, S.E.; Kujawski, M.; Yazaki, P.J.; Brown, C.; Shively, J.E. Tumor Regression and Immunity in Combination Therapy with Anti-CEA Chimeric Antigen Receptor T Cells and Anti-CEA-IL2 Immunocytokine. Oncoimmunology 2021, 10, 1899469. [Google Scholar] [CrossRef]
- Mayor, M.; Zeltsman, M.; McGee, E.; Adusumilli, P.S. A Regional Approach for CAR T-Cell Therapy for Mesothelioma: From Mouse Models to Clinical Trial. Immunotherapy 2016, 8, 491–494. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Cherkassky, L.; Villena-Vargas, J.; Colovos, C.; Servais, E.; Plotkin, J.; Jones, D.R.; Sadelain, M. Regional Delivery of Mesothelin-Targeted CAR T Cell Therapy Generates Potent and Long-Lasting CD4-Dependent Tumor Immunity. Sci. Transl. Med. 2014, 6, 261ra151. [Google Scholar] [CrossRef]
- Priceman, S.J.; Tilakawardane, D.; Jeang, B.; Aguilar, B.; Murad, J.P.; Park, A.K.; Chang, W.C.; Ostberg, J.R.; Neman, J.; Jandial, R.; et al. Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2 + Breast Cancer Metastasis to the Brain. Clin. Cancer Res. 2018, 24, 95–105. [Google Scholar] [CrossRef]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key Chemokines Direct Migration of Immune Cells in Solid Tumors. Cancer Gene Ther. 2022, 29, 10–21. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Carranza-Rua, O.; Alfaro, C.; Oñate, C.; Martín-Algarra, S.; Perez, G.; Landazuri, S.F.; Gonzalez, A.; Gross, S.; Rodriguez, I.; et al. Serum Interleukin-8 Reflects Tumor Burden and Treatment Response across Malignancies of Multiple Tissue Origins. Clin. Cancer Res. 2014, 20, 5697–5707. [Google Scholar] [CrossRef]
- Whilding, L.M.; Halim, L.; Draper, B.; Parente-Pereira, A.C.; Zabinski, T.; Davies, D.M.; Maher, J. CAR T-Cells Targeting the Integrin Avβ6 and Co-Expressing the Chemokine Receptor CXCR2 Demonstrate Enhanced Homing and Efficacy against Several Solid Malignancies. Cancers 2019, 11, 674. [Google Scholar] [CrossRef]
- Bughda, R.; Dimou, P.; D’souza, R.R.; Klampatsa, A. Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: Launching an Attack on Tumor Stroma. Immunotargets Ther. 2021, 10, 313–323. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, C.T.; Ma, T.T.; Li, Z.Y.; Zhou, L.N.; Mu, B.; Leng, F.; Shi, H.S.; Li, Y.O.; Wei, Y.Q. Immunotherapy Targeting Fibroblast Activation Protein Inhibits Tumor Growth and Increases Survival in a Murine Colon Cancer Model. Cancer Sci. 2010, 101, 2325–2332. [Google Scholar] [CrossRef]
- Lee, J.; Fassnacht, M.; Nair, S.; Boczkowski, D.; Gilboa, E. Tumor Immunotherapy Targeting Fibroblast Activation Protein, a Product Expressed in Tumor-Associated Fibroblasts. Cancer Res. 2005, 65, 11156–11163. [Google Scholar] [CrossRef]
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting Tumor-Associated Fibroblasts Improves Cancer Chemotherapy by Increasing Intratumoral Drug Uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, Y.; Yang, S.; Tu, Y.; Wang, C.; Li, J.; Yuan, Y.; Lian, Z. Bioorthogonal Equipping CAR-T Cells with Hyaluronidase and Checkpoint Blocking Antibody for Enhanced Solid Tumor Immunotherapy. ACS Cent. Sci. 2022, 8, 603–614. [Google Scholar] [CrossRef]
- Lim, A.R.; Rathmell, W.K.; Rathmell, J.C. The Tumor Microenvironment as a Metabolic Barrier to Effector T Cells and Immunotherapy. Elife 2020, 9, e55185. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Tang, N.; Cheng, C.; Zhang, X.; Qiao, M.; Li, N.; Mu, W.; Wei, X.F.; Han, W.; Wang, H. TGF-β Inhibition via CRISPR Promotes the Long-Term Efficacy of CAR T Cells against Solid Tumors. JCI Insight 2020, 5, e133977. [Google Scholar] [CrossRef]
- Narayan, V.; Barber-Rotenberg, J.S.; Jung, I.Y.; Lacey, S.F.; Rech, A.J.; Davis, M.M.; Hwang, W.T.; Lal, P.; Carpenter, E.L.; Maude, S.L.; et al. PSMA-Targeting TGFβ-Insensitive Armored CAR T Cells in Metastatic Castration-Resistant Prostate Cancer: A Phase 1 Trial. Nat. Med. 2022, 28, 724–734. [Google Scholar] [CrossRef]
- Akbari, B.; Soltantoyeh, T.; Shahosseini, Z.; Jadidi-Niaragh, F.; Hadjati, J.; Brown, C.E.; Mirzaei, H.R. PGE2-EP2/EP4 Signaling Elicits MesoCAR T Cell Immunosuppression in Pancreatic Cancer. Front. Immunol. 2023, 14, 1209572. [Google Scholar] [CrossRef]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-Secreting CAR-T Cell Therapy for Tumors with Positive Glypican-3 or Mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef]
- Larson, R.C.; Kann, M.C.; Bailey, S.R.; Haradhvala, N.J.; Llopis, P.M.; Bouffard, A.A.; Scarfó, I.; Leick, M.B.; Grauwet, K.; Berger, T.R.; et al. CAR T Cell Killing Requires the IFNγR Pathway in Solid but Not Liquid Tumours. Nature 2022, 604, 563–570. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Shi, J.; Liu, J.; Li, Q.; Chen, L. Combination Therapy: A Feasibility Strategy for Car-t Cell Therapy in the Treatment of Solid Tumors (Review). Oncol. Lett. 2018, 16, 2063–2070. [Google Scholar] [CrossRef]
- Srivastava, S.; Furlan, S.N.; Jaeger-Ruckstuhl, C.A.; Sarvothama, M.; Berger, C.; Smythe, K.S.; Garrison, S.M.; Specht, J.M.; Lee, S.M.; Amezquita, R.A.; et al. Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to Lung Tumors and Improves Antitumor Efficacy When Combined with Checkpoint Blockade. Cancer Cell 2021, 39, 193–208.e10. [Google Scholar] [CrossRef]
- Hovhannisyan, L.; Riether, C.; Aebersold, D.M.; Medová, M.; Zimmer, Y. CAR T Cell-Based Immunotherapy and Radiation Therapy: Potential, Promises and Risks. Mol. Cancer 2023, 22, 82. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, Y.O.; Park, S.W.; Bae, J.H.; Joo, S.C.; Hyung, H.K.; Chung, B.S.; Kim, S.H.; Kang, C.D. Increase of NKG2D Ligands and Sensitivity to NK Cell-Mediated Cytotoxicity of Tumor Cells by Heat Shock and Ionizing Radiation. Exp. Mol. Med. 2006, 38, 474–484. [Google Scholar] [CrossRef]
- Han, Y.; Xie, W.; Song, D.G.; Powell, D.J. Control of Triple-Negative Breast Cancer Using Ex Vivo Self-Enriched, Costimulated NKG2D CAR T Cells. J. Hematol. Oncol. 2018, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, L.; Sadagopan, A.; Ma, T.; Dotti, G.; Wang, Y.; Zheng, H.; Gao, X.; Wang, D.; DeLeo, A.B.; et al. Targeting Radiation-Resistant Prostate Cancer Stem Cells by B7-H3 CAR T Cells. Mol. Cancer Ther. 2021, 20, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Weller, M.; Guckenberger, M.; Sentman, C.L.; Roth, P. NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma. Cancer Res. 2018, 78, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-Armed Oncolytic Adenoviruses Enhance CAR-T Cell Therapeutic Efficacy and Reprogram Tumor Microenvironment in Glioblastoma. Mol. Ther. 2023, 31, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Siriwon, N.; Zhang, X.; Yang, S.; Jin, T.; He, F.; Kim, Y.J.; Mac, J.; Lu, Z.; Wang, S.; et al. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor–Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clin. Cancer Res. 2017, 23, 6982–6992. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Gibson, J.; Ou, K.; Lopez, L.S.; Ng, R.H.; Leggett, N.; Jonsson, V.D.; Zarif, J.C.; Lee, P.P.; Wang, X.; et al. PD-L1 Blockade Restores CAR T Cell Activity through IFN-Î 3-Regulation of CD163+ M2 Macrophages. J. Immunother. Cancer 2022, 10, e004400. [Google Scholar] [CrossRef] [PubMed]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-el-Enein, M. Overcoming On-Target, off-Tumour Toxicity of CAR T Cell Therapy for Solid Tumours. Nat. Rev. Clin. Oncol. 2023, 20, 49–62. [Google Scholar] [CrossRef]
- Li, K.; Qian, S.; Huang, M.; Chen, M.; Peng, L.; Liu, J.; Xu, W.; Xu, J. Original Article Development of GPC3 and EGFR-Dual-Targeting Chimeric Antigen. Receptor-T Cells for Adoptive T Cell Therapy. Am. J. Transl. Res. 2021, 13, 156. [Google Scholar] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bang, Y.-J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Chinsuwan, T.; Hirabayashi, K.; Mishima, S.; Hasegawa, A.; Tanaka, M.; Mochizuki, H.; Shimoi, A.; Murakami, T.; Yagyu, S.; Shimizu, K.; et al. Ligand-Based, PiggyBac-Engineered CAR-T Cells Targeting EGFR Are Safe and Effective against Non-Small Cell Lung Cancers. Mol. Ther. Oncolytics 2023, 31, 100728. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic Antigen (CEA) as Tumor Marker in Lung Cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J. ROR1: An Orphan Becomes Apparent. Blood 2022, 140, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Rebagay, G.; Yan, S.; Liu, C.; Cheung, N.K. ROR1 and ROR2 in Human Malignancies: Potentials for Targeted Therapy. Front. Oncol. 2012, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.M.; Diefenbach, A.; Mcmahon, C.W.; Xiong, N.; Carlyle, J.R.; Raulet, D.H. The Role of the NKG2D Immunoreceptor in Immune Cell Activation and Natural Killing. Immunity 2002, 17, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Rhinehart, R.; Secrist, H.; Bauer, S.; Grabstein, K.H.; Spies, T. Broad Tumor-Associated Expression and Recognition by Tumor-Derived T Cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 1999, 96, 6879–6884. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Jin, W.L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, Ø.; Tan, M. New Frontiers in Immune Checkpoint B7-H3 (CD276) Research and Drug Development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef]
- Lutfi, F.; Wu, L.; Sunshine, S.; Cao, X. Targeting the CD27-CD70 Pathway to Improve Outcomes in Both Checkpoint Immunotherapy and Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2021, 12, 715909. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Deschoolmeester, V.; Zwaenepoel, K.; Rolfo, C.; Silence, K.; Rottey, S.; Lardon, F.; Smits, E.; Pauwels, P. CD70: An Emerging Target in Cancer Immunotherapy. Pharmacol. Ther. 2015, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 Is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Z.; Hu, C.; Zhang, S.; Zi, M.; Yuan, L.; Cheng, X. Targeting CLDN18.2 in Cancers of the Gastrointestinal Tract: New Drugs and New Indications. Front. Oncol. 2023, 13, 1132319. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Application of Mesothelin Immunostaining in Tumor Diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Mao, L.; Wu, M.; Liu, J.; Yu, S. Challenges of Anti-Mesothelin CAR-T-Cell Therapy. Cancers 2023, 15, 1357. [Google Scholar] [CrossRef]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular Basis and Therapeutic Implications of CD40/CD40L Immune Checkpoint. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-Associated Carbohydrate Antigens. Annu. Rev. Immunol. 1984, 2, 103–126. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Lara Santos, L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 460344. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.G.; Pucci, M.; Venturi, G.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Glycosylation as a Main Regulator of Growth and Death Factor Receptors Signaling. Int. J. Mol. Sci. 2018, 19, 580. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed]
- Rossig, C.; Kailayangiri, S.; Jamitzky, S.; Altvater, B. Carbohydrate Targets for CAR T Cells in Solid Childhood Cancers. Front. Oncol. 2018, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Boyaval, F.; Van Zeijl, R.; Dalebout, H.; Holst, S.; Van Pelt, G.; Fariña-Sarasqueta, A.; Mesker, W.; Tollenaar, R.; Morreau, H.; Wuhrer, M.; et al. N-Glycomic Signature of Stage II Colorectal Cancer and Its Association with the Tumor Microenvironment. Mol. Cell. Proteom. 2021, 20, 100057. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.; Bäckström, M.; Dalziel, M.; Gschmeissner, S.; Karlsson, H.; Noll, T.; Gätgens, J.; Clausen, H.; Hansson, G.C.; Burchell, J.; et al. The ST6GalNAc-I Sialyltransferase Localizes throughout the Golgi and Is Responsible for the Synthesis of the Tumor-Associated Sialyl-Tn O-Glycan in Human Breast Cancer. J. Biol. Chem. 2006, 281, 3586–3594. [Google Scholar] [CrossRef]
- Livingston, P.O. Augmenting the Immunogenicity of Carbohydrate Tumor Antigens. Semin. Cancer Biol. 1995, 6, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.J.; Lees, A.; Snapper, C.M. T Cell-Independent Antigens Type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Nagarajan, A.; Malvi, P.; Wajapeyee, N. Heparan Sulfate and Heparan Sulfate Proteoglycans in Cancer Initiation and Progression. Front. Endocrinol. 2018, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast Growth Factor Signalling: From Development to Cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Bernfield, M.; Götte, M.; Park, W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef]
- Li, N.; Gao, W.; Zhang, Y.F.; Ho, M. Glypicans as Cancer Therapeutic Targets. Trends Cancer 2018, 4, 741–754. [Google Scholar] [CrossRef]
- Duan, L.; Hu, X.Q.; Feng, D.Y.; Lei, S.Y.; Hu, G.H. GPC-1 May Serve as a Predictor of Perineural Invasion and a Prognosticator of Survival in Pancreatic Cancer. Asian J. Surg. 2013, 36, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Maruyama, H.; Guo, F.; Kleeff, J.; Itakura, J.; Matsumoto, Y.; Lander, A.D.; Korc, M. Glypican-1 Is Overexpressed in Human Breast Cancer and Modulates the Mitogenic Effects of Multiple Heparin-Binding Growth Factors in Breast Cancer Cells. Cancer Res. 2001, 61, 5562–5569. [Google Scholar] [PubMed]
- Li, N.; Spetz, M.R.; Ho, M. The Role of Glypicans in Cancer Progression and Therapy. J. Histochem. Cytochem. 2020, 68, 841–862. [Google Scholar] [CrossRef]
- Li, N.; Fu, H.; Hewitt, S.M.; Dimitrov, D.S.; Ho, M. Therapeutically Targeting Glypican-2 via Single-Domain Antibody-Based Chimeric Antigen Receptors and Immunotoxins in Neuroblastoma. Proc. Natl. Acad. Sci. USA 2017, 114, E6623–E6631. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Friess, H.; Wang, L.; Abou-Shady, M.; Zimmermann, A.; Lander, A.D.; Korc, M.; Kleeff, J.; Büchler, M.W. Enhanced Glypican-3 Expression Differentiates the Majority of Hepatocellular Carcinomas from Benign Hepatic Disorders. Gut 2001, 48, 558–564. [Google Scholar] [CrossRef]
- Furini, S.; Falciani, C. Expression and Role of Heparan Sulfated Proteoglycans in Pancreatic Cancer. Front. Oncol. 2021, 11, 695858. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Sun, L.; Zhao, Z.; Liu, S.; Kuang, X.; Shu, J.; Luo, B. Glypican-4 Gene Polymorphism (Rs1048369) and Susceptibility to Epstein-Barr Virus-Associated and -Negative Gastric Carcinoma. Virus Res. 2016, 220, 52–56. [Google Scholar] [CrossRef]
- Dinccelik-Aslan, M.; Gumus-Akay, G.; Elhan, A.H.; Unal, E.; Tukun, A. Diagnostic and Prognostic Significance of Glypican 5 and Glypican 6 Gene Expression Levels in Gastric Adenocarcinoma. Mol. Clin. Oncol. 2015, 3, 584–590. [Google Scholar] [CrossRef]
- Munir, J.; Van Ngu, T.; Na Ayudthaya, P.D.; Ryu, S. Downregulation of Glypican-4 Facilitates Breast Cancer Progression by Inducing Cell Migration and Proliferation. Biochem. Biophys. Res. Commun. 2020, 526, 91–97. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Qiu, M.; Hu, J.; Fan, X.; Wang, J.; Xu, L.; Yin, R. Glypican-5 Is a Novel Metastasis Suppressor Gene in Non-Small Cell Lung Cancer. Cancer Lett. 2013, 341, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Zhang, D.; Zhang, Z.; Xu, Y.; Liu, S. A Lung Cancer Gene GPC5 Could Also Be Crucial in Breast Cancer. Mol. Genet. Metab. 2011, 103, 104–105. [Google Scholar] [CrossRef]
- Sawada, Y.; Yoshikawa, T.; Nobuoka, D.; Shirakawa, H.; Kuronuma, T.; Motomura, Y.; Mizuno, S.; Ishii, H.; Nakachi, K.; Konishi, M.; et al. Phase I Trial of a Glypican-3-Derived Peptide Vaccine for Advanced Hepatocellular Carcinoma: Immunologic Evidence and Potential for Improving Overall Survival. Clin. Cancer Res. 2012, 18, 3686–3696. [Google Scholar] [CrossRef]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; et al. Phase II Study of the GPC3-Derived Peptide Vaccine as an Adjuvant Therapy for Hepatocellular Carcinoma Patients. Oncoimmunology 2016, 5, e1129483. [Google Scholar] [CrossRef]
- Nakano, K.; Orita, T.; Nezu, J.; Yoshino, T.; Ohizumi, I.; Sugimoto, M.; Furugaki, K.; Kinoshita, Y.; Ishiguro, T.; Hamakubo, T.; et al. Anti-Glypican 3 Antibodies Cause ADCC against Human Hepatocellular Carcinoma Cells. Biochem. Biophys. Res. Commun. 2009, 378, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Gold, P.J.; El-Khoueiry, A.B.; Abrams, T.A.; Morikawa, H.; Ohishi, N.; Ohtomo, T.; Philip, P.A. First-in-Man Phase I Study of GC33, a Novel Recombinant Humanized Antibody against Glypican-3, in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 920–928. [Google Scholar] [CrossRef]
- Kato, D.; Yaguchi, T.; Iwata, T.; Katoh, Y.; Morii, K.; Tsubota, K.; Takise, Y.; Tamiya, M.; Kamada, H.; Akiba, H.; et al. GPC1 Specific CAR-T Cells Eradicate Established Solid Tumor without Adverse Effects and Synergize with Anti-PD-1 Ab. Elife 2020, 9, e49392. [Google Scholar] [CrossRef]
- Bailis, W.; Shyer, J.A.; Zhao, J.; Canaveras, J.C.G.; Al Khazal, F.J.; Qu, R.; Steach, H.R.; Bielecki, P.; Khan, O.; Jackson, R.; et al. Distinct Modes of Mitochondrial Metabolism Uncouple T Cell Differentiation and Function. Nature 2019, 571, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Hickman, T.L.; Choi, E.; Whiteman, K.R.; Muralidharan, S.; Pai, T.; Johnson, T.; Parikh, A.; Friedman, T.; Gilbert, M.; Shen, B.; et al. BOXR1030, an Anti-GPC3 CAR with Exogenous GOT2 Expression, Shows Enhanced T Cell Metabolism and Improved Anti-Cell Line Derived Tumor Xenograft Activity. PLoS ONE 2022, 17, e0266980. [Google Scholar] [CrossRef]
- Afratis, N.A.; Nikitovic, D.; Multhaupt, H.A.B.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key Regulators of Cell Signaling and Biological Functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef]
- Shriver, Z.; Capila, I.; Venkataraman, G.; Sasisekharan, R. Heparin and Heparan Sulfate: Analyzing Structure and Microheterogeneity. Handb. Exp. Pharmacol. 2012, 207, 159–176. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Biosynthesis and Function of Chondroitin Sulfate. Biochim. Biophys. Acta 2013, 1830, 4719–4733. [Google Scholar] [CrossRef] [PubMed]
- Barbareschi, M.; Maisonneuve, P.; Aldovini, D.; Cangi, M.G.; Pecciarini, L.; Mauri, F.A.; Veronese, S.; Caffo, O.; Lucenti, A.; Palma, P.D.; et al. High Syndecan-1 Expression in Breast Carcinoma Is Related to an Aggressive Phenotype and to Poorer Prognosis. Cancer 2003, 98, 474–483. [Google Scholar] [CrossRef]
- Davies, E.J.; Blackhall, F.H.; Shanks, J.H.; David, G.; McGown, A.T.; Swindell, R.; Slade, R.J.; Martin-Hirsch, P.; Gallagher, J.T.; Jayson, G.C. Distribution and Clinical Significance of Heparan Sulfate Proteoglycans in Ovarian Cancer. Clin. Cancer Res. 2004, 10, 5178–5186. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, D.; Chen, Y.; Li, W.; Liu, R.; He, Y.; Ren, L.; Zhou, L.; Deng, T.; Wang, X.; et al. Shed Syndecan-1 Is Involved in Chemotherapy Resistance via the EGFR Pathway in Colorectal Cancer. Br. J. Cancer 2014, 111, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, J.A.; Cho, I.S.; Ihm, C.H. Soluble Syndecan-1 at Diagnosis and during Follow up of Multiple Myeloma: A Single Institution Study. Korean J. Hematol. 2010, 45, 115–119. [Google Scholar] [CrossRef]
- Joensuu, H.; Anttonen, A.; Eriksson, M.; Mäkitaro, R.; Alfthan, H.; Kinnula, V.; Leppä, S. Soluble Syndecan-1 and Serum Basic Fibroblast Growth Factor Are New Prognostic Factors in Lung Cancer. Cancer Res. 2002, 62, 5210–5217. [Google Scholar]
- Hua, R.; Yu, J.; Yan, X.; Ni, Q.; Zhi, X.; Li, X.; Jiang, B.; Zhu, J. Syndecan-2 in Colorectal Cancer Plays Oncogenic Role via Epithelial-Mesenchymal Transition and MAPK Pathway. Biomed. Pharmacother. 2020, 121, 109630. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Osorio, J.C.; Chu, S.G.; Fernandez, I.E.; De Frias, S.P.; Sholl, L.; Cui, Y.; Tellez, C.S.; Siegfried, J.M.; Belinsky, S.A.; et al. Lung Adenocarcinoma Syndecan-2 Potentiates Cell Invasiveness. Am. J. Respir. Cell Mol. Biol. 2019, 60, 659–666. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.; Abiatari, I.; Raulefs, S.; Sauliunaite, D.; Erkan, M.; Kong, B.; Friess, H.; Michalski, C.W.; Kleeff, J. Syndecan-2 Promotes Perineural Invasion and Cooperates with K-Ras to Induce an Invasive Pancreatic Cancer Cell Phenotype. Mol. Cancer 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, W.Y.; Li, S.G.; Feng, X.S.; Gao, S.G. Midkine Promotes Perineural Invasion in Human Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Oka, Y.; Fujiki, F.; Morimoto, S.; Nakajima, H.; Nakae, Y.; Nakata, J.; Nishida, S.; Hosen, N.; Tatsumi, N.; et al. Syndecan-4 as a Biomarker to Predict Clinical Outcome for Glioblastoma Multiforme Treated with WT1 Peptide Vaccine. Future Sci. OA 2016, 2, FSO96. [Google Scholar] [CrossRef] [PubMed]
- Na, K.Y.; Bacchini, P.; Bertoni, F.; Kim, Y.W.; Park, Y.K. Syndecan-4 and Fibronectin in Osteosarcoma. Pathology 2012, 44, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, M.; Han, Q.; Hui, F.; Dai, H.; Zhang, W.; Zhang, Y.; Wang, Y.; Zhu, H.; Han, W. CD138-Directed Adoptive Immunotherapy of Chimeric Antigen Receptor (CAR)-Modified T Cells for Multiple Myeloma. J. Cell. Immunother. 2016, 2, 28–35. [Google Scholar] [CrossRef]
- Behera, S.K.; Praharaj, A.B.; Dehury, B.; Negi, S. Exploring the Role and Diversity of Mucins in Health and Disease with Special Insight into Non-Communicable Diseases. Glycoconj. J. 2015, 32, 575–613. [Google Scholar] [CrossRef]
- Jonckheere, N.; Vincent, A.; Neve, B.; Van Seuningen, I. Mucin Expression, Epigenetic Regulation and Patient Survival: A Toolkit of Prognostic Biomarkers in Epithelial Cancers. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188538. [Google Scholar] [CrossRef]
- Breloy, I.; Hanisch, F.G. Functional Roles of O-Glycosylation. Molecules 2018, 23, 3063. [Google Scholar] [CrossRef]
- Parry, S.; Hanisch, F.G.; Leir, S.H.; Sutton-Smith, M.; Morris, H.R.; Dell, A.; Harris, A. N-Glycosylation of the MUC1 Mucin in Epithelial Cells and Secretions. Glycobiology 2006, 16, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.S.; Yung, K.K.L. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, K.; Lam, A.K.Y.; Huang, J.; Qiu, F.; Qiao, B.; Zhang, Y. MUC1 as a Target for CAR-T Therapy in Head and Neck Squamous Cell Carinoma. Cancer Med. 2020, 9, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Maurstad, G.; Picco, G.; Arulappu, A.; Coleman, J.; Wandell, H.H.; Clausen, H.; Mandel, U.; Taylor-Papadimitriou, J.; Sletmoen, M.; et al. The Breast Cancer-Associated Glycoforms of MUC1, MUC1-Tn and Sialyl-Tn, Are Expressed in COSMC Wild-Type Cells and Bind the C-Type Lectin MGL. PLoS ONE 2015, 10, e0125994. [Google Scholar] [CrossRef] [PubMed]
- Posey, A.D.; Schwab, R.D.; Boesteanu, A.C.; Steentoft, C.; Mandel, U.; Engels, B.; Stone, J.D.; Madsen, T.D.; Schreiber, K.; Haines, K.M.; et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016, 44, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Delannoy, P. Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 6145. [Google Scholar] [CrossRef]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Miyazaki, S.; Hamamura, K.; Kambe, M.; Miyata, M.; Tajima, O.; Ohmi, Y.; Yamauchi, Y.; Furukawa, K.; Furukawa, K. Ganglioside GD3 Enhances Adhesion Signals and Augments Malignant Properties of Melanoma Cells by Recruiting Integrins to Glycolipid-Enriched Microdomains. J. Biol. Chem. 2010, 285, 27213–27223. [Google Scholar] [CrossRef]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.K.V. Oncotargets GD2 and GD3 Are Highly Expressed in Sarcomas of Children, Adolescents, and Young Adults. Pediatr. Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.H.; Furukawa, K.; Furukawa, K. Enhancement of Malignant Properties of Human Glioma Cells by Ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef]
- Wu, Z.L.; Schwartz, E.; Ladisch, S.; Seeger, R. Expression of GD2 Ganglioside by Untreated Primary Human Neuroblastomas. Cancer Res. 1986, 46, 440–443. [Google Scholar] [PubMed]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 Identifies Breast Cancer Stem Cells and Promotes Tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Merritt, W.D.; Casper, J.T.; Lauer, S.J.; Reaman, G.H. Expression of GD3 Ganglioside in Childhood T-Cell Lymphoblastic Malignancies. Cancer Res. 1987, 47, 1724–1730. [Google Scholar] [PubMed]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in Small Cell Lung Cancer Cell Lines: Enhancement of Cell Proliferation and Mediation of Apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar] [PubMed]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Shum, T.; Omer, B.; Tashiro, H.; Kruse, R.L.; Wagner, D.L.; Parikh, K.; Yi, Z.; Sauer, T.; Liu, D.; Parihar, R.; et al. Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells. Cancer Discov. 2017, 7, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, R. Alterations of Lewis Histo-Blood Group Antigen Expression in Cancer Cells. Postep. Hig. Med. Dosw. Online 2010, 64, 87–99. [Google Scholar]
- Ravindranath, M.H.; Amiri, A.A.; Bauer, P.M.; Kelley, M.C.; Essner, R.; Morton, D.L. Endothelial-Selectin Ligands Sialyl Lewis(x) and Sialyl Lewis(a) Are Differentiation Antigens Immunogenic in Human Melanoma. Cancer 1997, 79, 1686–1697. [Google Scholar] [CrossRef]
- Ugorski, M.; Laskowska, A. Sialyl Lewis(a): A Tumor-Associated Carbohydrate Antigen Involved in Adhesion and Metastatic Potential of Cancer Cells. Acta Biochim. Pol. 2002, 49, 303–311. [Google Scholar] [CrossRef]
- Yin, B.W.T.; Finstad, C.L.; Kitamura, K.; Federici, M.G.; Welshinger, M.; Kudryashov, V.; Hoskins, W.J.; Welt, S.; Lloyd, K.O. Serological and Immunochemical Analysis of Lewis y (Ley) Blood Group Antigen Expression in Epithelial Ovarian Cancer. Int. J. Cancer 1996, 65, 406–412. [Google Scholar] [CrossRef]
- Westwood, J.A.; Murray, W.K.; Trivett, M.; Haynes, N.M.; Solomon, B.; Mileshkin, L.; Ball, D.; Michael, M.; Burman, A.; Mayura-Guru, P.; et al. The Lewis-Y Carbohydrate Antigen Is Expressed by Many Human Tumors and Can Serve as a Target for Genetically Redirected T Cells despite the Presence of Soluble Antigen in Serum. J. Immunother. 2009, 32, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.S.; Neeson, P.J.; Khot, A.; Peinert, S.; Tai, T.; Tainton, K.; Chen, K.; Shin, M.; Wall, D.M.; Hönemann, D.; et al. Persistence and Efficacy of Second Generation CAR T Cell Against the LeY Antigen in Acute Myeloid Leukemia. Mol. Ther. 2013, 21, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
| Protein Antigens | |||
|---|---|---|---|
| Antigen | Total | CAR-T Interventions | Phase |
| Mesothelin (MSLN) | 20 | Anti-MSLN CAR-T cells, anti-CTLA4/PD-1 expressing MSLN CAR-T cells, CD40L expressing MSLN CAR-T cells, logic-gated MSLN CAR-T cells inhibited by HLA-A*02 | I/II |
| HER2 | 6 | Anti-HER2 CAR-T cells, oncolytic viruses + CAR-T cells, memory-enriched anti-HER2 CAR-T cells | I |
| CEA | 10 | Anti-CEA CAR-T cells, logic-gated CEA CAR-T cells inhibited by HLA-A*02 | I/II |
| ROR1 | 5 | Anti-ROR1 CAR-T cells, anti-ROR1 CAR-T cells expressing mbIL15 and PD-1 downregulation | I/II |
| B7-H3 | 7 | Anti-B7H3 CAR-T cells, dual anti-EGFR/B7H3 CAR-T cells, dual anti-CD19/B7H3 CAR-T cells with/without pembrolizumab | I |
| BT-001 | 2 | Anti BT-001 CAR-T cells | I |
| CD70 | 7 | Anti-CD70 CAR-T cells | I/II |
| NKG2DL | 8 | Anti-NKG2DL CAR-T cells, dual anti-NKG2DL/CLDN18.2 CAR-T cells, allogeneic/haploidentical anti-NKG2DL CAR γδ T cells, universal dual anti-NKG2DL-NKp44 CAR-T cells | I |
| EGFR | 6 | Dual anti-EGFR/B7H3 CAR-T cells, anti-CTLA-4/PD-1 expressing EGFR CAR-T cells, TGFβR knockout anti-EGFR CAR-T cells, dual anti-EGFR/CD19 CAR-T cells | I/II |
| CLDN18.2 | 14 | Anti-CLDN18.2 CAR-T cells, dual anti-CLDN18.2/PD-L1 CAR-T cells, dual anti-NKG2DL/CLDN18.2 CAR-T cells, anti-CLDN18.2 CAR-T cells with co-expression of cytokines, iPD-1-CLDN18.2 CAR-T cells | I/II |
| PD-L1 | 2 | dual anti-CLDN18.2/PD-L1 CAR-T cells, dual anti-VEGFR1/PD-L1 CAR-T cells | I |
| VEGFR1 | 1 | dual anti-VEGFR1/PD-L1 CAR-T cells | I |
| CD22 | 1 | Anti-PD-L1 armored anti-CD22 CAR-T/CAR-TILs | I |
| EpCAM | 2 | Anti-EpCAM CAR-T cells | I |
| TM4SF1 | 1 | Anti-TM4SF1 CAR-T cells | N/A |
| Nectin4 | 1 | Anti-Nectin4/FAP CAR-T cells expressing IL-7 and CCL19 or/and IL-12 | I |
| CLDN6 | 1 | Anti-CLDN6 CAR-T cells with or without a liposomal formulation containing CLDN6 RNA | I/II |
| PSMA | 1 | Dual anti-GD2/PSMA CAR-T cells | I/II |
| ROR2 | 1 | Anti-ROR2 CAR-T cells | I |
| EphA-2 | 2 | Anti-EphA-2 CAR-DCs loaded with TP53/KRAS mutant peptide combine with anti-PD-1/CTLA4 antibodies | I |
| HLA-G | 2 | Anti-HLA-G CAR-T cells, anti-HLA-G-BiTE γδ CAR-T cells, | I/II |
| GCC | 2 | Anti-GCC CAR-T cells | N/A |
| Carbohydrate antigens | |||
| Antigen | Total | CAR-T interventions | Phase |
| MUC1 | 7 | Anti-MUC1 CAR-T cells, anti-CTLA-4/PD-1 expressing MUC1 CAR-T cells, allogeneic anti-MUC1 CAR-T cells, PD-1 knockout anti-MUC1 CAR-T cells | I/II |
| GPC3 | 6 | Anti-GPC3 CAR-T cells, IL-15 armored anti-GPC3 CAR-T cells, IL-15 and IL-21 armored anti-GPC3 CAR-T cells, anti-GPC3 CAR-T cells with exogenous GOT2 expression | I/II |
| Lewis Y | 1 | Anti-Lewis Y CAR-T cells | I |
| GD2 | 6 | Anti-GD2 CAR-T cells, Dual anti-GD2/PSMA CAR-T cells, anti-GD2 CAR-T cells with an inducible apoptotic caspase 9 domain (iC9), OX40/CD28 expressing iC9 anti-GD2 CAR-T cells, C7R expressing anti-GD2 CAR-T Cells | I/II |
| Trop-2 | 1 | Anti-Trop-2 CAR-T cells | I/II |
| CD138 | 1 | Anti-CD138 CAR-T cells | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorós-Pérez, B.; Rivas-Pardo, B.; Gómez del Moral, M.; Subiza, J.L.; Martínez-Naves, E. State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future? Cells 2024, 13, 725. https://doi.org/10.3390/cells13090725
Amorós-Pérez B, Rivas-Pardo B, Gómez del Moral M, Subiza JL, Martínez-Naves E. State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future? Cells. 2024; 13(9):725. https://doi.org/10.3390/cells13090725
Chicago/Turabian StyleAmorós-Pérez, Beatriz, Benigno Rivas-Pardo, Manuel Gómez del Moral, José Luis Subiza, and Eduardo Martínez-Naves. 2024. "State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future?" Cells 13, no. 9: 725. https://doi.org/10.3390/cells13090725